Consequences of Micro- and Macronutrient Deficiencies on Physiological and Growth Metrics in Hydroponic ‘Thompson Seedless’ Grapevines
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of New Shoot Length
2.2. Determination of Leaf Area
2.3. Determination of Photosynthesis
2.4. Determination of Chlorophyll Content
2.5. Sample Collection and Processing
2.6. Determination of Mineral Element Contents
2.7. Statistical Analysis
3. Results
3.1. Leaf and Root Visual Symptoms Under Deficiency Conditions
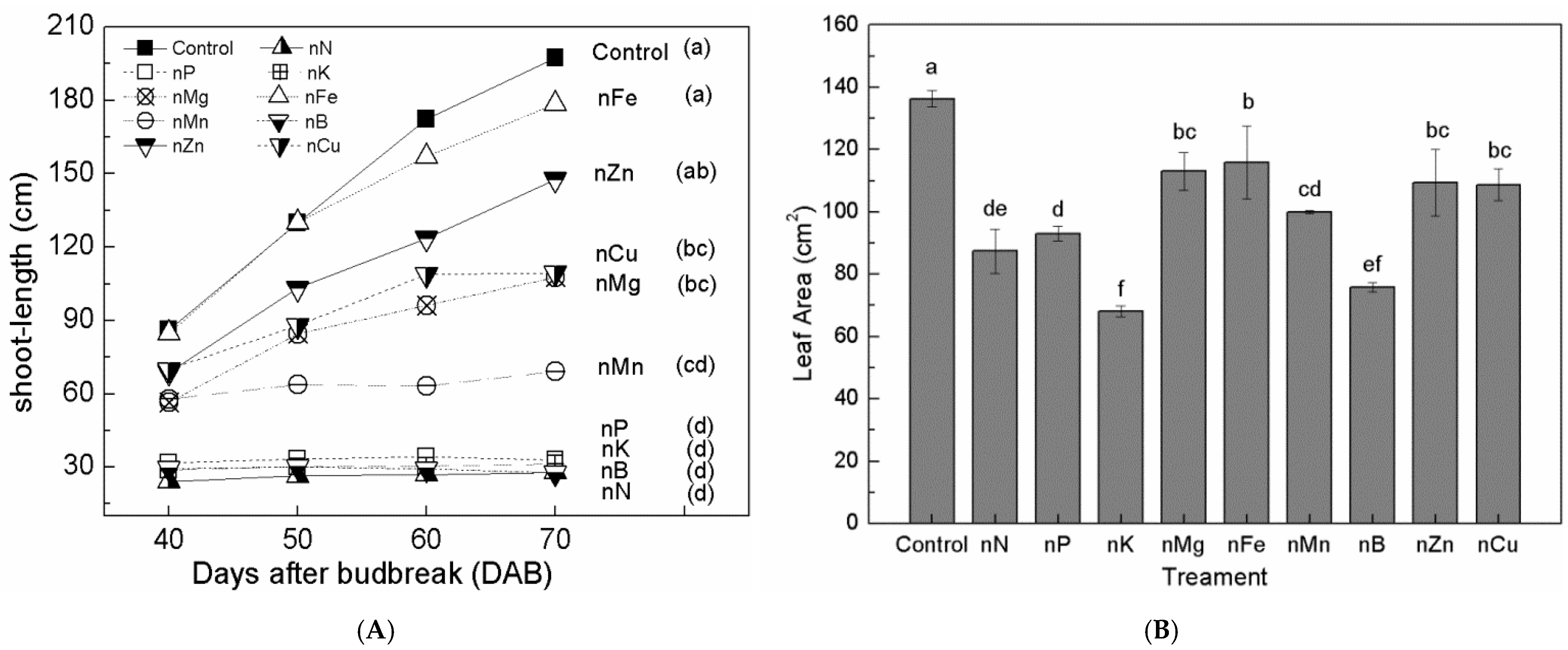
3.2. Effect of Nutrient Deficiency on Shoot Length
3.3. Effects of Nutritional Deficiency on the Leaf Area
3.4. Biomass of Leaves, Stems, and Roots of Thompson Seedless Plants
3.5. Effects of Nutrient Deficiency on Chlorophyll Content
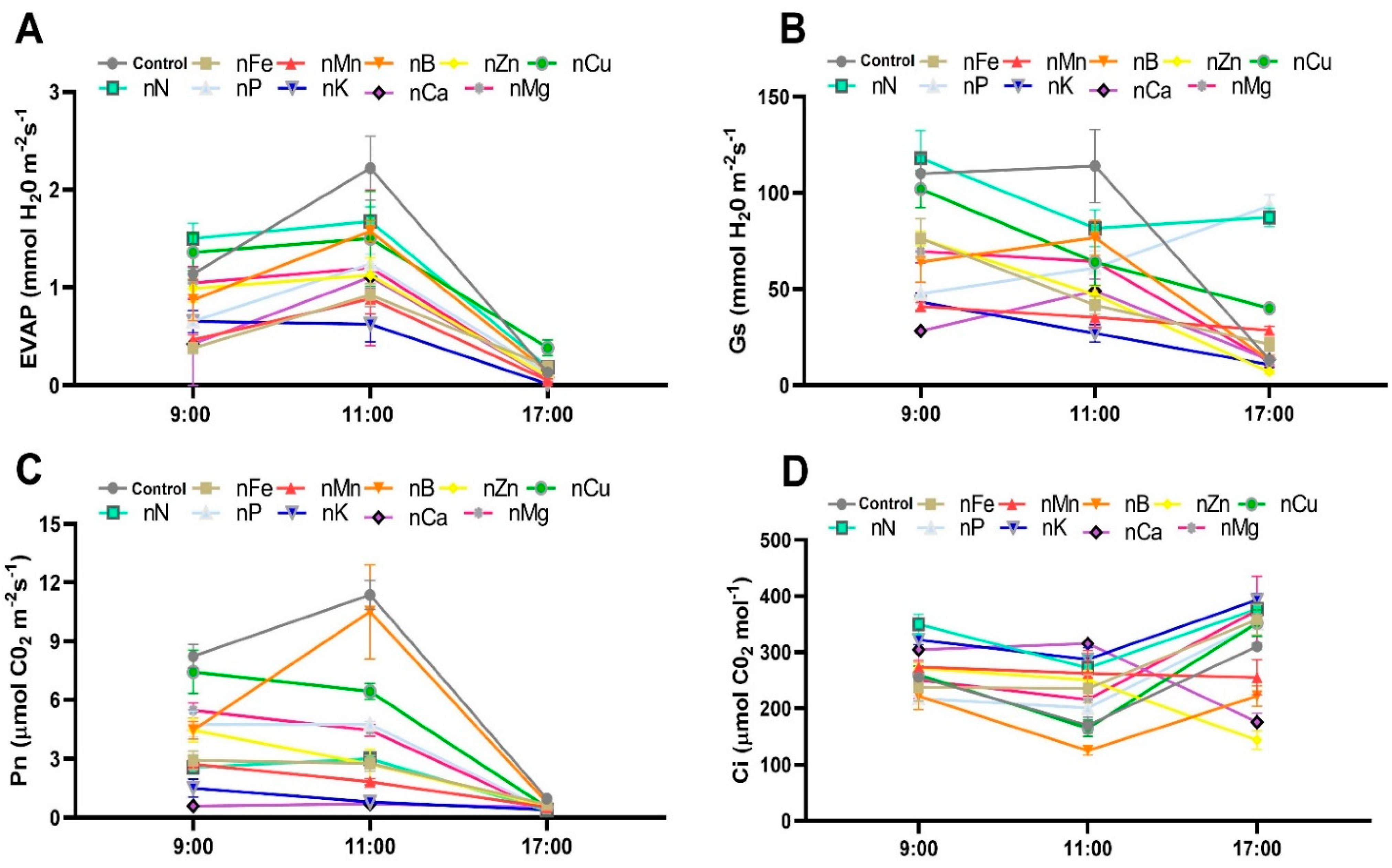
3.6. Effect of Nutritional Deficiency on Respiration
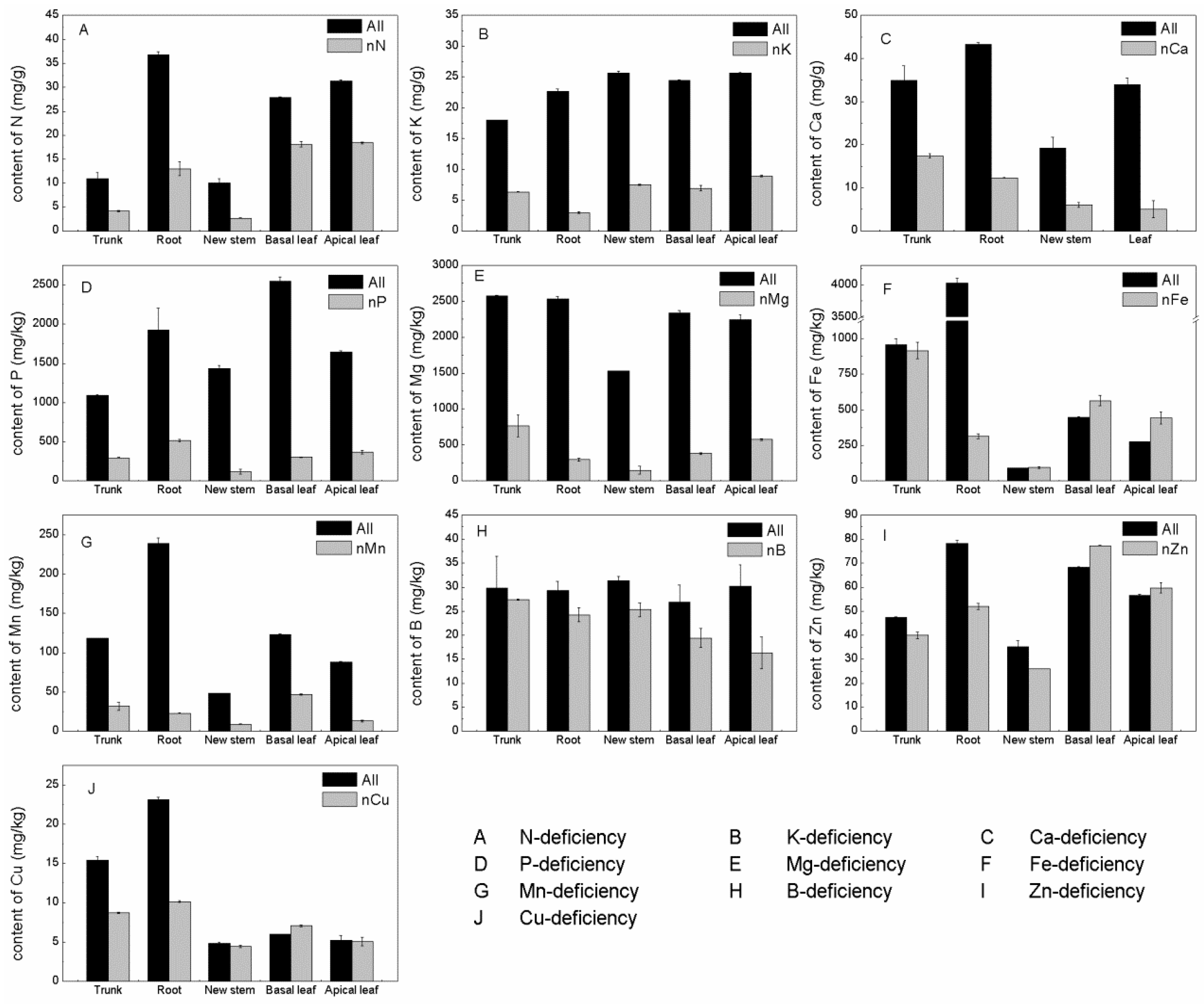
3.7. Effect of Nutrient Deficiency on the Mineral Content of Thompson Seedless
3.7.1. Effect of Nitrogen Deficiency
