Metabolomic and Transcriptomic Analyses Reveal the Response Mechanism of Seed Germination in Macadamia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Determination of Physiological Indexes
2.3. Metabolite Profiling and Data Analysis
2.4. Transcriptome Sequencing and Analysis
2.5. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
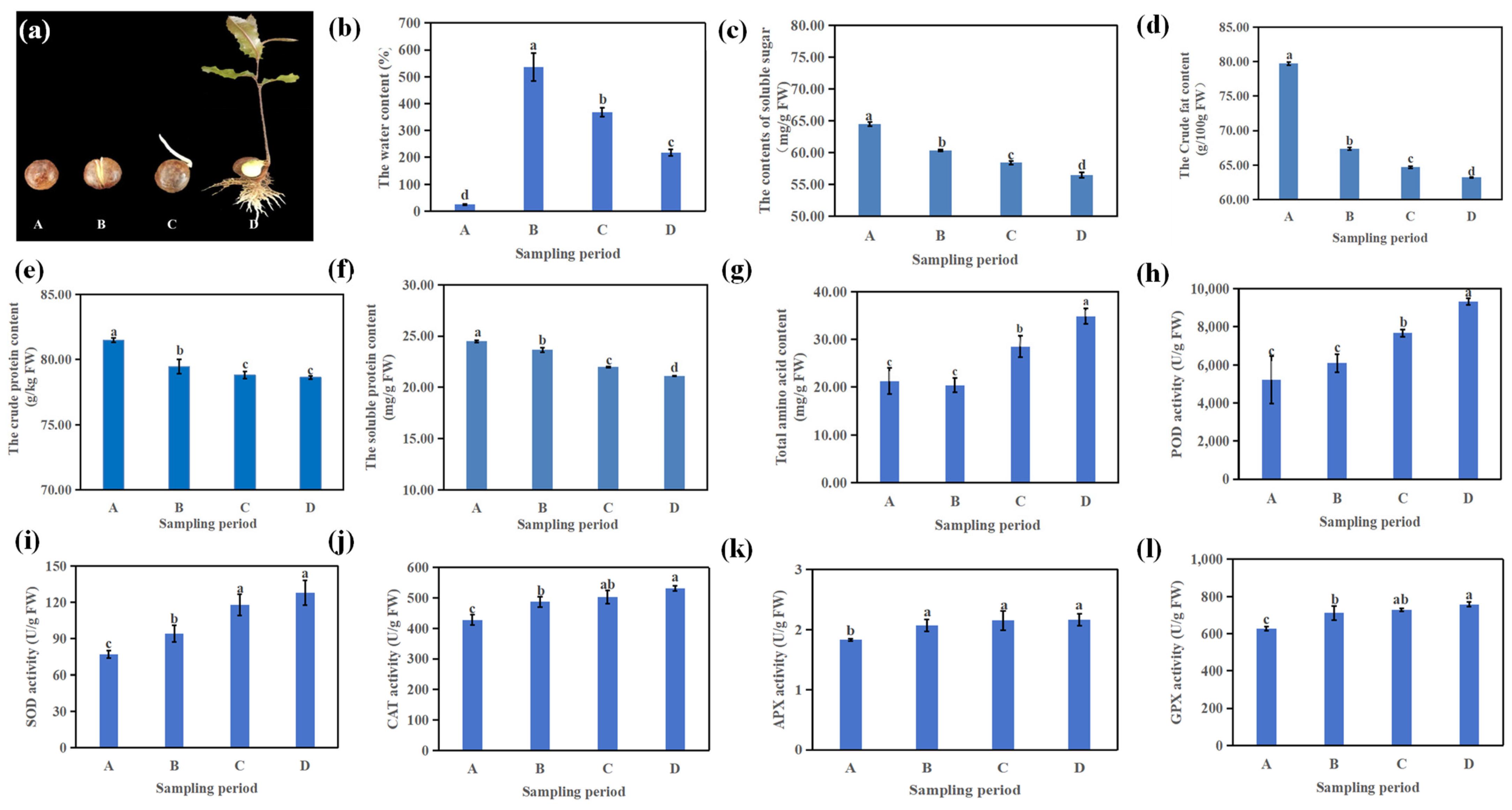
3.1. Physiological Changes During Macadamia Seed Germination
3.2. Metabolomic Analysis During Macadamia Seed Germination
3.3. Transcriptome Analysis During Macadamia Seed Germination
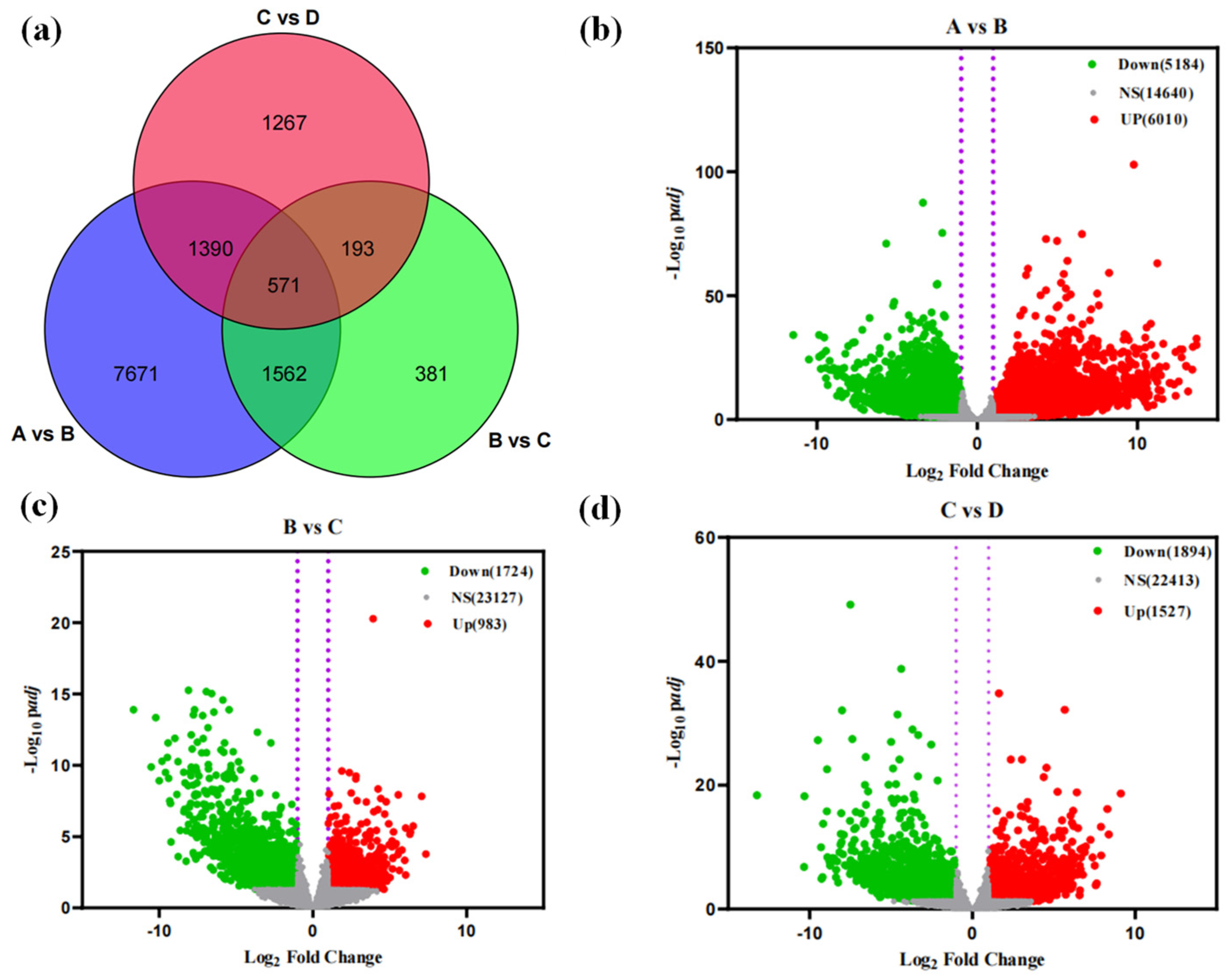
3.3.1. Analysis of Transcriptome Sequencing Results During Seed Germination
3.3.2. Differential Gene Screening and Functional Annotation
3.3.3. GO and KEGG Enrichment Analyses
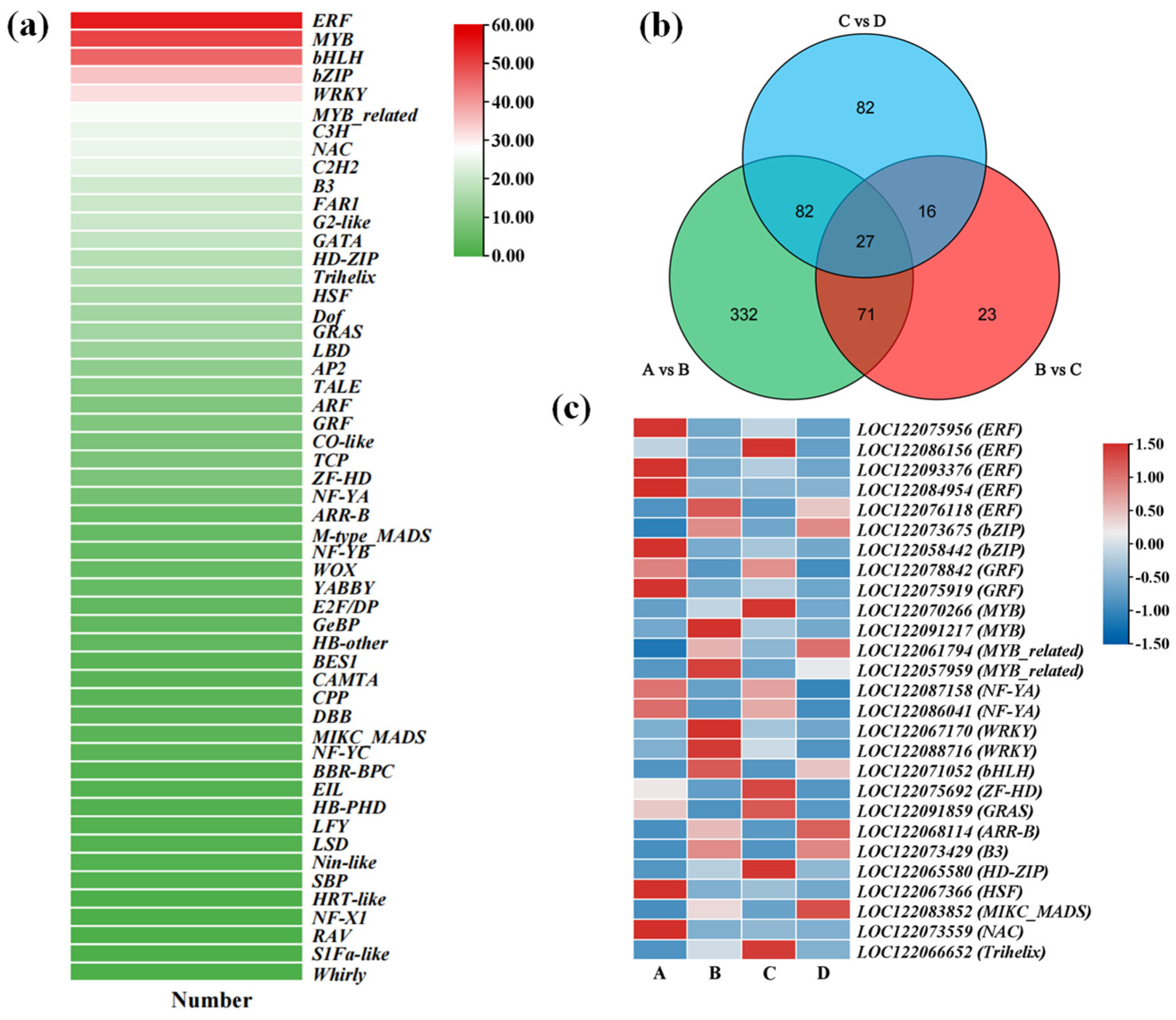
3.3.4. Expression Patterns of Transcription Factors During Macadamia Seed Germination
