Phosphoproteomic Profiling Deciphers Heat-Stress-Responsive Mechanisms in Passion Fruit
Abstract
1. Introduction
2. Materials and Methods
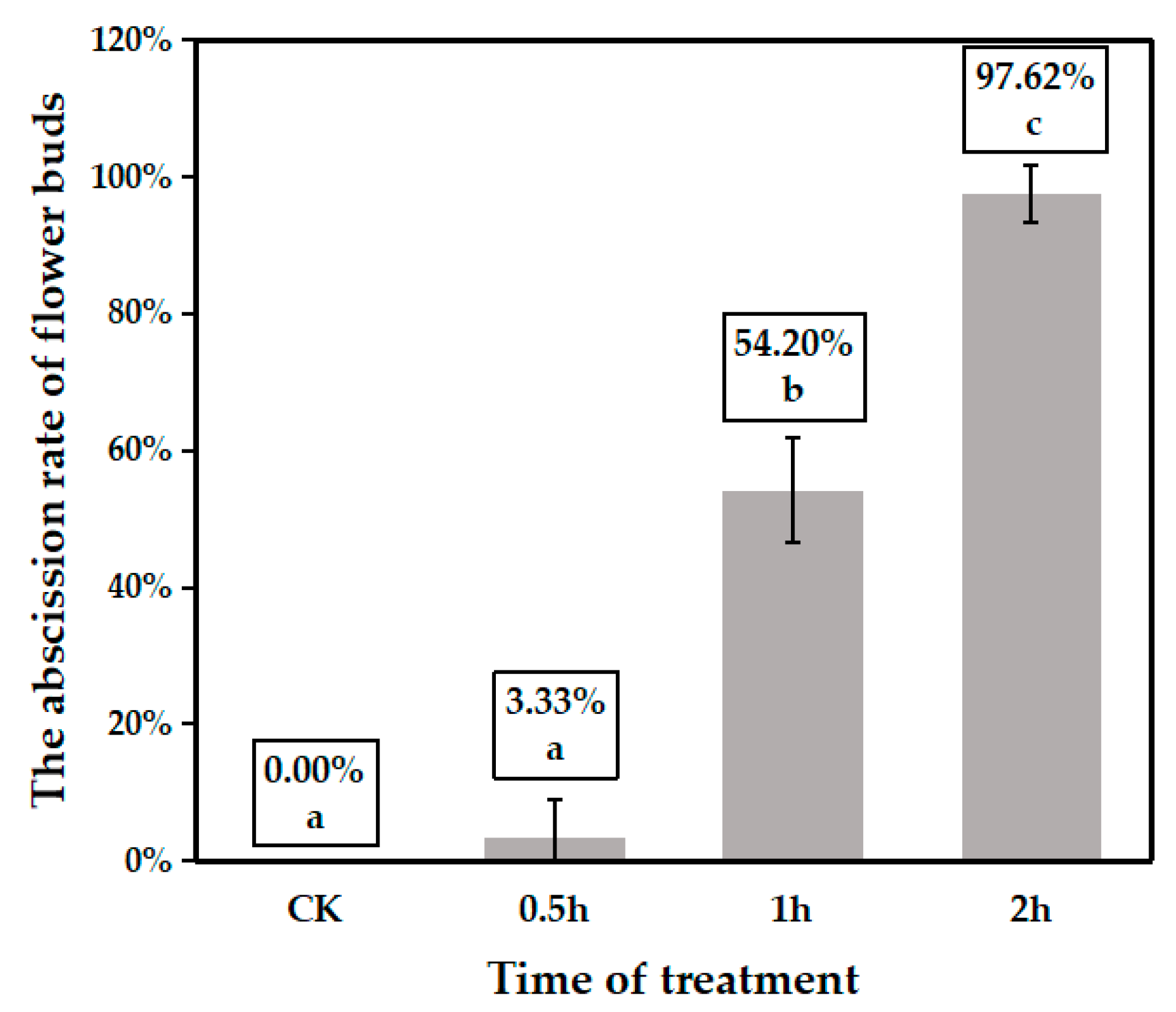
2.1. Plant Material and Treatment
2.2. Protein Extraction and Trypsin Digestion
2.3. Enrichment for Phosphorylated Peptides and LC-MS/MS Analysis
2.4. Database Search
2.5. Bioinformatics Annotation Analysis
2.6. Detection of ABA and H2O2 Contents
2.7. Quantitative Real-Time PCR
2.8. Transient Transformation Assays
2.9. Statistical Analyses
3. Results
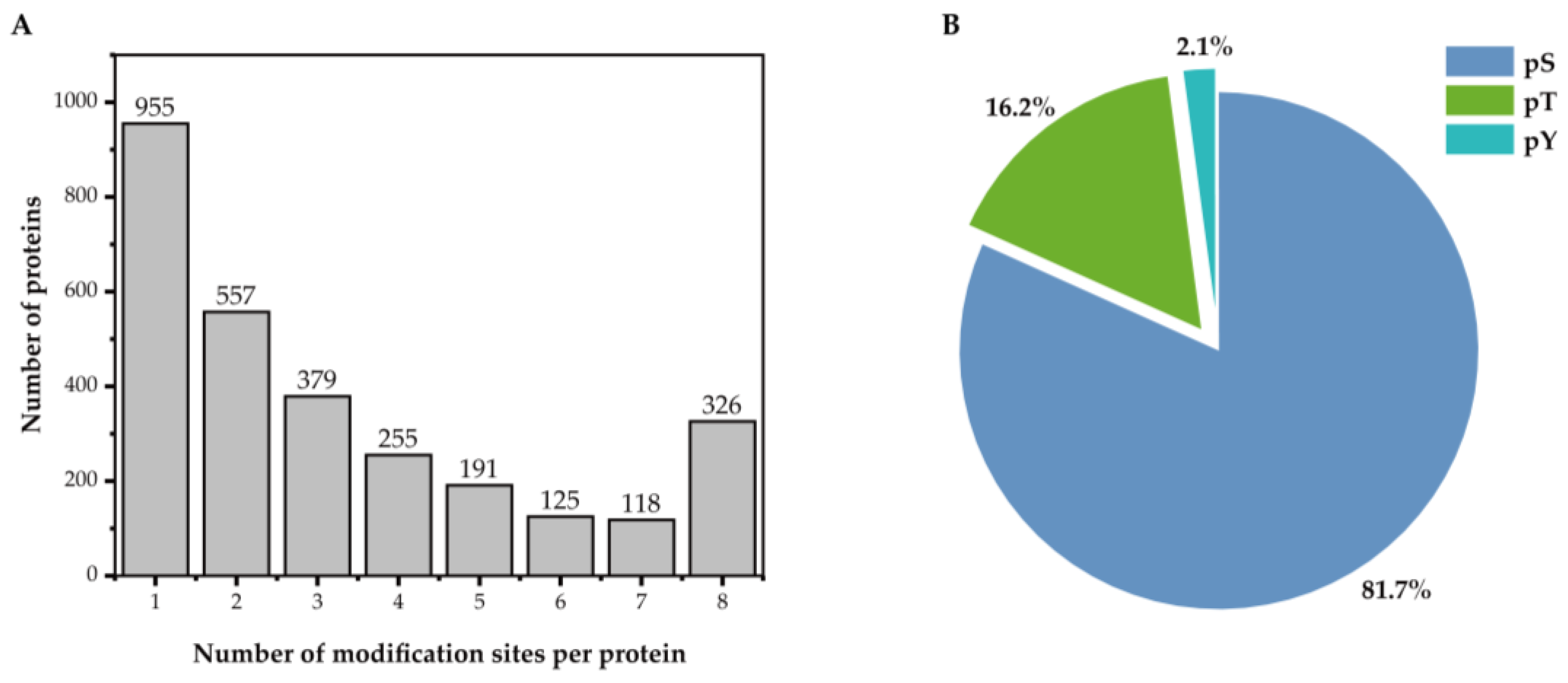
3.1. Identification of Proteins and Phosphorylated Sites in Floral Buds Under Heat Stress
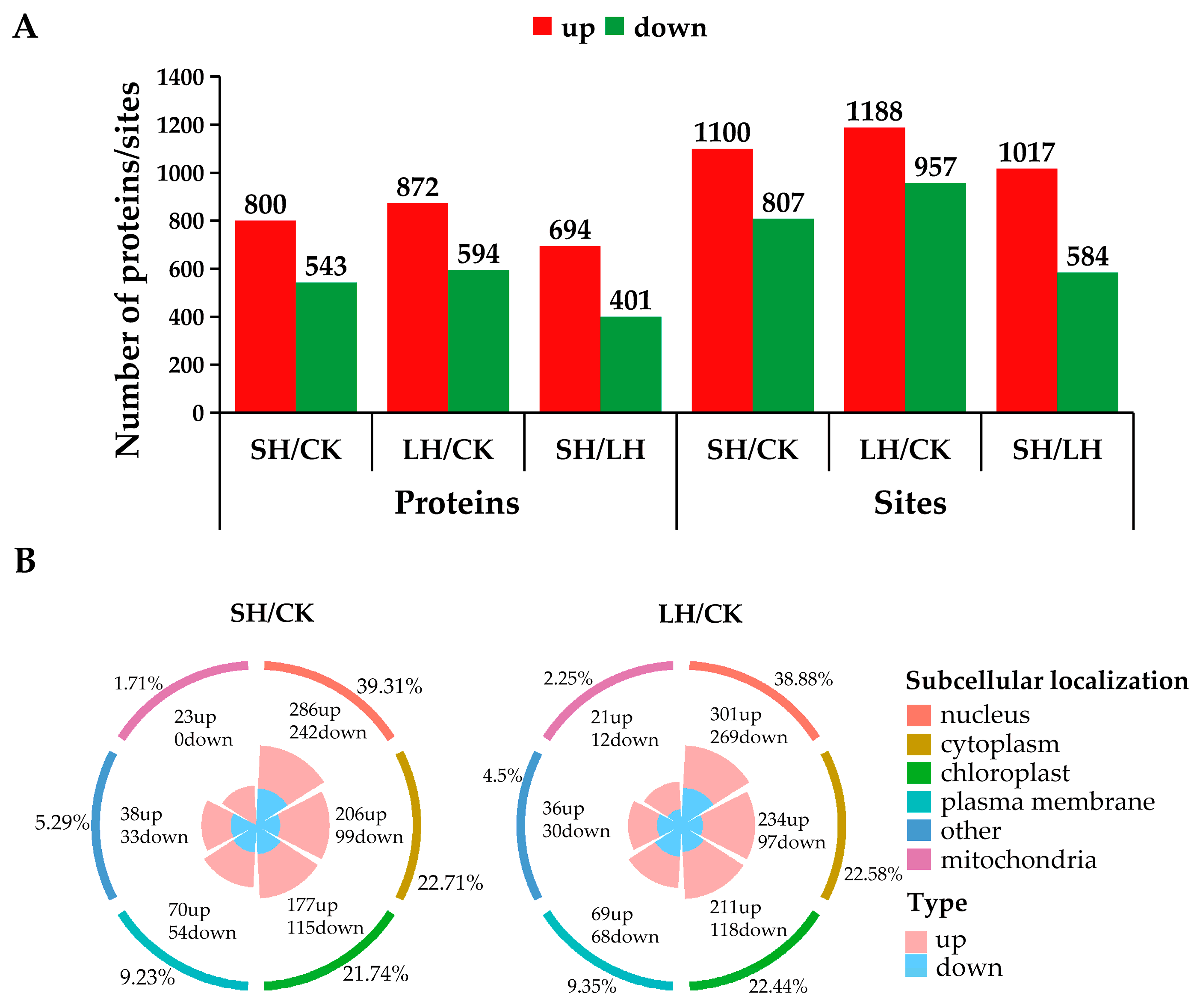
3.2. Analysis of Phosphoproteins in Response to Heat Stress
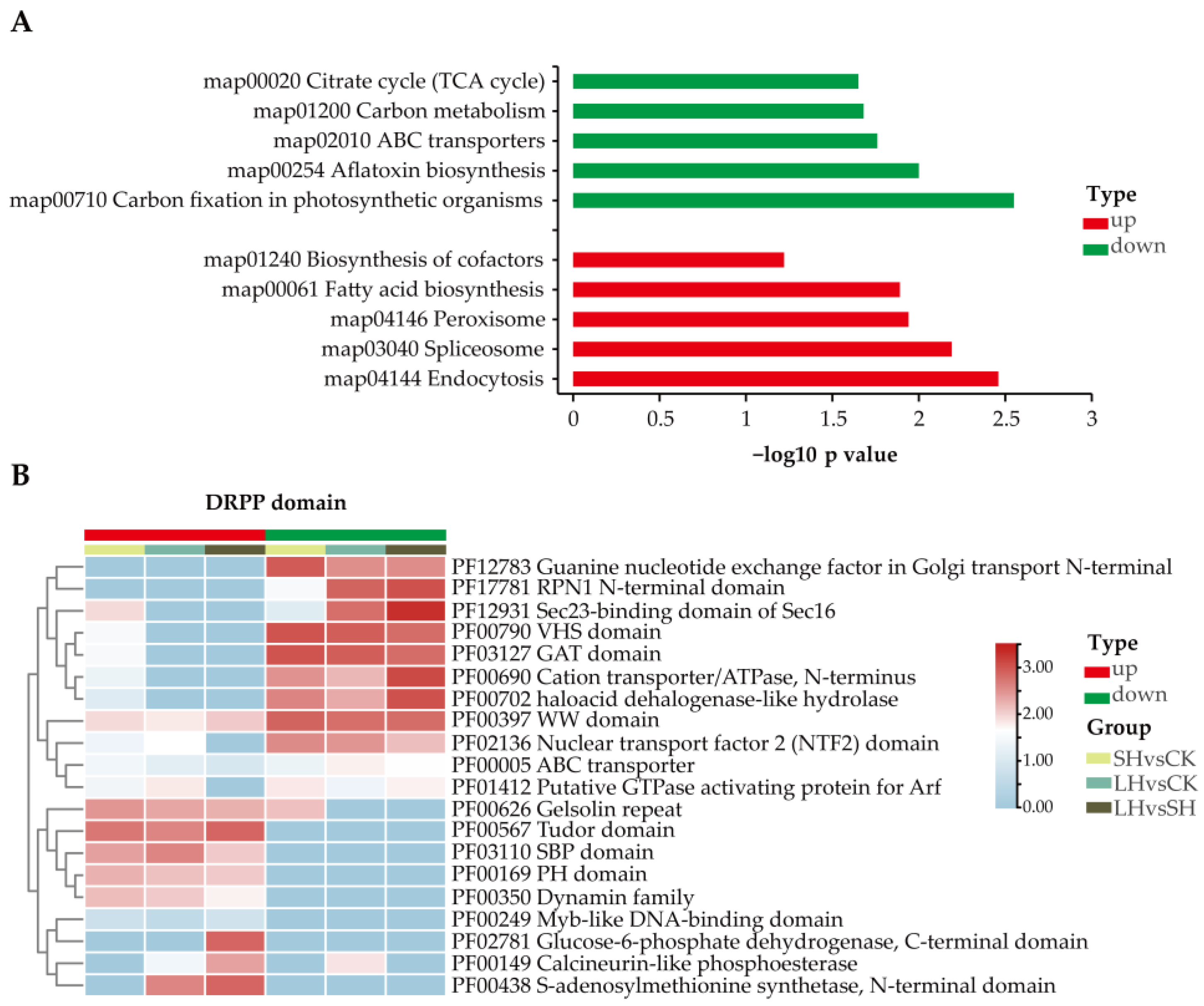
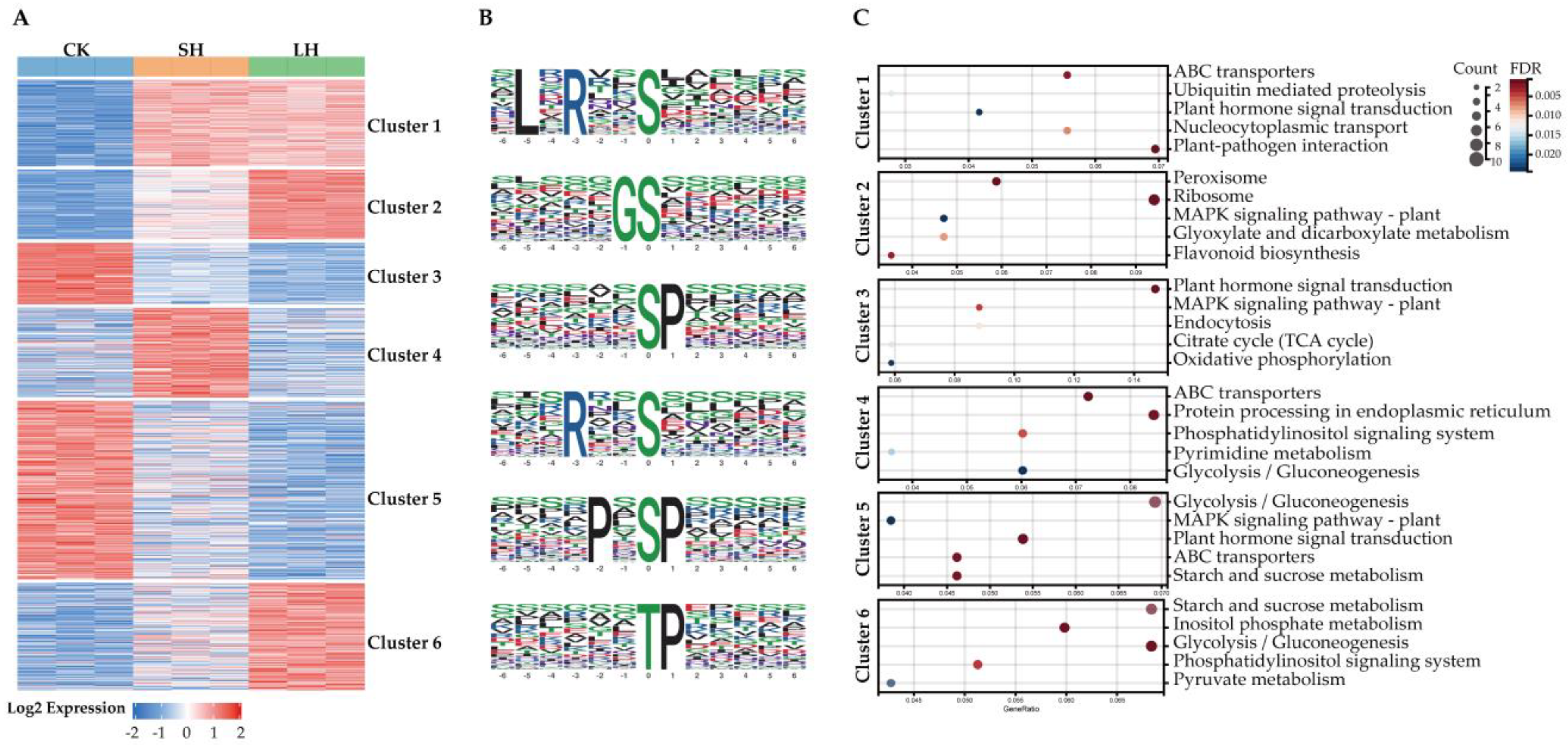
3.3. Cluster Analysis of Phosphoproteins
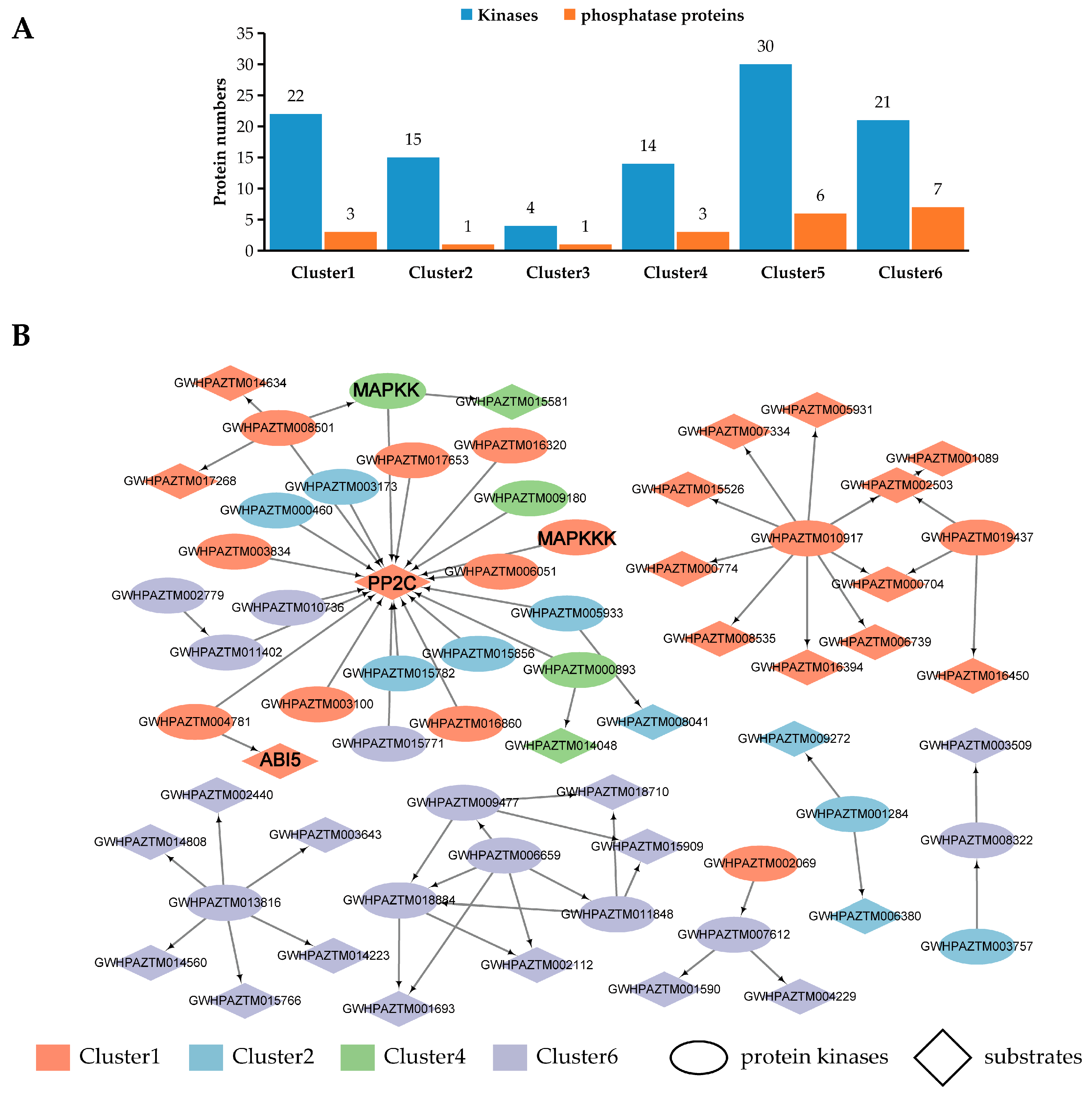
3.4. Identification of Protein Kinases and Phosphatases
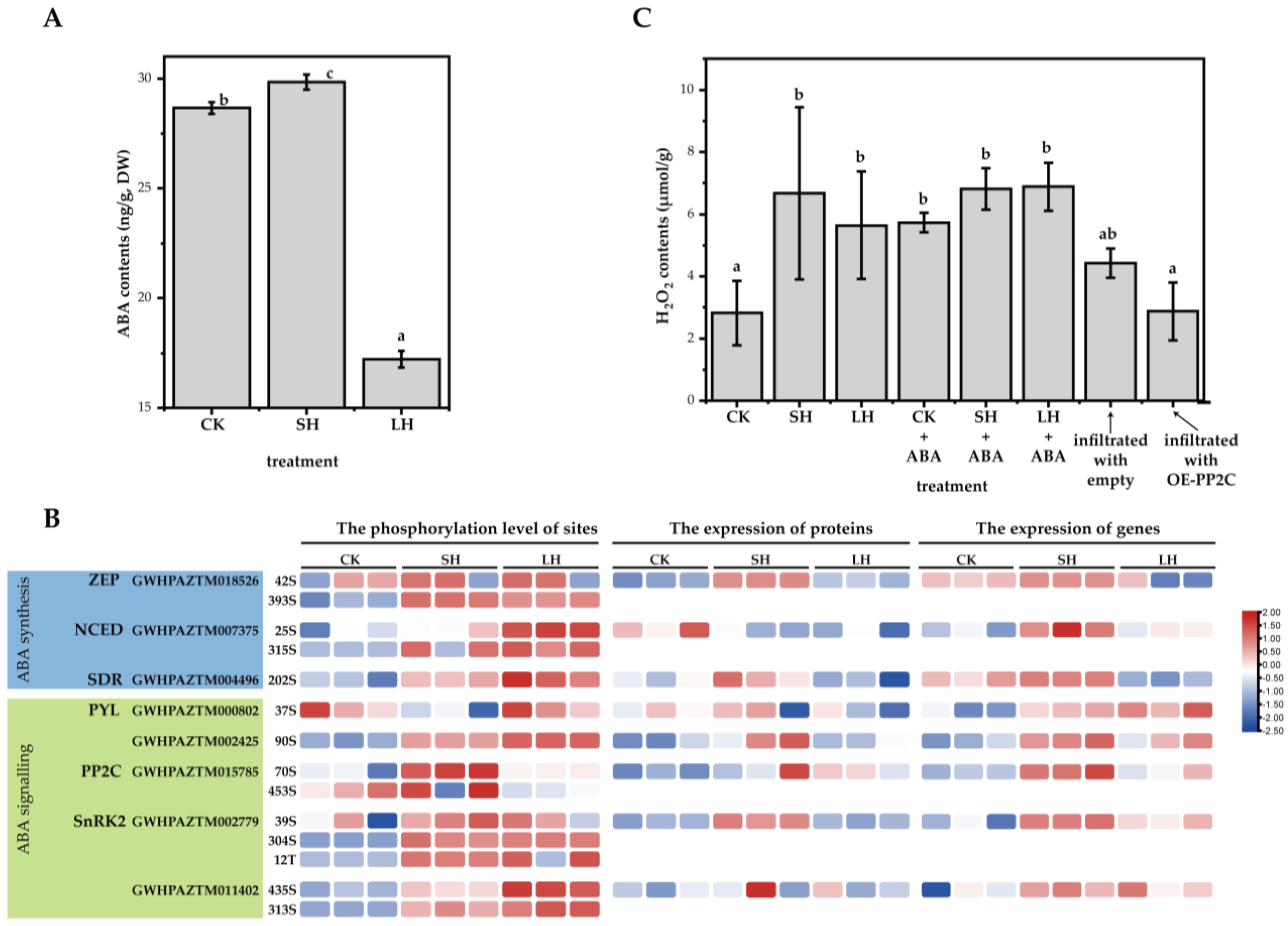
3.5. The Levels of Expression and Phosphorylation of Phosphoproteins Related to ABA Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, E.A.; Souza, M.M.; Abreu, P.P.; da Conceição, L.D.H.C.S.; Araújo, I.S.; Viana, A.P.; de Almeida, A.-A.F.; Freitas, J.C.d.O. Confirmation and characterization of interspecific hybrids of Passiflora L. (Passifloraceae) for ornamental use. Euphytica 2012, 184, 389–399. [Google Scholar] [CrossRef]
- Dong, L.; Wang, X.; Cai, Z.; Su, W.; Chen, Q.; Qiu, W.; Reng, H.; Wang, J.; Fang, W.; Huang, Z. Net chamber cultivation technique of healthy virus-free seedlings of passion fruit. S. China Fruits 2021, 50, 141–143. [Google Scholar] [CrossRef]
- Sobol, S.; Chayut, N.; Nave, N.; Kafle, D.; Hegele, M.; Kaminetsky, R.; Wünsche, J.N.; Samach, A. Genetic variation in yield under hot ambient temperatures spotlights a role for cytokinin in protection of developing floral primordia. Plant Cell Environ. 2014, 37, 643–657. [Google Scholar] [CrossRef]