3.7.2. Effect of Phosphorus Deficiency
3.7.3. Effect of Potassium Deficiency
3.7.4. Effect of Calcium Deficiency
3.7.5. Effect of Magnesium Deficiency
3.7.6. Effect of Iron Deficiency
3.7.7. Effect of Boron Deficiency
3.7.8. Effect of Zinc Deficiency
3.7.9. Effect of Copper Deficiency
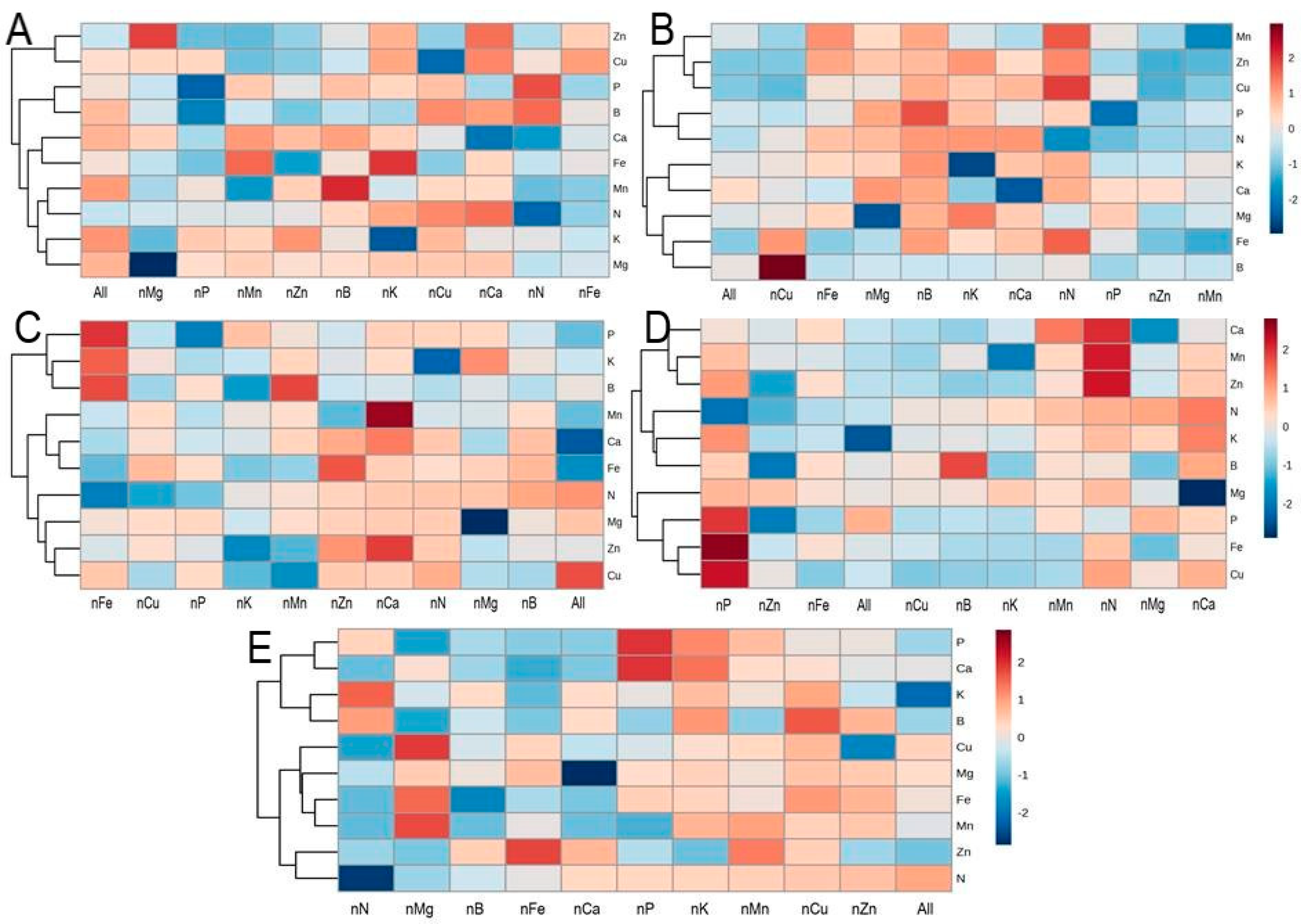
3.8. Hierarchical Cluster Analysis of Nutrient Deficit Elements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| N | Nitrogen |
| P | Phosphorus |
| K | Potassium |
| Ca | Calcium |
| Mg | Magnesium |
| Fe | Iron |
| Mn | Manganese |
| B | Boron |
| Zn | Zinc |
| Cu | Copper |
| SPAD | Soil Plant Analysis Development |
| RCBD | Randomized Complete Block Design |
| Mo | Molybdenum |
| Ni | Nickel |
| Cl | Chloride |
| ppm | Parts Per Million |
References
- Dobermann, A.; Bruulsema, T.; Cakmak, I.; Gerard, B.; Majumdar, K.; McLaughlin, M.; Reidsma, P.; Vanlauwe, B.; Wollenberg, L.; Zhang, F.; et al. Responsible Plant Nutrition: A New Paradigm to Support Food System Transformation. Glob. Food Secur. 2022, 33, 100636. [Google Scholar] [CrossRef]