3.4. Transcriptome and Metabolome Association Analysis
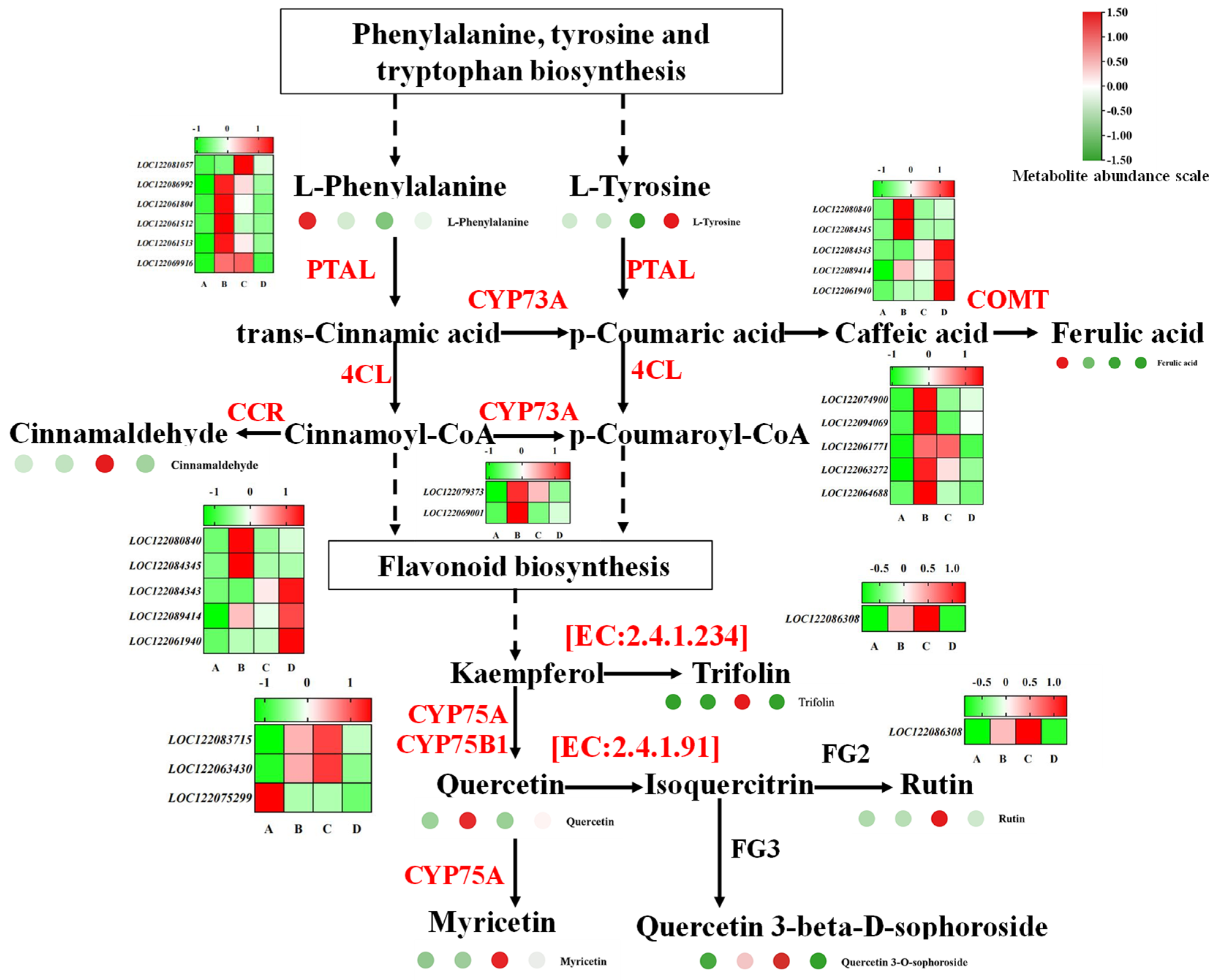
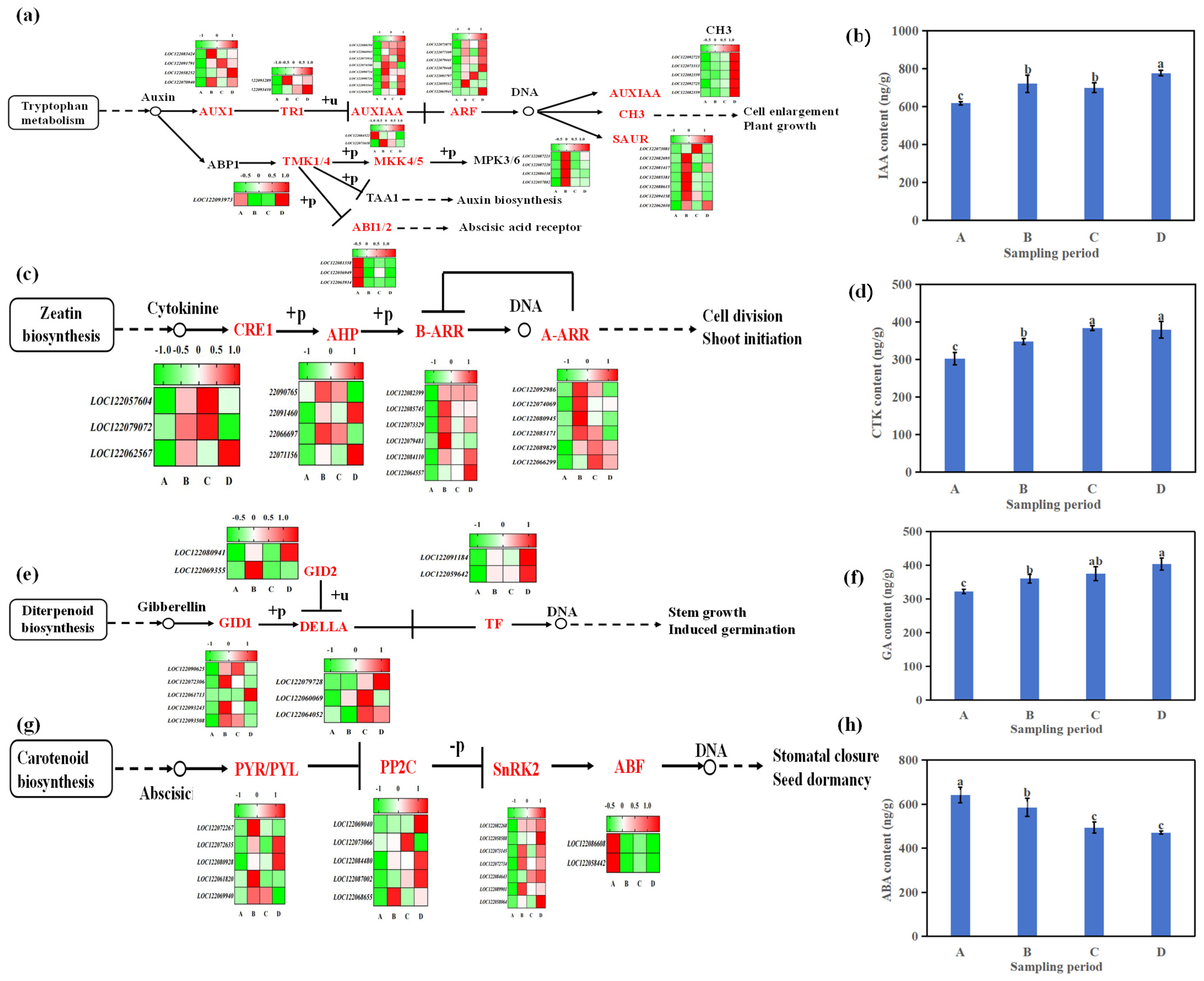
3.5. Phenylpropanoid Biosynthesis and Plant Hormone Signal Transduction Related to Macadamia Seed Germination
4. Discussion
4.1. Physiological Changes Related to Macadamia Seed Germination
4.2. Phenylpropanoid Biosynthesis Related to Macadamia Seed Germination
4.3. Hormonal Levels and Signal Transduction Related to Macadamia Seed Germination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittle, C.A.; Otto, S.P.; Johnston, M.O.; Krochko, J.E. Adaptive epigenetic memory of ancestral temperature regime in Arabidopsis thaliana. Botany 2009, 87, 650–657. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Varaillas, C.; Debaeke, P. Seedling emergence and biomass production of soybean cultivars under wheat-soybean relay cropping. PLoS ONE 2023, 18, e0293671. [Google Scholar] [CrossRef]