- Chayut, N.; Sobol, S.; Nave, N.; Samach, A. Shielding Flowers Developing under Stress: Translating Theory to Field Application. Plants 2014, 3, 304–323. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wu, Y.; Huang, W.; Liu, J.; Wei, S.; Mou, H.; Wei, D.; Huang, Y.; Xiong, X.; Zhang, Y. Flower formation and fruit setting in ‘Tainong No.1’ passion fruit and its relationship with meteorological factors. J. Fruit Sci. 2020, 37, 1358–1370. [Google Scholar] [CrossRef]
- Lan, Y.; Shi, Z.; Li, L. Preliminary Report on Test of High Temperature Affecting Fruit Set Rate of Passiflora caerulea. S. China Fruits 2021, 50, 66–68. [Google Scholar] [CrossRef]
- Huang, X.; Chen, G.; Huang, Y.; Gui, J.; Huang, C.; Ju, Y.; Sheng, J.; Jiang, P.; Yang, L. Effents of continuous high-temperature on flowering and fruit-setting habits and photosynthesis of four passion fruit varieties. J. Fruit Sci. 2021, 41, 1401–1409. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Abiko, M.; Akibayashi, K.; Sakata, T.; Kimura, M.; Kihara, M.; Itoh, K.; Asamizu, E.; Sato, S.; Takahashi, H.; Higashitani, A. High-temperature induction of male sterility during barley (Hordeum vulgare L.) anther development is mediated by transcriptional inhibition. Sex. Plant Reprod. 2005, 18, 91–100. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Zhu, D.; Xiang, J.; Chen, X. Effects of air humidity and soil moisture on rice spikelet sterility induced by high temperature. Acta Agric. Jiangxi 2017, 29, 24–27. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, W.; Lai, J.; Tang, L.; Lin, T.; Li, L.; Wei, X.; Xu, J. Determination of viability of passion fruit pollens. Fujian J. Agric. Sci. 2024, 39, 302–309. [Google Scholar] [CrossRef]
- Ren, S.; Ma, K.; Lu, Z.; Chen, G.; Cui, J.; Tong, P.; Wang, L.; Teng, N.; Jin, B. Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr. Forests 2019, 10, 383. [Google Scholar] [CrossRef]