- Nath, M.; Tuteja, N. NPKS Uptake, Sensing, and Signaling and MiRNAs in Plant Nutrient Stress. Protoplasma 2016, 253, 767–786. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N.; Gorelick, J.; Zerahia, R.; Koch, S. Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L). Front. Plant Sci. 2019, 10, 736. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Luo, H.; Jiang, Y.; Wang, L.; Xiao, C.; Weng, L. Influence of Nutrient (NPK) Factors on Growth, and Pharmacodynamic Component Biosynthesis of Atractylodes Chinensis: An Insight on Acetyl-CoA Carboxylase (ACC), Reductase (HMGR), and Farnesyl Pyrophosphate Synthase (FPPS) Signaling Responses. Front. Plant Sci. 2022, 13, 799201. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Reddy, P.R.V.; Aski, M.S.; Prakash, G.; Id, M.; Id, K.D.; Singh, A.; Id, R.P.; Singh, M.P. Genetic Variation for Root Architectural Traits in Response to Phosphorus Deficiency in Mungbean at the Seedling Stage. PLoS ONE 2020, 15, e0221008. [Google Scholar] [CrossRef]
- Grechi, I.; Vivin, P.; Hilbert, G.; Milin, S.; Robert, T.; Gaudill, J. Effect of Light and Nitrogen Supply on Internal C: N Balance and Control of Root-to-Shoot Biomass Allocation in Grapevine. Environ. Exp. Bot. 2007, 59, 139–149. [Google Scholar] [CrossRef]
- Terra, M.M.; Brasil Sobrinho, M.O.C.; Pires, E.J.P.; Nagai, V. Six years of NPK fertilizer experimentation with grapevine cultivar Niagara rosada, growing in a podzol soil, in Indaiatuba, SP, Brazil. Acta Hortic. 2000, 526, 235–240. [Google Scholar] [CrossRef]
- Frost, S.; Lerno, L.A.; Zweigenbaum, J.; Heymann, H.; Ebeler, S.E. Characterization of Red Wine Proanthocyanidins Using a Putative Proanthocyanidin Database, Amide Hydrophilic Interaction Liquid Chromatography (HILIC), and Time-of-Flight Mass Spectrometry. Molecules 2018, 23, 2687. [Google Scholar] [CrossRef]
- Zhao, D.; Oosterhuis, D.M. Cotton Growth and Physiological Responses to Boron Deficiency. J. Plant Nutr. 2003, 26, 855–867. [Google Scholar] [CrossRef]
- Singh, P.; Misra, A.; Srivastava, N. Influence of Mn Deficiency on Growth, Chlorophyll Content, Physiology, and Essential Monoterpene Oil(s) in Genotypes of Spearmint (Mentha spicata L.). Photosynthetica 2001, 39, 473–476. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.; Jiang, H.; Smith, B.R.; Yang, L.; Xie, C. Boron Deficiency Decreases Growth and Photosynthesis, and Increases Starch and Hexoses in Leaves of Citrus Seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef]
- Zhao, Z.; Chu, C.; Zhou, D.; Sha, Z.; Wu, S. Soil Nutrient Status and the Relation with Planting Area, Planting Age and Grape Varieties in Urban Vineyards in Shanghai. Heliyon 2019, 5, e02362. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Kalaiarasi, K.; Shanmugam, J.; Dhairiyasamy, R. Comparative Analysis of Spectroradiometric and Chemical Methods for Nutrient Detection in Black Gram Leaves. Results Eng. 2024, 24, 103065. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil. California Agricultural Ecperiment Station, Circular-347. 1950. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19500302257 (accessed on 20 March 2025).
