Effect of Cutting Phenological Stage, Chemical Treatments, and Substrate on Rooting Softwood Cuttings of Tree Peony
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
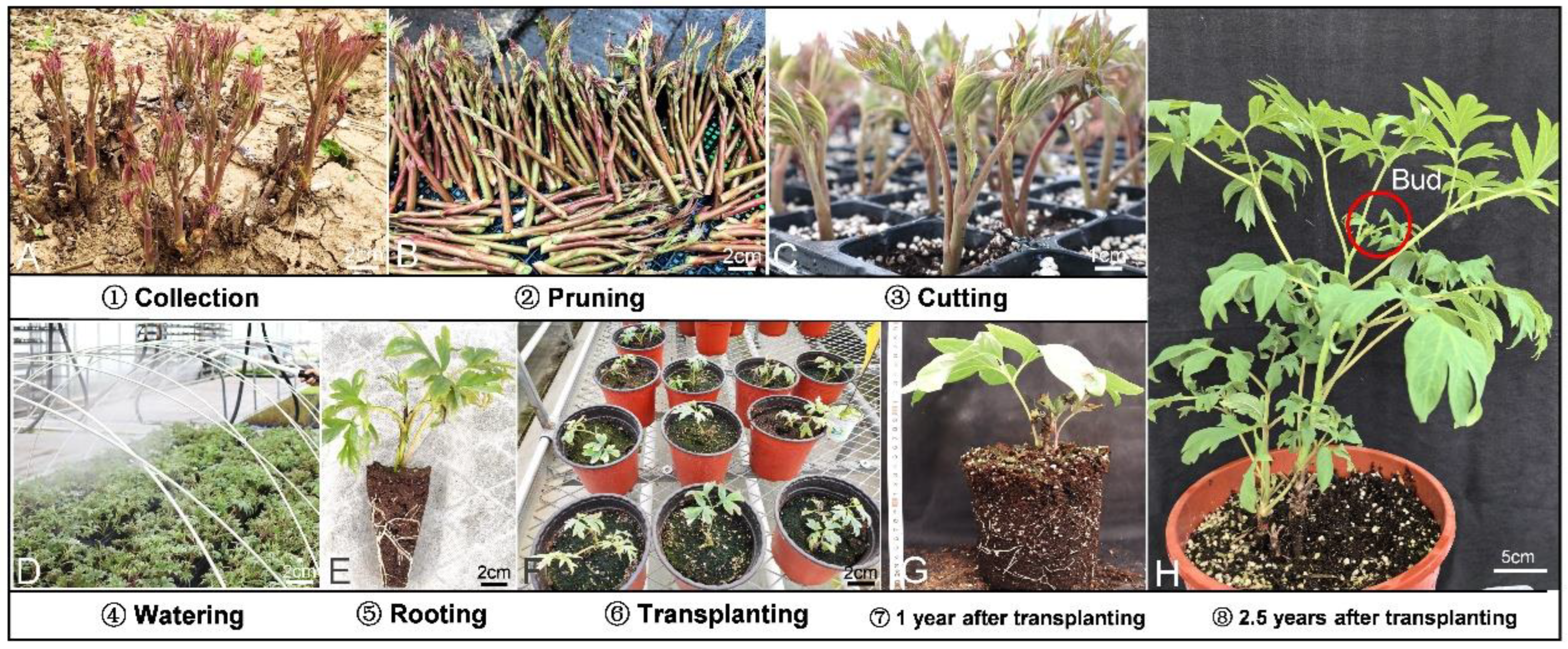
2.2. Cutting Treatments and Experimental Conditions
2.2.1. Cutting Phenological Stage
2.2.2. Plant Growth Regulators
2.2.3. Polyamines
2.2.4. Substrates
2.2.5. Experimental Conditions
2.3. Evaluation of Rooting Cuttings
2.4. Morphological and Anatomical Observation on Shoots Development and Rooting Process
2.5. Statistical Analysis
3. Results
3.1. Effect of Cutting Phenological Stage on the Rooting of Tree Peony
3.2. Effect of Plant Growth Regulators on Rooting
3.3. Effect of Polyamines on Rooting
3.4. Effect of Substrates on Rooting
3.5. Softwood Development and Rooting of Softwood Cuttings in Tree Peony
3.5.1. Morphological and Anatomical Observation of ‘HN’ Softwood Development
3.5.2. Rooting Process and Rooting Type of ‘HN’ Softwood Cuttings
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhang, L.; Wu, D.; Jiang, S.; Wu, Q.; Dai, M. Paeonol attenuates nonalcoholic steatohepatitis by regulating intestinal flora and AhR/NLRP3/Caspase-1 metabolic pathway. J. Ethnopharmacol. 2024, 329, 118147. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, J.; Chen, D.; Zhang, Z. Chinese Flare Mudan; China Forestry Publishing House: Beijing, China, 2005; Volume 5, pp. 44–46. [Google Scholar]
- John, M.D.; James, L.G. Cutting Propagation: A Guide to Propagating and Producing Floriculture Crops; Ball Publishing: Batavia, IL, USA, 2006; pp. 3–4. [Google Scholar]
- Monder, M.J.; Pacholczak, A. Rhizogenesis and concentration of carbohydrates in cuttings harvested at different phenological stages of once-blooming rose shrubs and treated with rooting stimulants. Biol. Agric. Hortic. 2020, 36, 53–70. [Google Scholar] [CrossRef]
- Morales-Orellana, R.J.; Winkelmann, T.; Bettin, A.; Rath, T. Stimulation of adventitious root formation by laser wounding in rose cuttings: A matter of energy and pattern. Front. Plant Sci. 2022, 13, 1009085. [Google Scholar] [CrossRef]
- Hejazi, Z.; Safi, S.; Barakzai, A.L.; Karimi, B.K.; Qurashi, F.M.; Tetsumura, T. Cultivar effect on root development in pomegranate hardwood cuttings treated with auxin. Rhizosphere 2023, 25, 100661. [Google Scholar] [CrossRef]
- Li, S. Molecular bases for the regulation of adventitious root generation in plants. Front. Plant Sci. 2021, 12, 614072. [Google Scholar] [CrossRef]
- Guan, L.; Murphy, A.; Peer, W.; Gan, L.; Li, Y.; Cheng, Z. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Johnson, E.P.; Preece, J.E.; Aradhya, M.; Gradzeil, T. Rooting response of Prunus wild relative semi-hardwood cuttings to indole-3-butyric acid potassium salt (KIBA). Sci. Hortic. 2020, 263, 109144. [Google Scholar] [CrossRef]