- Wang, H.; Gu, J.; Fang, W.; Chen, F.; Zhang, D. Response of cell membrane stability and osmotic adjustment substances in leaves of different cultivars of Anthurium andraeanum under high temperature stress. Acta Agric. Shanghai 2020, 36, 24–27. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, J.; Hu, Y.; Chi, Y.; Jin, S. Physiological and biochemical responses of leaves of four rhododendron cultivars under heat stress. Mol. Plant Breed. 2022, 20, 6877–6884. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Wang, Q.; Shang, L.; Zhao, Y.; Zhang, G.; Ma, Q.; Hong, S.; Gu, C. Physiological and transcriptional responses to heat stress and functional analyses of PsHSPs in tree peony (Paeonia suffruticosa). Front. Plant Sci. 2022, 13, 926900. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Cheng, Y.; Takano, T.; Liu, S. rHsp90 gene expression in response to several environmental stresses in rice (Oryza sativa L.). Plant Physiol. Biochem. 2006, 44, 380–386. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhou, Y.; Zhang, Y.; Su, Y.H.; Xu, T. Protein phosphorylation: A molecular switch in plant signaling. Cell Rep. 2023, 42, 112729. [Google Scholar] [CrossRef]
- Mergner, J.; Frejno, M.; List, M.; Papacek, M.; Chen, X.; Chaudhary, A.; Samaras, P.; Richter, S.; Shikata, H.; Messerer, M.; et al. Mass-spectrometry-based draft of the Arabidopsis proteome. Nature 2020, 579, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, J. Protein Phosphorylation in Plant Cell Signaling. Methods Mol. Biol. 2021, 2358, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.K. In vitro reconstitution of an abscisic acid signalling pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Y.; Li, Z.; Hsu, C.C.; Liu, X.; Fu, L.; Hou, Y.J.; Du, Y.; Xie, S.; Zhang, C.; et al. Reciprocal Regulation of the TOR Kinase and ABA Receptor Balances Plant Growth and Stress Response. Mol. Cell 2018, 69, 100–112.e106. [Google Scholar] [CrossRef]
- Takahashi, Y.; Zhang, J.; Hsu, P.K.; Ceciliato, P.H.O.; Zhang, L.; Dubeaux, G.; Munemasa, S.; Ge, C.; Zhao, Y.; Hauser, F.; et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat. Commun. 2020, 11, 12. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.S.; Bahk, S.; An, J.; Yoo, Y.; Kim, J.Y.; Chung, W.S. Phosphorylation of the transcriptional repressor MYB15 by mitogen-activated protein kinase 6 is required for freezing tolerance in Arabidopsis. Nucleic Acids Res. 2017, 45, 6613–6627. [Google Scholar] [CrossRef]
