Dynamic Pollen–Stigma Coordination in Dendrobium Hybridization: A Strategy to Maximize Fruit Set and Hybrid Seed Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Parent Materials
2.2. Measurement of Pollen Viability at Different Stages
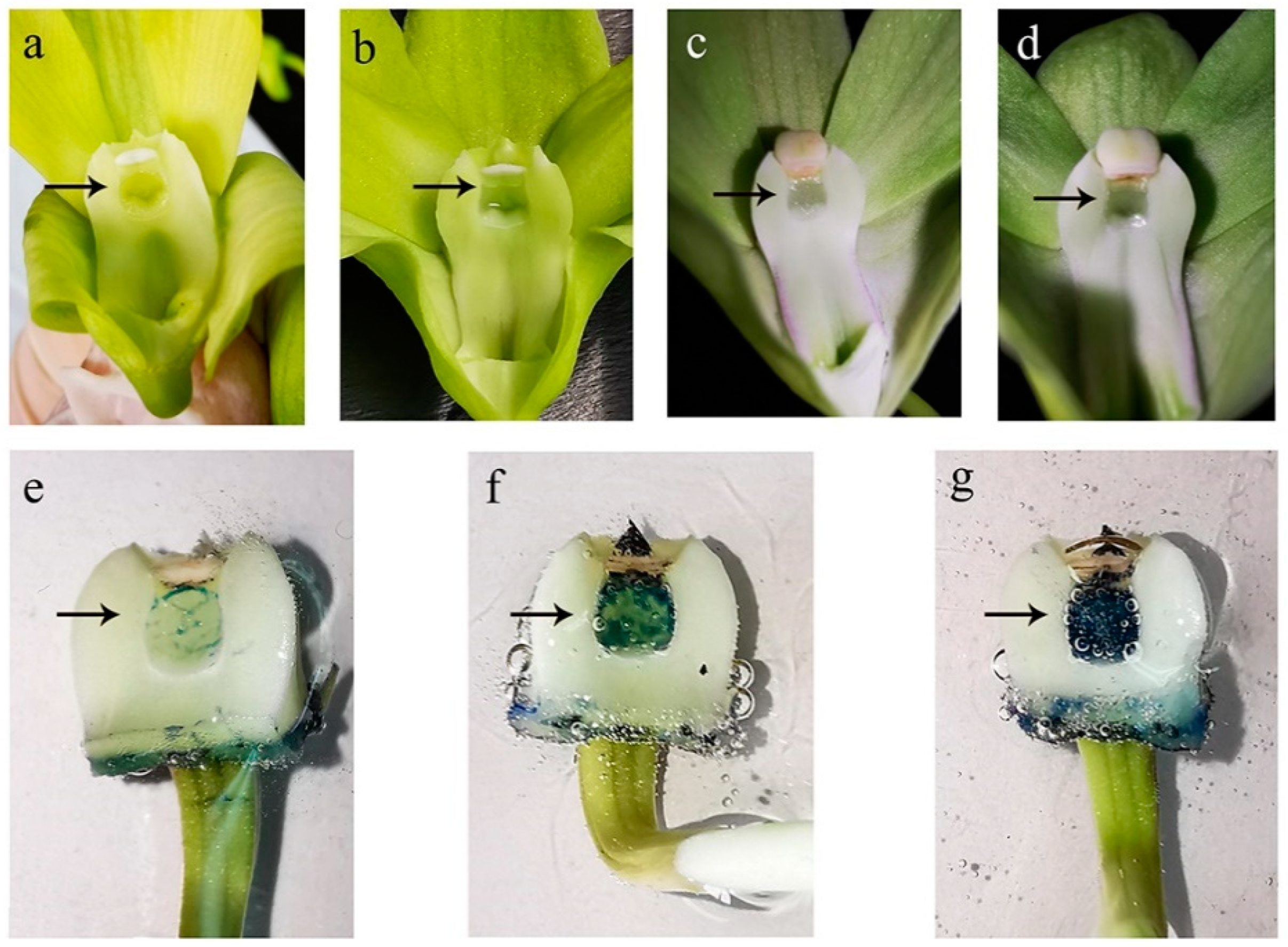
2.3. Estimation of Stigma Development
2.4. Precise Artificial Pollination
2.5. Assessment of Fruit Set
2.6. Evaluation of Cross-Seed Development and Viability
2.7. Statistical Analysis
3. Results
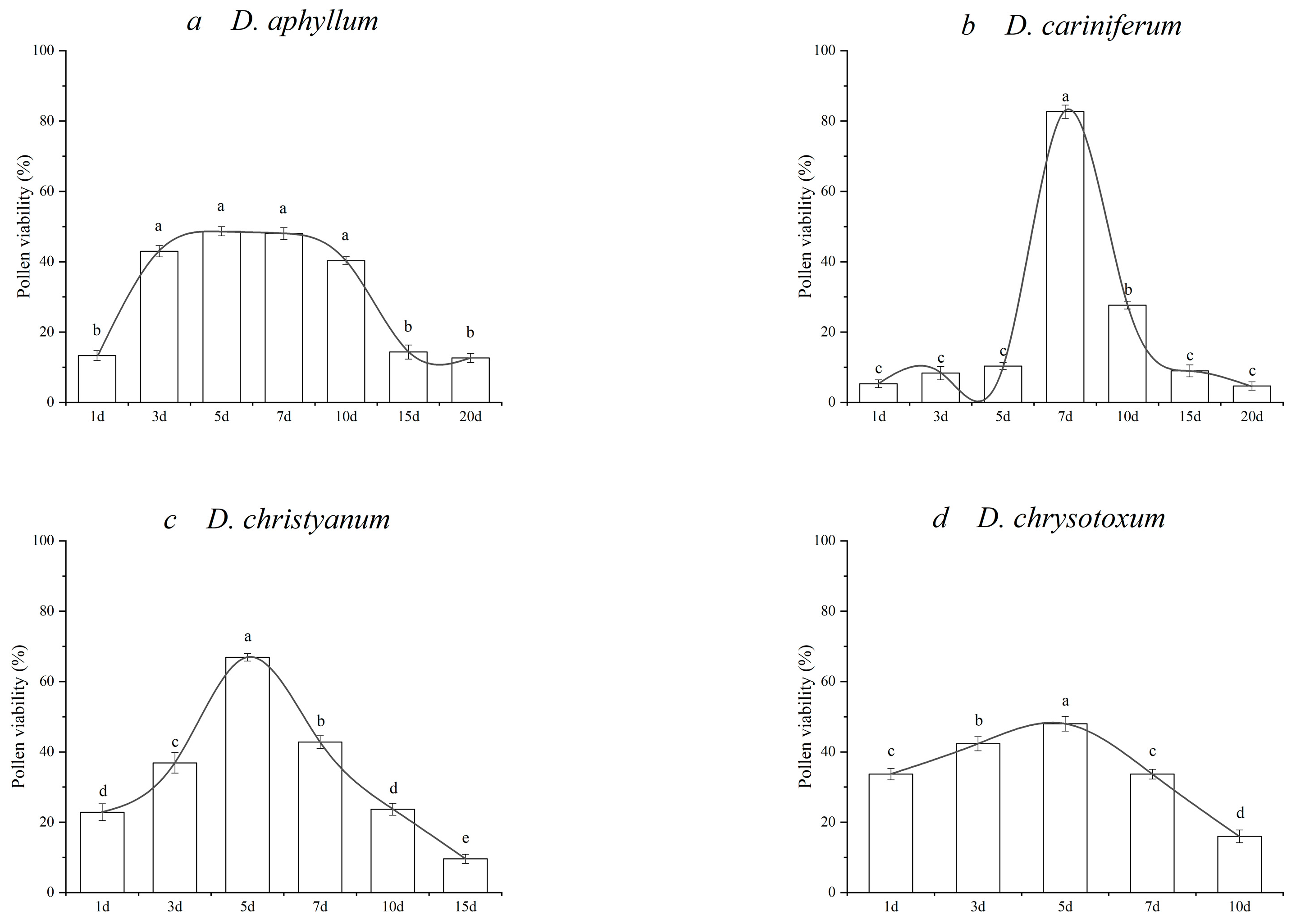
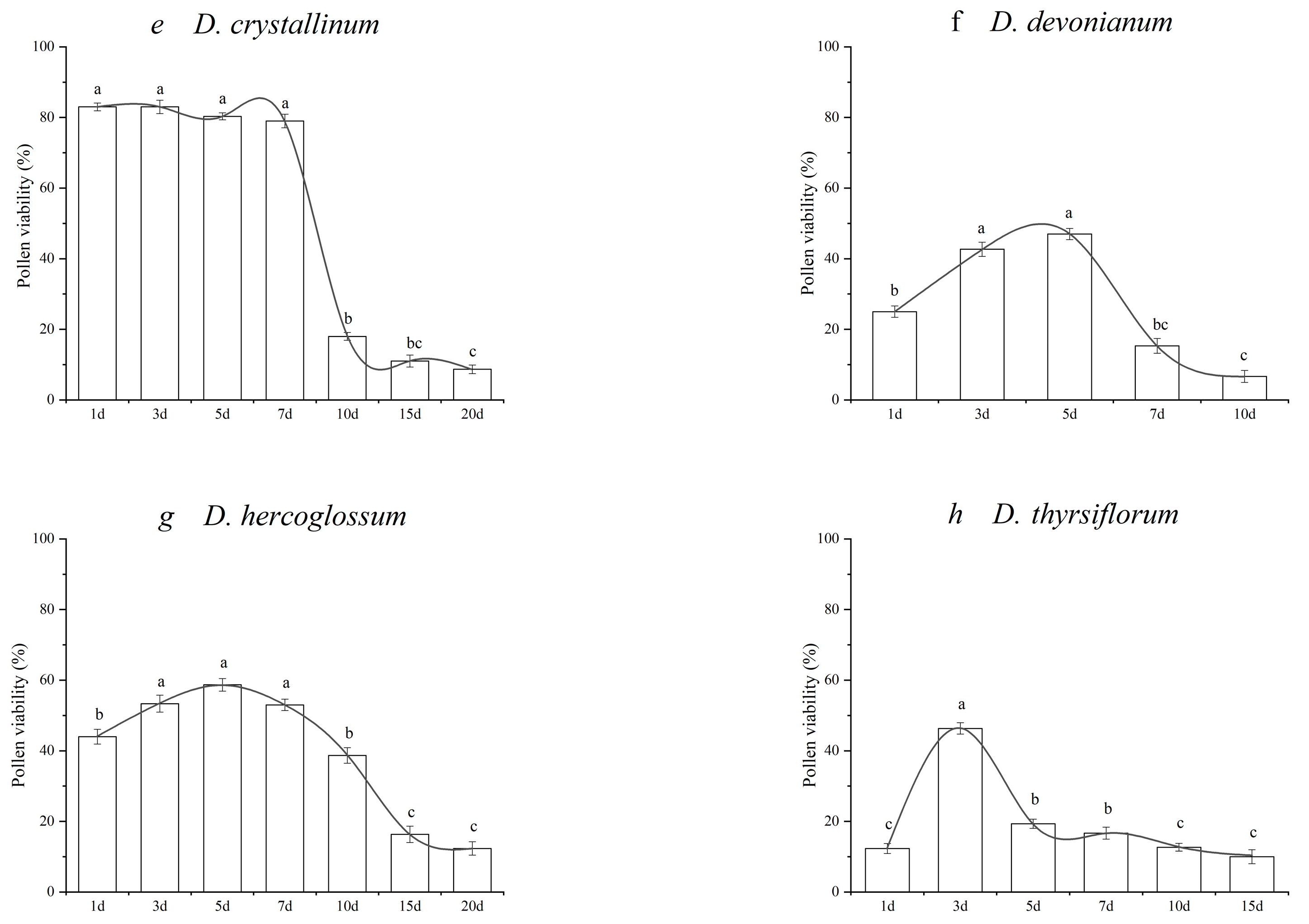
3.1. Dynamic Changes in and Duration of Pollen Viability
3.2. Stigma Receptivity at the Different Development Stages
3.3. Fruit Set of Eight Cross Combinations
3.4. Hybrid Seed Development and Viability
4. Discussion
4.1. Vigor Dynamics of the Pollen and Stigma
4.2. Determinators of the Creation of Interspecific Hybrids
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | superoxide dismutase |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| MS | Murashige and Skoog |
| TZ | modified tetrazolium |
References
- Ketsa, S.; Warrington, I.J. The Dendrobium Orchid: Botany, Horticulture, and Utilization. Crop Sci. 2023, 63, 1829–1888. [Google Scholar] [CrossRef]
- Hayati, N.Q.; Nugrahapsari, R.A.; Sastro, Y. Analysis of Competitive Assessment of Consumer Requirements and Technical Requirements on The Quality Development of Dendrobium Orchid Potted Flowers. In Proceedings of the International Symposia on Horticulture (ISH) on Emerging Challenges and Opportunities in Horticulture Supporting Sustainable Development Goals, Bali, Indonesia, 27–30 November 2018. [Google Scholar]