- Pan, T.; Chen, X.; Hao, Y.; Jiang, C.; Wang, S.; Wang, J.; Wei, Q.; Chen, S.; Yu, X.; Cheng, F.; et al. Optimization of factors affecting the rooting of pine wilt disease resistant Masson pine (Pinus massoniana) stem cuttings. PLoS ONE 2021, 16, e0251937. [Google Scholar] [CrossRef]
- Kumar, A.; Nagar, P.K.; Palni, L.M.S. The role of indole-3-butyric acid and polyamines on in vitro rooting of microshoots of Rosa damascena Mill. and Gladiolus hybridus Hort. S. Afr. J. Bot. 2024, 172, 1–7. [Google Scholar] [CrossRef]
- Tsafouros, A.; Frantzeskaki, A.; Assimakopoulo, A.; Roussos, P.A. Spatial and temporal changes of mineral nutrients and carbohydrates in cuttings of four stone fruit rootstocks and their contribution to rooting potential. Sci. Hortic. 2019, 253, 227–240. [Google Scholar] [CrossRef]
- Hartmann, H.P.; Kester, D.E.; Davies, J.T. Plant Propagation: Principle and Practices; Prentice Hall: Hoboken, NJ, USA, 1990. [Google Scholar]
- Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Vasileiadis, A.; Magklaras, P.; Smyrnioudis, I.; Vasilakis, T.; Chazakis, M.; Anastasiadi, V.; Ziogou, F.T. Propagation of Pistacia lentiscus var. Chia genotypes and determination of their ornamental traits combined with a genetic analysis using ISSR markers. Agronomy 2021, 11, 205. [Google Scholar] [CrossRef]
- Izhaki, A.; Yitzhak, Y.; Blau, T.; David, I.; Rotbaum, A.; Riov, J.; Zilkah, S. Rooting of cuttings of selected Diospyros virginiana clonal rootstocks and bud growth in rooted cuttings. Sci. Hortic. 2018, 232, 13–21. [Google Scholar] [CrossRef]
- Sabatino, L.; D’Anna, F.; Iapichino, G. Improved propagation and growing techniques for Oleander nursery production. Horticulturae 2019, 5, 55. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Liu, F.; Rosenqvist, E. Does constant or changing light give the best rooting of hibiscus cuttings of two sizes? Sci. Hortic. 2023, 309, 111675. [Google Scholar] [CrossRef]
- Nasri, F.; Fadakar, A.; Saba, M.K.; Yousefi, B. Study of indole butyric acid (IBA) effects on cutting rooting improving some of wild genotypes of damask roses (Rosa damascena Mill.). J. Agric. Sci. 2015, 60, 263–275. [Google Scholar] [CrossRef]
- Azad, M.S.; Alam, M.J.; Mollick, A.S.; Khan, M.N.I. Rooting of cuttings of the wild Indian almond tree (Sterculia foetida) enhanced by the application of indole-3-butyric acid (IBA) under leafy and non-leafy conditions. Rhizosphere 2018, 5, 8–15. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, M.; Li, J.; Ge, W. Key regulatory pathways, microRNAs, and target genes participate in adventitious root formation of Acer rubrum L. Sci. Rep. 2022, 12, 12057. [Google Scholar] [CrossRef]