- Andrási, N.; Rigó, G.; Zsigmond, L.; Pérez-Salamó, I.; Papdi, C.; Klement, E.; Pettkó-Szandtner, A.; Baba, A.I.; Ayaydin, F.; Dasari, R.; et al. The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J. Exp. Bot. 2019, 70, 4903–4918. [Google Scholar] [CrossRef]
- Yip Delormel, T.; Boudsocq, M. Properties and functions of calcium-dependent protein kinases and their relatives in Arabidopsis thaliana. New Phytol. 2019, 224, 585–604. [Google Scholar] [CrossRef]
- Brandt, B.; Brodsky, D.E.; Xue, S.; Negi, J.; Iba, K.; Kangasjärvi, J.; Ghassemian, M.; Stephan, A.B.; Hu, H.; Schroeder, J.I. Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 2012, 109, 10593–10598. [Google Scholar] [CrossRef]
- Li, Z.; Takahashi, Y.; Scavo, A.; Brandt, B.; Nguyen, D.; Rieu, P.; Schroeder, J.I. Abscisic acid-induced degradation of Arabidopsis guanine nucleotide exchange factor requires calcium-dependent protein kinases. Proc. Natl. Acad. Sci. USA 2018, 115, E4522–E4531. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2020, 181, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, Z.; Wang, Q.; Garrell, A.K.; Liu, M.; Guan, Y.; Zhou, W.; Liu, W. Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018, 18, 315. [Google Scholar] [CrossRef]
- Hogrebe, A.; von Stechow, L.; Bekker-Jensen, D.B.; Weinert, B.T.; Kelstrup, C.D.; Olsen, J.V. Benchmarking common quantification strategies for large-scale phosphoproteomics. Nat. Commun. 2018, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Liang, Q.; Zhang, Y.; Kang, K.; Wang, W.; Feng, Y.; Wu, S.; Yang, C.; Li, Y. Genome-wide analysis of long noncoding RNAs affecting floral bud dormancy in pears in response to cold stress. Tree Physiol. 2021, 41, 771–790. [Google Scholar] [CrossRef]
- Zhang, D.; Du, L.; Lin, J.; Wang, L.; Zheng, P.; Deng, B.; Zhang, W.; Su, W.; Liu, Y.; Lu, Y.; et al. Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in passion fruit (Passiflora edulis) and their involvement in flower and fruit development. BMC Plant Biol. 2024, 24, 626. [Google Scholar] [CrossRef]