- Deng, Y.; Chen, S.; Chen, F.; Cheng, X.; Zhang, F. The Embryo Rescue Derived Intergeneric Hybrid between Chrysanthemum and Ajania Przewalskii Shows Enhanced Cold Tolerance. Plant Cell Rep. 2011, 30, 2177–2186. [Google Scholar] [CrossRef]
- Zhu, W.; Lu, M.; He, Q.; Zhu, G. A Review: Orchid Industry and Scientific Research Achievements in China. In Proceedings of the Paper presented at the IV International Orchid Symposium 1414, Guangzhou, China, 9 December 2024. [Google Scholar]
- Lester, R.N.; Kang, J.H. Embryo and Endosperm Function and Failure inSolanumSpecies and Hybrids. Ann. Bot. 1998, 82, 445–453. [Google Scholar] [CrossRef]
- Oneal, E.; Willis, J.H.; Franks, R.G. Disruption of Endosperm Development Is a Major Cause of Hybrid Seed Inviability between Mimulus Guttatus and Mimulus Nudatus. New Phytol. 2016, 210, 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.L.; Semir, J.; Shepherd, G.J. Self-Incompatibility, Inbreeding Depression and Crossing Potential in Five Brazilianpleurothallis (Orchidaceae) Species. Ann. Bot. 2001, 88, 89–99. [Google Scholar] [CrossRef]
- Ricci, N.A.P.; Bento, J.P.S.P.; Mayer, J.L.S.; Singer, R.B.; Koehler, S. Gametophytic Self-Incompatibility in Maxillariinae Orchids. Protoplasma 2024, 261, 271–279. [Google Scholar] [CrossRef]
- Johansen, B. Incompatibility in Dendrobium (Orchidaceae). Bot. J. Linn. Soc. 1990, 103, 165–196. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y.; Liu, Y.; Chen, D.; Luo, Y.; Niu, S. Challenges and Perspectives in the Study of Self-Incompatibility in Orchids. Int. J. Mol. Sci. 2021, 22, 12901. [Google Scholar] [CrossRef]