- Loconsole, D.; Sdao, A.E.; Cristiano, G.; Lucia, B.D. Different responses to adventitious rhizogenesis under indole-3-butyric acid and seaweed extracts in ornamental’s cuttings: First Results in Photinia x fraseri ‘Red Robin’. Agriculture 2023, 13, 513. [Google Scholar] [CrossRef]
- Song, X.; Huang, R.; Liu, H.; Zhang, J.; Chang, Y.; Pei, D. Transcriptome profiling of indole-3-butyric acid–induced adventitious root formation in softwood cuttings of walnut. Hortic. Plant J. 2024, 10, 1336–1348. [Google Scholar] [CrossRef]
- Sousa, C.E.D.; Oliveria, F.L.R.; Sant’Anna-Santos, B.F.; Zuffellato-Ribas, K.C. Physiological and anatomical aspects of the rooting of Brunfelsia pauciflora cuttings. Sci. Hortic. 2023, 307, 111491. [Google Scholar] [CrossRef]
- Frick, E.M.; Strader, L.C. Roles for IBA-derived auxin in plant development. J. Exp. Bot. 2018, 69, 169–177. [Google Scholar] [CrossRef]
- Li, S.; Huang, P.; Ding, G.; Zhou, L.; Tang, P.; Sun, M.; Zheng, Y.; Lin, S. Optimization of hormone combinations for root growth and bud germination in Chinese fir (Cunninghamia lanceolata) clone leaf cuttings. Sci. Rep. 2017, 7, 5046. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Shen, H.; Yang, L. Effect of Exogenous Plant Growth Regulators and Rejuvenation Measures on the Endogenous Hormone and Enzyme Activity Responses of Acer mono Maxim in Cuttage Rooting. Int. J. Mol. Sci. 2023, 24, 11883. [Google Scholar] [CrossRef]
- Cristofori, V.; Rouphael, Y.; Rugini, E. Collection time, cutting age, IBA and putrescine effects on root formation in Corylus avellana L. cuttings. Sci. Hortic. 2010, 124, 189–194. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Roussos, P.A.; Vemmos, S.N. The possible role of polyamines to the recalcitrance of “Kalamata’’ olive leafy cuttings to root. J. Plant Growth Regul. 2014, 33, 579–589. [Google Scholar] [CrossRef]
- Tsafouros, A.; Roussos, P.A. The possible bottleneck effect of polyamines’ catabolic enzymes in efficient adventitious rooting of two stone fruit rootstocks. J. Plant Physiol. 2020, 244, 152999. [Google Scholar] [CrossRef]
- Niu, L.; Tang, Y.; Zhu, B.; Huang, Z.; Wang, D.; Chen, Q.; Yu, J. Nitric oxide promotes adventitious root formation in cucumber under cadmium stress through improving antioxidant system, regulating glycolysis pathway and polyamine homeostasis. Front. Plant Sci. 2023, 14, 1126606. [Google Scholar] [CrossRef]