- Yang, J.; Xie, M.-Y.; Yang, X.-L.; Liu, B.-H.; Lin, H.-H. Phosphoproteomic Profiling Reveals the Importance of CK2, MAPKs and CDPKs in Response to Phosphate Starvation in Rice. Plant Cell Physiol. 2019, 60, 2785–2796. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Li, C.; Ren, P.; Yao, L.; Li, B.; Meng, Y.; Ma, X.; Si, E.; Yang, K.; et al. Global Profiling of Phosphorylation Reveals the Barley Roots Response to Phosphorus Starvation and Resupply. Front. Plant Sci. 2021, 12, 676432. [Google Scholar] [CrossRef]
- van Wijk, K.J.; Friso, G.; Walther, D.; Schulze, W.X. Meta-Analysis of Arabidopsis thaliana Phospho-Proteomics Data Reveals Compartmentalization of Phosphorylation Motifs. Plant Cell 2014, 26, 2367–2389. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Yao, X.; Tian, Y.; He, Y.; Lin, Y.; Lei, W.; Peng, S.; Pan, G.; Shi, H.; Zhang, D.; et al. Ubiquitin-specific protease UBP14 stabilizes HY5 by deubiquitination to promote photomorphogenesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2024, 121, e2404883121. [Google Scholar] [CrossRef]
- Wang, D.; Wei, L.; Liu, T.; Ma, J.; Huang, K.; Guo, H.; Huang, Y.; Zhang, L.; Zhao, J.; Tsuda, K.; et al. Suppression of ETI by PTI priming to balance plant growth and defense through an MPK3/MPK6-WRKYs-PP2Cs module. Mol. Plant 2023, 16, 903–918. [Google Scholar] [CrossRef]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, Q.; Wu, C. Effects of high temperature stress flowering on flower organ development, reproduction and physiological mechanism of fruit trees. North. Fruits 2022, 31, 1–4. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Zhao, F.; Zhang, D.; Li, N.; Zhu, G.; Li, C.; Wang, W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015, 6, 298. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, H.; Ma, T.; Shen, S.; Zheng, H.; Yang, L.; Liu, L.; Wei, Z.; Xin, W.; Zou, D.; et al. Global Phosphoproteomic Analysis Reveals the Defense and Response Mechanisms of Japonica Rice under Low Nitrogen Stress. Int. J. Mol. Sci. 2023, 24, 7699. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Su, Y.; Song, Z.; Wang, J. The Posttranscriptional Mechanism in Salvia miltiorrhiza Bunge Leaves in Response to Drought Stress Using Phosphoproteomics. Agronomy 2022, 12, 781. [Google Scholar] [CrossRef]