- Eyles, A.; Close, D.C.; Quarrell, S.R.; Allen, G.R.; Spurr, C.J.; Barry, K.M.; Whiting, M.D.; Gracie, A.J. Feasibility of Mechanical Pollination in Tree Fruit and Nut Crops: A Review. Agronomy 2022, 12, 1113. [Google Scholar] [CrossRef]
- Xing, G.; Qu, L.; Zhang, W.; Zhang, Y.; Yuan, X.; Lei, J. Study on Interspecific Hybridization between Tulip Cultivars and Wild Species Native to China. Euphytica 2020, 216, 1–17. [Google Scholar] [CrossRef]
- Liu, P.; Quan, X.; Song, Z.; Li, W.; Wang, Y.; Gu, H.; Xie, D.; Yang, W.; Dresselhaus, T.; Zhong, S.; et al. A Two-Step Self-Pollination Mechanism Maximizes Fertility in Brassicaceae. Cell 2025, 24, 3550–3558. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Yadav, N.S.; Sorokin, A.; Bilichak, A.; Kovalchuk, I. Development and Optimization of a Germination Assay and Long-Term Storage for Cannabis Sativa Pollen. Plants 2020, 9, 665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Lorne, M.W. Stigma Receptivity over the Lifetime of the Hermaphroditic Flower of Elsholtzia Rugulosa Was Negatively Correlated with Pollen Viability. Plant Signal. Behav. 2016, 11, e1259052. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Sun, D.; Gao, C. Flower Opening Dynamics, Pollen-Ovule Ratio, Stigma Receptivity and Stigmatic Pollen Germination (in-Vivo) in Chaenomeles Speciosa (Sweet) Nakai. Sci. Rep. 2024, 14, 7127. [Google Scholar] [CrossRef]
- Qiu, J.; Gao, C.; Wei, H.; Wang, B.; Hu, Y.; Guo, Z.; Long, L.; Yang, L.; Li, H. Flowering Biology of Rhododendron Pulchrum. Horticulturae 2021, 7, 508. [Google Scholar] [CrossRef]