- Powlson, D.S.; Prookes, P.C.; Christensen, B.T. Measurement of Soil Microbial Biomass Provides an Early Indication of Changes in Total Soil Organic Matter Due to Straw Incorporation. Soil. Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Mu, W.; Gong, S.; Stankovski, S.; Zhang, X.; Kanianska, R.; Feng, J. WebGIS-Based Suitability Evaluation System for Chinese Table Grape Production. Comput. Electron. Agric. 2019, 165, 104945. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-Talks Between Macro- and Micronutrient Uptake and Signaling in Plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Javed, H.U.; Zahid, M.S.; Wu, Z.; Ma, C.; Jiu, S.; Xu, W.; Zhang, C.; Wang, S. Nutrient Solution with High Nitrogen Content, a Suitable Facilitator of Growth and Berry Quality in Hydroponic ‘Shine Muscat’ Grapevine (Vitis vinifera × V. Labrusca). Sci. Hortic. 2023, 310, 111749. [Google Scholar] [CrossRef]
- Zareei, E.; Javadi, T.; Aryal, R. Biochemical Composition and Antioxidant Activity Affected by Spraying Potassium Sulfate in Black Grape (Vitis vinifera L. Cv. Rasha). J. Sci. Food Agric. 2018, 98, 5632–5638. [Google Scholar] [CrossRef]
- Veazie, P.; Cockson, P.; Henry, J.; Perkins-veazie, P.; Whipker, B. Characterization of Nutrient Disorders and Impacts on Chlorophyll and Anthocyanin Concentration of Brassica Rapa Var. Chinensis. Agronomy 2020, 10, 461. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The Molecular–Physiological Functions of Mineral Macronutrients and Their Consequences for Deficiency Symptoms in Plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Peng, F.; Wang, X. Effect of Bag-Controlled Release Fertelizer on Nitrogen Utilization Rate, Growth and Fruiting of the ‘Fuji ’Apple. J. Plant Nutr. 2010, 33, 1904–1913. [Google Scholar] [CrossRef]
- Bai, L.; Deng, H.; Zhang, X.; Yu, X.; Li, Y. Gibberellin Is Involved in Inhibition of Cucumber Growth and Nitrogen Uptake at Suboptimal Root-Zone Temperatures. PLoS ONE 2016, 5, 0156188. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Effects of Nitrogen Application Rate on Dry Matter Redistribution, Grain Yield, Nitrogen Use Efficiency and Photosynthesis in Malting Barley. Acta Agric. Scand. B Soil. Plant Sci. 2012, 62, 410–419. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in Plants: From Nutrition to the Modulation of Abiotic Stress Adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 185–268. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.-H.; Huang, D.; Dong, Q.-L.; Li, P.-M.; van Nocker, S.; Ma, F.-W. Physiological Evaluation of Nitrogen Use Efficiency of Different Apple Cultivars under Various Nitrogen and Water Supply Conditions. J. Integr. Agric. 2020, 19, 709–720. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; Meyer, R.C.; Altmann, T.; von Wirén, N. Natural Variation of BSK3 Tunes Brassinosteroid Signaling to Regulate Root Foraging under Low Nitrogen. Nat. Commun. 2019, 10, 2378. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, Z.; Wang, W.; Qiu, Y.; Zhang, Z.; Liu, Y.; Li, A.; Gao, X.; Liu, L.; Qian, Y.; et al. Nitrate–NRT1.1B–SPX4 Cascade Integrates Nitrogen and Phosphorus Signalling Networks in Plants. Nat. Plants 2019, 5, 401–413. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Xia, H.; Xu, T.; Zhang, J.; Shen, K.; Li, Z. Drought-Induced Responses of Nitrogen Metabolism in Ipomoea Batatas. Plants 2020, 9, 1341. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Bosa, K.; Ko, J.; Zuk-gołaszewska, K. Effects of Salt Stress on Photosystem II Efficiency and CO2 Assimilation of Two Syrian Barley Landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Pietkiewicz, S. Review: Some Physiological Indices to Be Exploited as a Crucial Tool in Plant Breeding. Plant Breed. Seed Sci. 2004, 49, 19–39. [Google Scholar]
- Liu, B.H.; Cheng, L.; Liang, D.; Zou, Y.J.; Ma, F.W. Growth, Gas Exchange, Water-Use Efficiency, and Carbon Isotope Composition of ‘Gale Gala’ Apple Trees Grafted onto 9 Wild Chinese Rootstocks in Response to Drought Stress. Photosynthetica 2012, 50, 401–410. [Google Scholar] [CrossRef]
- Bottrill, D.E.; Possingham, J.V.; Kriedemann, P.E. The effect of nutrient deficiencies on photosynthesis and respiration in spinach. Plant Soil. 1970, 32, 424–438. [Google Scholar] [CrossRef]
- Humble, G.D.; Raschke, K. Stomatal Opening Quantitatively Related to Potassium Transport: Evidence from Electron Probe Analysis. Plant Physiol. 1971, 48, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. High Light Intensity Enhances Chlorosis and Necrosis in Leaves of Zinc, Potassium, and Magnesium Deficient Bean (Phaseolus vulgaris) Plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Peoples, T.R.; Koch, D.W. Role of Potassium in Carbon Dioxide Assimilation in Medicago sativa L. Plant Physiol. 1979, 63, 878–881. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; Gutiérrez-Díez, A.; Vázquez-Alvarado, R.E.; López-Jiménez, A. Visual Symptoms, Vegetative Growth, and Mineral Concentration in Fig Tree (Ficus carica L.) under Macronutrient Deficiencies. Agronomy 2019, 9, 787. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of Magnesium Deficiency on Photosynthesis and Carbohydrate Partitioning. Acta Physiol. Plant 2016, 38, 145. [Google Scholar] [CrossRef]
- Lima, T.C.; Freitas, M.S.M.; Mendonça, L.V.P.; de Souza Gonçalves, Y.; Pereira, J.A.P.; Lima, K.F. Visual Symptoms of Macronutrient and Boron Deficiency in Cymbopogon citratus. J. Plant Nutr. 2019, 42, 1430–1438. [Google Scholar] [CrossRef]
- Rhodes, R.; Miles, N.; Hughes, J.C. Interactions between Potassium, Calcium and Magnesium in Sugarcane Grown on Two Contrasting Soils in South Africa. Field Crops Res. 2018, 223, 1–11. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium Deficiency in Plants: An Urgent Problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]



| Treatments | mg/L | N | P | K | Ca | Mg | Fe | Mn | B | Zn | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Applied | 210 | 31 | 234 | 160 | 48 | 2.8 | 0.5 | 0.5 | 0.05 | 0.02 |
| Measured | 192.6 | 31.5 | 249.1 | 133.2 | 45.4 | 2.6 | 0.46 | 0.41 | 0.049 | 0.021 | |