- Arefian, M.; Bhagya, N.; Prasad, T.S.K. Phosphorylation-mediated signalling in flowering: Prospects and retrospects of phosphoproteomics in crops. Biol. Rev. 2021, 96, 2164–2191. [Google Scholar] [CrossRef]
- Li, Q.; Li, M.; Ma, H.; Xue, M.; Chen, T.; Ding, X.; Zhang, S.; Xiao, J. Quantitative Phosphoproteomic Analysis Provides Insights into the Sodium Bicarbonate Responsiveness of Glycine max. Biomolecules 2023, 13, 1520. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, J.; Lai, M.; Zhang, Y.; Qiu, W.; Li, Y.; Tu, H.; Ling, Q.; Fu, X. Differential gene expression analysis and physiological response characteristics of passion fruit (Passiflora edulis) buds under high-temperature stress. PeerJ 2023, 11, e14839. [Google Scholar] [CrossRef] [PubMed]
- Castroverde, C.D.M.; Dina, D. Temperature regulation of plant hormone signaling during stress and development. J. Exp. Bot. 2021, 72, 7436–7458. [Google Scholar] [CrossRef]
- Gilbert, G.A.; Knight, J.D.; Vance, C.P.; Allan, D.L. Proteoid Root Development of Phosphorus Deficient Lupin is Mimicked by Auxin and Phosphonate. Ann. Bot. 2000, 85, 921–928. [Google Scholar] [CrossRef]
- Liang, Y.; Gong, Z.; Wang, J.; Zheng, J.; Ma, Y.; Min, L.; Chen, Q.; Li, Z.; Qu, Y.; Chen, Q.; et al. Nanopore-Based Comparative Transcriptome Analysis Reveals the Potential Mechanism of High-Temperature Tolerance in Cotton (Gossypium hirsutum L.). Plants 2021, 10, 2517. [Google Scholar] [CrossRef]
- Qiao, H.; Shen, Z.; Huang, S.S.; Schmitz, R.J.; Urich, M.A.; Briggs, S.P.; Ecker, J.R. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 2012, 338, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Merchante, C.; Brumos, J.; Yun, J.; Hu, Q.; Spencer, K.R.; Enríquez, P.; Binder, B.M.; Heber, S.; Stepanova, A.N.; Alonso, J.M. Gene-specific translation regulation mediated by the hormone-signaling molecule EIN2. Cell 2015, 163, 684–697. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhang, F.; Shao, Z.; Zhao, B.; Huang, A.; Tran, J.; Hernandez, F.V.; Qiao, H. EIN2-directed histone acetylation requires EIN3-mediated positive feedback regulation in response to ethylene. Plant Cell 2020, 33, 322–337. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef]
- Prerostova, S.; Dobrev, P.I.; Kramna, B.; Gaudinova, A.; Knirsch, V.; Spichal, L.; Zatloukal, M.; Vankova, R. Heat Acclimation and Inhibition of Cytokinin Degradation Positively Affect Heat Stress Tolerance of Arabidopsis. Front. Plant Sci. 2020, 11, 87. [Google Scholar] [CrossRef]
- Qin, P.; Zhang, G.; Hu, B.; Wu, J.; Chen, W.; Ren, Z.; Liu, Y.; Xie, J.; Yuan, H.; Tu, B.; et al. Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci. Adv. 2021, 7, eabc8873. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Wang, Q.; Guan, Y.; Li, X.; Huangfu, Y.; Meng, F.; Li, J.; Dai, S.; Liu, W. Phosphoproteomic Profiling Reveals Early Salt-Responsive Mechanisms in Two Foxtail Millet Cultivars. Front. Plant Sci. 2021, 12, 712257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, W.; Guo, D.; Li, Y.; Li, J.; Liu, H.; Wei, L.; Yu, N.; Wang, B.; Zheng, Y.; et al. An Activity-Based Sensing Fluorogenic Probe for Monitoring Ethylene in Living Cells and Plants. Angew. Chem. Int. Ed. Engl. 2021, 60, 21934–21942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mu, Q.; Wang, X.; Zhang, J.; Yu, H.; Huang, T.; He, Y.; Dai, S.; Meng, X. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant Cell 2022, 34, 3066–3087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chiang, Y.H.; Toruño, T.Y.; Lee, D.; Ma, M.; Liang, X.; Lal, N.K.; Lemos, M.; Lu, Y.J.; Ma, S.; et al. The MAP4 Kinase SIK1 Ensures Robust Extracellular ROS Burst and Antibacterial Immunity in Plants. Cell Host Microbe 2018, 24, 379–391.e375. [Google Scholar] [CrossRef]