- Buchner, L.; Eisen, A.-K.; Šikoparija, B.; Jochner-Oette, S. Pollen Viability of Fraxinus Excelsior in Storage Experiments and Investigations on the Potential Effect of Long-Range Transport. Forests 2022, 13, 600. [Google Scholar] [CrossRef]
- Al-Najm, A.; Brauer, S.; Trethowan, R.; Merchant, A.; Ahmad, N. Optimization of in Vitro Pollen Germination and Viability Testing of Some Australian Selections of Date Palm (Phoenix Dactylifera L.) and Their Xenic and Metaxenic Effects on the Tissue Culture—Derived Female Cultivar Barhee. Vitr. Cell. Dev. Biol. Plant 2021, 57, 771–785. [Google Scholar] [CrossRef]
- Breygina, M.; Klimenko, E.; Shilov, E.; Podolyan, A.; Mamaeva, A.; Zgoda, V.; Fesenko, I. Hydrogen Peroxide in Tobacco Stigma Exudate Affects Pollen Proteome and Membrane Potential in Pollen Tubes. Plant Biol. 2021, 23, 592–602. [Google Scholar] [CrossRef]
- Breygina, M.; Luneva, O.; Schekaleva, O.; Lazareva, N.; Babushkina, K.; Kirilyuk, I.A. Pattern of Ros Generation and Interconversion on Wet Stigmas in Basal and Divergent Angiosperms. Plant Growth Regul. 2023, 101, 463–472. [Google Scholar] [CrossRef]
- Schekaleva, O.; Luneva, O.; Klimenko, E.; Shaliukhina, S.; Breygina, M. Dynamics of Ros Production, Sod, Pod and Cat Activity During Stigma Maturation and Pollination in Nicotiana Tabacum and Lilium Longiflorum. Plant Biol. 2024, 26, 1240–1246. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Wei, J. Study on Flowering Dynamics and Pollination Habits of Monochasma Savatieri under Artificial Cultivation Conditions. Plants 2025, 14, 715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yu, Y.; Liu, Y.; Yang, S.; Chen, Y. Gradual Pollen Presentation in Vaccinium Corymbosum ‘Bluecrop’: An Adaptive Mechanism to Improve Pollination Efficiency and Outcrossing. PeerJ 2024, 12, e17273. [Google Scholar] [CrossRef]