- Campbell, S.M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Evaluation of substrate composition and exogenous hormone application on vegetative propagule rooting success of essential oil hemp (Cannabis sativa L.). PLoS ONE 2021, 16, e0249160. [Google Scholar] [CrossRef]
- Nemati, R.; Fortin, J.P.; Craig, J.; Donald, S. Growing Mediums for Medical Cannabis Production in North America. Agronomy 2021, 11, 1366. [Google Scholar] [CrossRef]
- Weingarten, M.; Mattson, N.; Grab, H. Evaluating Propagation Techniques for Cannabis sativa L. Cultivation: A Comparative Analysis of Soilless Methods and Aeroponic Parameters. Plants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, P.; Vethamoni, P.I.; Gomathi, M. Aeroponics soilless cultivation system for vegetable crops. Chem. Sci. Rev. Lett. 2017, 6, 838–849. [Google Scholar]
- Eldridge, B.M.; Manzoni, L.R.; Graham, C.A.; Rodgers, B.; Farmer, J.R.; Dodd, A.N. Getting to the roots of aeroponic indoor farming. New Phytol. 2020, 228, 1183–1192. [Google Scholar] [CrossRef]
- Blaney, L.T. Tree peonies rooted from leaf-bud cuttings. Or. Ornam. Nurs. Dig. 1958, 2, 1+2+4. [Google Scholar]
- Zeng, D.; Yin, W.; Wang, Y.; Zhao, X.; Wang, H. Propagation with Etiolated Softwood Cuttings of Five Dwarf Cultivars of Chinese Tree Peony. Acta Hortic. Sin. 2005, 32, 725. [Google Scholar]
- Chen, C. Study on the Cutting Rooting and Stem Anatomical Structure Development of Tree Peony. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019. [Google Scholar]
- Meng, X.; Wang, Z.; He, S.; Shi, L.; Song, Y.; Lou, X.; He, D. Endogenous hormone levels and activities of IAA-modifying enzymes during adventitious rooting of tree peony cuttings and grafted scions. Hortic. Environ. Biotechnol. 2019, 60, 187–197. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, F.; Zhong, Y. Histological and cytological study on meristematic nodule induction and shoot organogenesis in Paeonia ostii ‘Feng Dan’. Plant Cell Tiss. Org. 2022, 149, 609–620. [Google Scholar] [CrossRef]
- Wen, S.; Miao, D.; Cui, H.; Li, S.; Gu, Y.; Jia, R.; Leng, Y. Physiology and transcriptomic analysis of endogenous hormones regulating in vitro adventitious root formation in tree peony. Sci. Hortic. 2023, 318, 112122. [Google Scholar] [CrossRef]
- Monder, M.J.; Niedzielski, M.; Woliński, K. The pivotal role of phenological stages enhanced by plant origin preparations in the process of rhizogenesis of Rosa ‘Hurdal’stem cuttings. Agriculture 2022, 12, 158. [Google Scholar] [CrossRef]
- Tong, N.; Shu, Q.; Wang, B.; Peng, L.; Liu, Z. Histology, physiology, and transcriptomic and metabolomic profiling reveal the developmental dynamics of annual shoots in tree peonies (Paeonia suffruticosa Andr.). Hortic. Res. 2023, 10, uhad152. [Google Scholar] [CrossRef]