- Pan, L.; De Smet, I. Expanding the Mitogen-Activated Protein Kinase (MAPK) Universe: An Update on MAP4Ks. Front. Plant Sci. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T.; Ding, L.; Wang, C.; Teng, R.; Xu, S.; Cao, X.; Teng, N. Lily LlHSFC2 coordinates with HSFAs to balance heat stress response and improve thermotolerance. New Phytol. 2024, 241, 2124–2142. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Chai, G.; Zhang, D.; Fang, Y.; Deng, K.; Aslam, M.; Niu, X.; Zhang, W.; Qin, Y.; et al. Identification of passion fruit HSF gene family and the functional analysis of PeHSF-C1a in response to heat and osmotic stress. Plant Physiol. Biochem. 2023, 200, 107800. [Google Scholar] [CrossRef]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.; Xiao, N.; Lu, Y.; Fu, Y.; Yang, C.; Li, M.; Wu, S.; et al. iProX in 2021: Connecting proteomics data sharing with big data. Nucleic Acids Res. 2022, 50, D1522–D1527. [Google Scholar] [CrossRef] [PubMed]
| Titles | Number |
|---|---|
| Peptides | 15,367 |
| Modified peptides | 11,827 |
| Identified proteins | 4909 |
| Identified sites | 17,765 |
| Comparable proteins | 2906 |
| Comparable sites | 10,614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Tang, Y.; Yu, D.; Zhou, P.; Liu, Z.; Wei, X.; Xu, J. Phosphoproteomic Profiling Deciphers Heat-Stress-Responsive Mechanisms in Passion Fruit. Horticulturae 2025, 11, 553. https://doi.org/10.3390/horticulturae11050553
Li L, Tang Y, Yu D, Zhou P, Liu Z, Wei X, Xu J. Phosphoproteomic Profiling Deciphers Heat-Stress-Responsive Mechanisms in Passion Fruit. Horticulturae. 2025; 11(5):553. https://doi.org/10.3390/horticulturae11050553
Chicago/Turabian StyleLi, Liang, Yajun Tang, Dong Yu, Ping Zhou, Zhicheng Liu, Xiuqing Wei, and Jiahui Xu. 2025. "Phosphoproteomic Profiling Deciphers Heat-Stress-Responsive Mechanisms in Passion Fruit" Horticulturae 11, no. 5: 553. https://doi.org/10.3390/horticulturae11050553
APA StyleLi, L., Tang, Y., Yu, D., Zhou, P., Liu, Z., Wei, X., & Xu, J. (2025). Phosphoproteomic Profiling Deciphers Heat-Stress-Responsive Mechanisms in Passion Fruit. Horticulturae, 11(5), 553. https://doi.org/10.3390/horticulturae11050553