- Hu, S. Experimental Methods in Plant Embryology (I) Determination of Pollen Viability. Chin. Bull. Bot. 1993, 10, 60. [Google Scholar]
- Breygina, M.; Schekaleva, O.; Klimenko, E.; Luneva, O. The Balance between Different Ros on Tobacco Stigma During Flowering and Its Role in Pollen Germination. Plants 2022, 11, 993. [Google Scholar] [CrossRef]
- Soares, J.S.; Rosa, Y.B.C.J.; Tatara, M.B.; Sorgato, J.C.; Lemes, C.S.R. Orchid Seeds Viability Identification by Tetrazolium Test. Dir. Open Access J. 2014, 35, 2275–2284. [Google Scholar]
- Kılıç, T.; Sinanoğlu, E.; Kırbay, E.; Kazaz, S.; Ercişli, S. Determining Appropriate Methods for Estimating Pollen Viability and Germination Rates in Lisianthus. Acta Sci. Pol. Hortorum Cultus 2024, 23, 33–42. [Google Scholar] [CrossRef]
- Li, M.; Jiang, F.; Huang, L.; Wang, H.; Song, W.; Zhang, X.; Zhang, Y.; Niu, L. Optimization of in Vitro Germination, Viability Tests and Storage of Paeonia Ostii Pollen. Plants 2023, 12, 2460. [Google Scholar] [CrossRef]
- Wizenberg, S.B.; Dang, M.; Campbell, L.G. Methods for Characterizing Pollen Fitness in Cannabis Sativa L. PLoS ONE 2022, 17, e0270799. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.M.; Zheng, B.Q.; Guo, X.; Wang, Y. Pollen Viability and Preservation of Dendrobium Lindleyi/ Pollen viability and preservation of Dendrobium lindleyi. Lin Ye Ke Xue Yan Jiu 2014, 27, 657. [Google Scholar]