- Uwe, D.; Alexander, H.; Manuel, P.P.J.; Yvonne, K.; Manuel, A.; Fahimeh, S.; Siegfried, Z.; Philipp, F.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar]
- Shi, Q.; Wang, Y.; Zhou, L.; Huang, G. Influence of Rooting Powder and Culture Substrata on Rooting of Different Traditional Cultivars of Paeonia suffruticosa. North. Hortic. 2012, 1, 91–96. [Google Scholar]
- Karam, N.S.; Gebre, G.H. Rooting of Cercis siliquastrum cuttings influenced by cutting position on the branch and indole-butyric acid. J. Hortic. Sci. Biotechnol. 2004, 79, 792–796. [Google Scholar] [CrossRef]
- Wen, S.; Cheng, F.; Zhong, Y.; Wang, X.; Li, L.; Zhang, Y.; Qiu, J. Efficient protocols for the micropropagation of tree peony (Paeonia suffruticosa ‘Jin Pao Hong’, P. suffruticosa ‘Wu Long Peng Sheng’, and P. × lemoinei ‘High Noon’) and application of arbuscular mycorrhizal fungi to improve plantlet establishment. Sci. Hortic. 2016, 201, 10–17. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Vemmos, S.N.; Roussos, P.A. Shoot girdling improves rooting performance of kalamata olive cuttings by upregulating carbohydrates, polyamines and phenolic compounds. Agriculture 2021, 11, 71. [Google Scholar] [CrossRef]
- Matam, P.; Parvatam, G. Putrescine and polyamine inhibitors in culture medium alter in vitro rooting response of Decalepis hamiltonii Wight&Arn. Plant Cell Tiss. Org. 2017, 128, 273–282. [Google Scholar]
- Abass, M.M.; Thabet, R.S.; Lasheen, F.F.; Abdelhamid, A.N.; Hassan, K.M.; Saudy, H.S.; Boghdady, M.S. Modulating the rhizosphere medium and indole−3−butyric acid supply influence rooting, nutrients and biochemical constituents and histological features of Pedilanthus tithymaloids. J. Soil Sci. Plant Nut. 2024, 24, 6880–6892. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Kim, S.H.; Yu, C.Y.; Chung, I.M. Biochemical and physiological changes during early adventitious root formation in Chrysanthemum indicum Linné cuttings. Plants 2022, 11, 1440. [Google Scholar] [CrossRef]
- Malviya, N.K.; Chaurasiya, R.; Maji, S. Use of cocopeat for soilless cultivation of tomato. South Asian J. Exp. Biol. 2020, 10, 169–175. [Google Scholar] [CrossRef]
- Baronti, S.; Montagnoli, A.; Beatrice, P.; Danieli, A.; Maienza, A.; Vaccari, F.P.; Casini, D.; Di Gennaro, S.F. Above- and below-ground morpho-physiological traits indicate that biochar is a potential peat substitute for grapevine cuttings nursery production. Sci. Rep. 2024, 14, 17185. [Google Scholar] [CrossRef]
- Erdal, İ.; Hakan, A. Comparison of the perlite, leonardite, vermicompost and peat moss and their combinations with cocopeat as tomato growing media. J. Soil Sci. Plant Nutr. 2025. [Google Scholar] [CrossRef]