| N-deficient | Applied | 0 | 31 | 234 | 160 | 48 | 2.8 | 0.5 | 0.5 | 0.05 | 0.02 |
| Applied | −0.10 | 35.1 | 264.6 | 118.3 | 44.3 | 2.9 | 0.53 | 0.48 | 0.041 | 0.028 | |
| P-deficient | Applied | 210 | 0 | 234 | 160 | 48 | 2.8 | 0.5 | 0.5 | 0.05 | 0.02 |
| Measured | 220.5 | −1.1 | 241.0 | 165.1 | 45.2 | 2.7 | 0.47 | 0.47 | 0.055 | 0.026 | |
| K-deficient | Applied | 210 | 31 | 0 | 160 | 48 | 2.8 | 0.5 | 0.5 | 0.05 | 0.02 |
| Measured | 204.6 | 33.5 | 1.6 | 139.1 | 45.2 | 2.5 | 0.50 | 0.46 | 0.06 | 0.028 | |
| Ca-deficient | Applied | 210 | 31 | 234 | 0 | 48 | 2.8 | 0.5 | 0.5 | 0.05 | 0.02 |
| Measured | 205.0 | 35.3 | 227.8 | −1.76 | 43.2 | 2.9 | 0.49 | 0.49 | 0.041 | 0.022 | |
| Mg-deficient | Applied | 210 | 31 | 234 | 160 | 0 | 2.8 | 0.5 | 0.5 | 0.05 | 0.02 |
| Measured | 207.4 | 31.3 | 244.9 | 173.7 | 0.041 | 2.3 | 0.58 | 0.49 | 0.041 | 0.024 | |
| Fe-deficient | Applied | 210 | 31 | 234 | 160 | 48 | 0 | 0.5 | 0.5 | 0.05 | 0.02 |
| Measured | 212.0 | 34.0 | 247.7 | 159.2 | 44.7 | 0.13 | 0.52 | 0.49 | 0.05 | 0.022 | |
| Mn-deficient | Applied | 210 | 31 | 234 | 160 | 48 | 2.8 | 0 | 0.5 | 0.05 | 0.02 |
| Measured | 197.9 | 34.9 | 240.0 | 148.2 | 44.6 | 2.5 | 0.017 | 0.51 | 0.052 | 0.024 | |
| B-deficient | Applied | 210 | 31 | 234 | 160 | 48 | 2.8 | 0.5 | 0 | 0.05 | 0.02 |
| Measured | 220.5 | 35.6 | 248.1 | 143.9 | 44.6 | 2.7 | 0.53 | 0.03 | 0.042 | 0.023 | |
| Zn-deficient | Applied | 210 | 31 | 234 | 160 | 48 | 2.8 | 0.5 | 0.5 | 0 | 0.02 |
| Measured | 220.3 | 17.6 | 251.9 | 154.9 | 44.7 | 2.3 | 0.51 | 0.49 | 0.012 | 0.023 | |
| Cu-deficient | Applied | 210 | 31 | 234 | 160 | 48 | 2.8 | 0.5 | 0.5 | 0.05 | 0 |
| Measured | 217.8 | 32.8 | 242.3 | 151.3 | 44.4 | 2.6 | 0.50 | 0.52 | 0.057 | 0.0098 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; He, J.; Suolang, J.; Liu, L.; Hayat, F.; Wang, S.; Umer Javed, H.; Wang, L. Consequences of Micro- and Macronutrient Deficiencies on Physiological and Growth Metrics in Hydroponic ‘Thompson Seedless’ Grapevines. Horticulturae 2025, 11, 543. https://doi.org/10.3390/horticulturae11050543
Zhao M, He J, Suolang J, Liu L, Hayat F, Wang S, Umer Javed H, Wang L. Consequences of Micro- and Macronutrient Deficiencies on Physiological and Growth Metrics in Hydroponic ‘Thompson Seedless’ Grapevines. Horticulturae. 2025; 11(5):543. https://doi.org/10.3390/horticulturae11050543
Chicago/Turabian StyleZhao, Maoxiang, Jianjun He, Jiebu Suolang, Luyao Liu, Faisal Hayat, Shipping Wang, Hafiz Umer Javed, and Lei Wang. 2025. "Consequences of Micro- and Macronutrient Deficiencies on Physiological and Growth Metrics in Hydroponic ‘Thompson Seedless’ Grapevines" Horticulturae 11, no. 5: 543. https://doi.org/10.3390/horticulturae11050543
APA StyleZhao, M., He, J., Suolang, J., Liu, L., Hayat, F., Wang, S., Umer Javed, H., & Wang, L. (2025). Consequences of Micro- and Macronutrient Deficiencies on Physiological and Growth Metrics in Hydroponic ‘Thompson Seedless’ Grapevines. Horticulturae, 11(5), 543. https://doi.org/10.3390/horticulturae11050543