- Hao, Q.; Xu, L.; Wang, H.; Liu, Q.; Wang, K. Evaluation of Pollen Viability, Stigma Receptivity, and the Cross Barrier between Tropical and Hardy Water Lily Cultivars. Flora 2022, 290, 152046. [Google Scholar] [CrossRef]
- Shao, F.; Wang, S.; Chen, J.; Chen, J.; Hong, R.; Tang, Y.; Wang, J. Stigma Shape Development and Receptivity Of’zhongqiu Sucui’chinese Jujube. Acta Hortic. Sin. 2019, 46, 2309–2322. [Google Scholar]
- Zulkarnain, Z.; Eliyanti, E.; Swari, E.I. Pollen Viability Stigma Receptivity in Swainsona Formosa (G. Don) J. Thompson (Fabaceae), an Ornamental Legume Native to Australia. Ornam. Hortic. 2019, 25, 158–167. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Wang, X.; Zeng, H.; Qiao, Z. Germination Characteristics of Fruiting Nonfruiting Lagerstroemia Indica Pollen. J. Cent. South. Univ. For. Technol. 2023, 43, 74–81. [Google Scholar]
- Lestari, N.K.D.; Deswiniyanti, N.W.; Sari, N.K.Y.; Murna, I.M.; Rizqy, A.N. Morphological Relationships and Cross Compatibility of Seven Dendrobium Species in Indonesia. Biodiversitas J. Biol. Divers. 2023, 24, 6. [Google Scholar] [CrossRef]
- Maulida, D.; Yusnita, Y.; Hapsoro, d.; Agustiansyah, A.; Karyanto, A. Interspecific Hybridization of Dendrobium Mirbelianum X D. Nindii or D. Discolor, in Vitro Seed Germination, Seedling Growth and Plantlet Acclimatization. Biodiversitas J. Biol. Divers. 2023, 24, 5. [Google Scholar] [CrossRef]
- Yuan, S.-C.; Lekawatana, S.; Amore, T.D.; Chen, F.-C.; Chin, S.-W.; Vega, D.M.; Wang, Y.-T. The Global Orchid Market. In Orchid Genome; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–28. [Google Scholar]