- He, M.; Lv, L.; Li, H.; Meng, W.; Zhao, N. Analysis on soil seed bank diversity characteristics and its relation with soil physical and chemical properties after substrate addition. PLoS ONE 2016, 11, e0147439. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vijayaraghavan, K.; Franklin, D.R. Design and development of green roof substrate to improve runoff water quality: Plant growth experiments and adsorption. Water Res. 2014, 63, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Xu, L. De novo root regeneration from leaf explants: Wounding, auxin, and cell fate transition. Curr. Opin. Plant Biol. 2018, 41, 39–45. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Huang, K.; Cheng, Z. Auxin regulation and MdPIN expression during adventitious root initiation in apple cuttings. Hortic. Res. 2020, 7, 143. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Wang, Y.P. The propagation method of peony by its twig and the cytohistological observation on the root regeneration. Acta Hortic. Sin. 1993, 20, 176–180. [Google Scholar]






| Treatment Code | Plant Growth Regulator | Concentration (mg·L−1/Dilution Factor) | Soaking Time (min/s) |
|---|---|---|---|
| IBA-1000 | IBA | 1000 | 15 |
| IBA-2000 | IBA | 2000 | 15 |
| IBA-3000 | IBA | 3000 | 15 |
| GP-150 | GenPan | 150 | 15 |
| GP-250 | GenPan | 250 | 15 |
| GP-350 | GenPan | 350 | 15 |
| RP | Rooting Powder | 0.25% IBA | 3 s |
| CK | Water | - | 15 |
| Treatment Code | Polyamine | Concentration (mM) | Soaking Time (min) |
|---|---|---|---|
| Put-0.05 | Put | 0.05 | 5 |
| Put-0.1 | Put | 0.1 | 5 |
| Put-1.0 | Put | 1.0 | 5 |
| Spd-0.05 | Spd | 0.05 | 5 |
| Spd-0.1 | Spd | 0.1 | 5 |
| Spd-1.0 | Spd | 1.0 | 5 |
| Spm-0.05 | Spm | 0.05 | 5 |
| Spm-0.1 | Spm | 0.1 | 5 |
| Spm-1.0 | Spm | 1.0 | 5 |
| CK | Water | - | 5 |
| Year | Cultivar | DBF/DAF | Rooting Rate (%) | Callus Rate (%) | Rot Rate (%) |
|---|---|---|---|---|---|
| 2021 | HN | 10DBF | 38.33 ± 10.27 ab | 100.00 a | 6.67 ± 4.71 j |
| 28DAF | 50.00 ± 8.16 a | 90 ± 8.16 ab | 33.33 ± 4.71 g | ||
| 80DAF | 6.67 ± 4.71 c | 40 ± 8.16 e | 70 ± 8.16 d | ||
| JHQX | 10DBF | 31.67 ± 6.24 ab | 100.00 a | 50 ± 8.16 f | |
| 29DAF | 13.33 ± 4.17 bc | 20 ± 8.16 h | 70 ± 8.16 d | ||
| 80DAF | 0.00 c | 16.67 ± 4.71 hi | 73.33 ± 9.43 d | ||
| JYG | 10DBF | 21.67 ± 8.50 | 76.67 ± 12.47 c | 63.33 ± 4.71 e | |
| 29DAF | 6.67 ± 4.71 c | 13.33 ± 4.71 j | 81.67 ± 6.24 bc | ||
| 81DAF | 0.00 c | 6.67 ± 4.71 j | 93.33 ± 4.71 ab | ||
| GYSH | 10DBF | 16.67 ± 9.43 bc | 80 ± 8.16 d | 66.67 ± 4.71 de | |
| 30DAF | 6.67 ± 4.71 c | 13.33 ± 4.71 j | 83.33 ± 4.71 bc | ||