- Kamemoto, H.; Wilfret, G.J. Inter-and multi-sectional Dendrobium hybrids. Proc. 9th World Orchid Conf. 1980, 255, 261. [Google Scholar]
- Devadas, R.; Pattanayak, S.R.; Singh, D.R. Studies on Cross Compatibility in Dendrobium Species Hybrids. Indian J. Genet. Plant Breed. 2016, 76, 344–355. [Google Scholar] [CrossRef]
- Sirilak, I.; Bundithya, W.; Kuanprasert, N.; Apavatjrut, P. Analysis of Intersectional Hybrids of Dendrobiumby RAPD Technique. Kasetsart J. Nat. Sci. 2006, 40, 456–461. [Google Scholar]
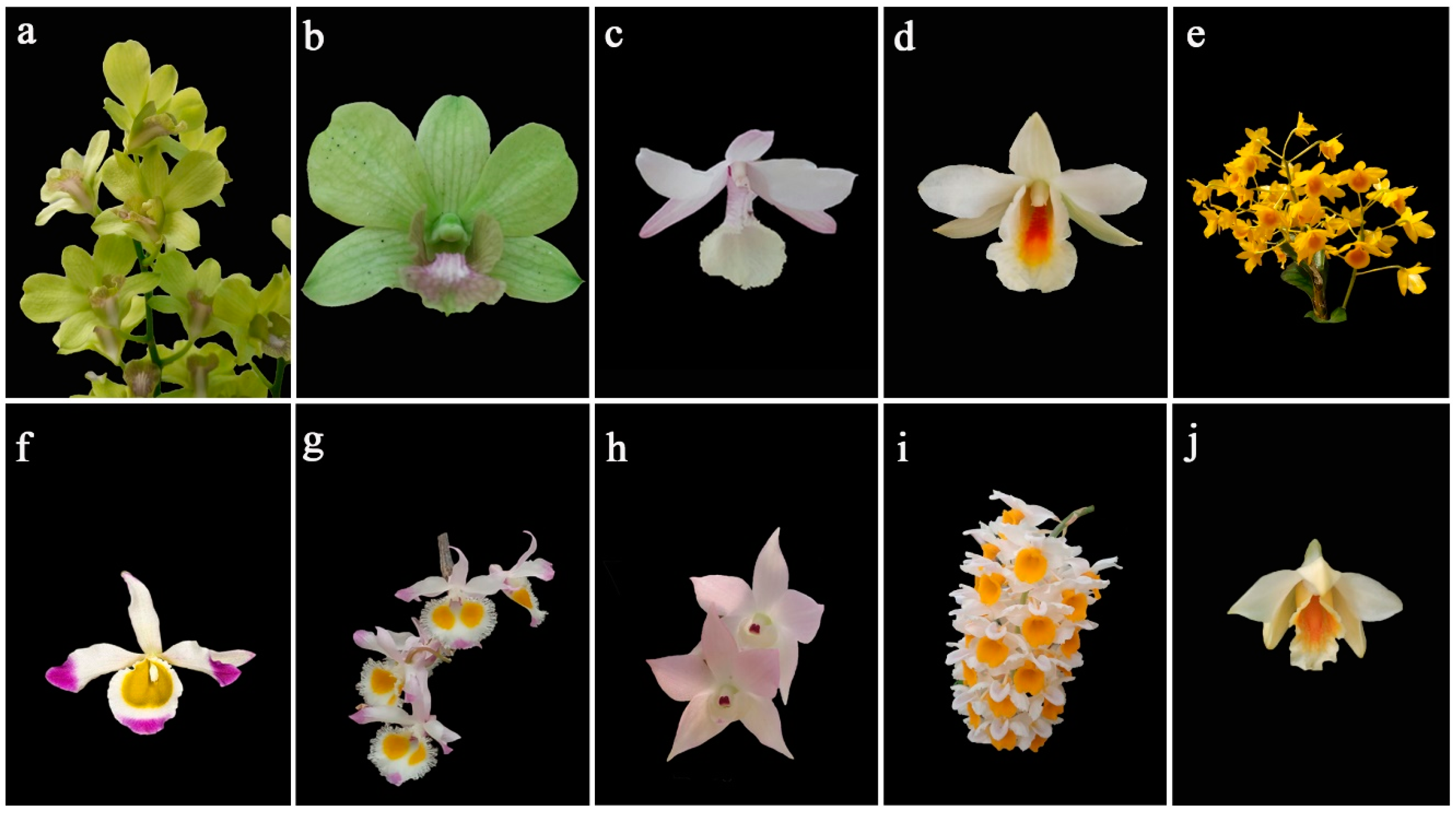




| Female Parent | Male Parent | Empirical Pollination | Precision Pollination | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pollinated Flowers | Number of Maturity Pod | Fruit-Setting Rate (%) | Pollinated Flowers | Number of Masturity Pod | Fruit-Setting Rate (%) | Fruit Maturity Percentage (%) | Days for Pods to Ripen (d) | ||
| D. ‘Burana Jade’ | D. aphyllum | 11 | 0 | 0 | 11 | 2 | 18.18 | 40.0 | 138 |
| D. christyanum | 15 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | |
| D. chrysotoxum | 10 | 0 | 0 | 10 | 1 | 10 | 25.0 | 252 | |
| D. crystallinum | 15 | 0 | 0 | 15 | 0 | 0 | 0 | - | |
| D. devonianum | 10 | 0 | 0 | 10 | 0 | 0 | 0 | - | |
| D. hercoglossum | 13 | 1 | 7.69 | 13 | 4 | 30.77 | 57.14 | 220 | |
| D. thyrsiflorum | 10 | 0 | 0 | 10 | 1 | 10 | 50 | 121 | |
| D. cariniferum | 11 | 0 | 0 | 11 | 0 | 0 | 0 | - | |
| Total | 95 | 1 | 1.06 | 95 | 8 | 8.62 | 43.04 | 182.75 | |
| Female Parent | Male Parent | Percentage of Seed with Visible Embryo (%) | Percentage of Viability Seed (%) | Mean Length of Seed (μm ± SD) | Mean Width of Seed (μm ± SD) |
|---|---|---|---|---|---|
| D. ‘Burana Jade’ | D. aphyllum | 42.78 b | 13.97 a | 442.56 ± 84.35 a | 40.17 ± 13.89 b |
| D. chrysotoxum | 25.49 c | 16.20 a | 238.96 ± 97.62 c | 28.84 ± 6.84 c | |
| D. thyrsiflorum | 54.81 a | 22.38 a | 450.80 ± 63.19 a | 79.65 ± 16.44 a | |
| D.hercoglossum | 21.21 c | — | 337.50 ± 41.53 b | 35.36 ± 6.45 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Qian, Y.; Guan, A.; Yue, Y.; Li, Z.; Dunn, B.; Yang, J.; Yi, S.; Liao, Y.; Yin, J. Dynamic Pollen–Stigma Coordination in Dendrobium Hybridization: A Strategy to Maximize Fruit Set and Hybrid Seed Viability. Horticulturae 2025, 11, 544. https://doi.org/10.3390/horticulturae11050544
Wu Q, Qian Y, Guan A, Yue Y, Li Z, Dunn B, Yang J, Yi S, Liao Y, Yin J. Dynamic Pollen–Stigma Coordination in Dendrobium Hybridization: A Strategy to Maximize Fruit Set and Hybrid Seed Viability. Horticulturae. 2025; 11(5):544. https://doi.org/10.3390/horticulturae11050544
Chicago/Turabian StyleWu, Qian, Yanbing Qian, Ao Guan, Yan Yue, Zongyan Li, Bruce Dunn, Jianwei Yang, Shuangshuang Yi, Yi Liao, and Junmei Yin. 2025. "Dynamic Pollen–Stigma Coordination in Dendrobium Hybridization: A Strategy to Maximize Fruit Set and Hybrid Seed Viability" Horticulturae 11, no. 5: 544. https://doi.org/10.3390/horticulturae11050544
APA StyleWu, Q., Qian, Y., Guan, A., Yue, Y., Li, Z., Dunn, B., Yang, J., Yi, S., Liao, Y., & Yin, J. (2025). Dynamic Pollen–Stigma Coordination in Dendrobium Hybridization: A Strategy to Maximize Fruit Set and Hybrid Seed Viability. Horticulturae, 11(5), 544. https://doi.org/10.3390/horticulturae11050544