| 81DAF | 0 | 6.67 ± 4.71 j | 96.67 ± 4.71 a | ||
| 2022 | HN | 18DAF | 16.67 ± 4.71 bc | 96.67 ± 4.71 a | 10.00 ± 8.16 i |
| 35DAF | 10.00 ± 8.16 c | 93.33 ± 4.71 bc | 23.33 ± 4.71 h | ||
| 55DAF | 6.67 ± 4.71 c | 40.00 ± 8.16 fg | 70.00 ± 8.16 d | ||
| 70DAF | 0.00 c | 21.67 ± 8.50 gh | 83.33 ± 4.71 bc | ||
| BZL | 36DAF | 30.00 ± 8.16 ab | 100.00 a | 13.33 ± 4.71 i | |
| 58DAF | 6.67 ± 4.71 c | 80.00 ± 8.16 c | 23.33 ± 12.47 h | ||
| 72DAF | 0.00 c | 16.67 ± 9.43 j | 80.00 ± 8.16 bc | ||
| JHQX | 19DAF | 53.33 ± 12.47 a | 96.67 ± 4.71 ab | 6.67 ± 4.71 j | |
| 36DAF | 7.50 ± 5.40 c | 85.83 ± 4.25 bc | 25.00 ± 4.08 h | ||
| 56DAF | 3.33 ± 4.71 c | 39.17 ± 8.25 fg | 71.67 ± 2.36 d | ||
| GYSH | 20DAF | 6.67 ± 4.71 c | 46.67 ± 4.71 e | 66.67 ± 9.43 de | |
| 38DAF | 3.33 ± 4.71 c | 25.00 ± 4.08 gh | 75.00 ± 4.08 cd | ||
| 58DAF | 0.00 c | 10.00 ± 8.16 ij | 80.00 ± 8.16 bc | ||
| JYG | 10DAF | 0.00 c | 10.00 ± 8.16 ij | 86.67 ± 4.71 ab | |
| 22DAF | 10.83 ± 1.18 c | 35.83 ± 4.25 ef | 78.33 ± 2.36 cd | ||
| 37DAF | 0.00 c | 6.67 ± 4.71 j | 85.83 ± 4.25 b | ||
| JZM | 10DAF | 0.00 c | 17.50 ± 3.54 hi | 78.33 ± 2.36 cd | |
| 45DAF | 6.67 ± 4.71 c | 29.17 ± 7.17 fg | 70.83 ± 7.17 d | ||
| 68DAF | 0.00 c | 3.33 ± 4.71 k | 83.33 ± 4.71 bc | ||
| JH | 40DAF | 3.33 ± 4.71 c | 21.67 ± 2.36 hi | 85.83 ± 4.25 b | |
| 62DAF | 0.00 c | 3.33 ± 4.71 k | 93.33 ± 4.71 ab | ||
| JHL | 41DAF | 6.67 ± 4.71 c | 20.83 ± 7.16 h | 89.17 ± 1.18 b | |
| 63DAF | 0.00 c | 3.33 ± 4.71 k | 96.67 ± 4.71 a | ||
| LYH | 44DAF | 3.33 ± 4.71 c | 20.00 ± 8.16 h | 75.00 ± 4.08 d | |
| 65DAF | 0.00 c | 3.33 ± 4.71 k | 93.33 ± 4.71 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Cheng, F.; Tao, X.; Zhong, Y. Effect of Cutting Phenological Stage, Chemical Treatments, and Substrate on Rooting Softwood Cuttings of Tree Peony. Horticulturae 2025, 11, 552. https://doi.org/10.3390/horticulturae11050552
Li D, Cheng F, Tao X, Zhong Y. Effect of Cutting Phenological Stage, Chemical Treatments, and Substrate on Rooting Softwood Cuttings of Tree Peony. Horticulturae. 2025; 11(5):552. https://doi.org/10.3390/horticulturae11050552
Chicago/Turabian StyleLi, Dongli, Fangyun Cheng, Xiwen Tao, and Yuan Zhong. 2025. "Effect of Cutting Phenological Stage, Chemical Treatments, and Substrate on Rooting Softwood Cuttings of Tree Peony" Horticulturae 11, no. 5: 552. https://doi.org/10.3390/horticulturae11050552
APA StyleLi, D., Cheng, F., Tao, X., & Zhong, Y. (2025). Effect of Cutting Phenological Stage, Chemical Treatments, and Substrate on Rooting Softwood Cuttings of Tree Peony. Horticulturae, 11(5), 552. https://doi.org/10.3390/horticulturae11050552