- Steinbrecher, T.; Leubner-Metzger, G. The biomechanics of seed germination. J. Exp. Bot. 2017, 68, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying Biochemical and Molecular Mechanisms for Seed Germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef]
- Zhu, M.; Zang, Y.; Zhang, X.; Shang, S.; Xue, S.; Chen, J.; Tang, X. Insights into the regulation of energy metabolism during the seed-to-seedling transition in marine angiosperm Zostera marina L.: Integrated metabolomic and transcriptomic analysis. Front. Plant Sci. 2023, 14, 1130292. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Ponnaiah, M.; Cueff, G.; Rajjou, L.; Prodhomme, D.; Gibon, Y.; Bailly, C.; Corbineau, F.; Meimoun, P.; Bouteau, H. Integrating proteomics and enzymatic profiling to decipher seed metabolism affected by temperature in seed dormancy and germination. Plant Sci. 2018, 269, 118–125. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Ipek, A.; Kaya, M.; Guerbuez, B. Effects of Seed Age and GA (3) Application on Germination of Fenugreek (Trigonella foenum-graecum L.) and Cumin (Cuminum cyminum L.) Seeds. J. Agric. Sci.-Tarim Bilim. Derg. 2008, 14, 57–61. [Google Scholar] [CrossRef]
- Peng, J.; Harberd, N.P. The role of GA-mediated signalling in the control of seed germination. Curr. Opin. Plant Biol. 2002, 5, 376–381. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Li, Z.; Luo, X.; Wang, L.; Shu, K.; Zhang, J. Abscisic Acid Insensitive 5 mediates light–ABA/gibberellin crosstalk networks during seed germination. J. Exp. Bot. 2022, 73, 4674–4682. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Su, L.; Li, D.; Luo, L.; Sun, K.; Yang, M.; Gu, F.W.; Xia, A.Y.; Liu, Y.Z.; Wang, H. Dynamic transcriptome and metabolome analyses of two types of rice during the seed germination and young seedling growth stages. BMC Genom. 2020, 21, 603. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.A.; Suo, J.T.; Liu, S.; Xu, J.D.; Yang, T.G.; Xiang, N.Y.; Wu, Y.; Liu, B.J.; Qin, R.; Liu, H. Combined transcriptomic and metabolomic analysis reveals the potential mechanism of seed germination and young seedling growth in Tamarix hispida. BMC Genom. 2022, 23, 109. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Hong, Y.; Guo, H.; Qin, P.; Huang, A.; Yang, X.; Ren, G. Transcriptomic and metabolomic landscape of quinoa during seed germination. BMC Plant Biol. 2022, 22, 237. [Google Scholar] [CrossRef]
- Liu, R.; Lu, J.; Xing, J.Y.; Du, M.; Wang, M.X.; Zhang, L.; Li, Y.F.; Zhang, C.H.; Wu, Y. Transcriptome and metabolome analyses revealing the potential mechanism of seed germination in Polygonatum cyrtonema. Sci. Rep. 2021, 11, 12161. [Google Scholar] [CrossRef]
- Qu, C.P.; Zhao, H.C.; Chen, J.Y.; Zuo, Z.; Sun, X.; Huang, J.H.; Yang, C.J.; Zhang, X.L.; Zhang, P.; Quan, X.K.; et al. The transcriptional events and their relationship to physiological changes during poplar seed germination and post-germination. BMC Genom. 2019, 20, 801. [Google Scholar] [CrossRef]
- Zhou, W.X.; Duan, Y.Y.; Jiang, X.G.; Tan, X.H.; Li, Q.; Wang, H.; Zhang, Y.J.; Zhang, M.D. Transcriptome and metabolome analyses reveal novel insights into the seed germination of Michelia chapensis, an endangered species in China. Plant Sci. 2023, 328, 111568. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Mapel-Velazco, L.; Martín-del-Campo, S.T.; Chávez-Servia, J.L.; Martínez, A.J.; Verdalet-Guzmán, I. Fatty acids profile of oil from nine varieties of Macadamia nut. Int. J. Food Prop. 2017, 20, 1262–1269. [Google Scholar] [CrossRef]
- Kang, Z.M.; Li, C.X.; Song, X.M.; Cai, Y.B.; Guo, G.Z.; Zeng, H.; Tu, X.H.; Pan, X.J.; Zhang, W.E. Metabolomic and transcriptomic analysis of macadamia seedling responses to drought stress and the role of MiGST in enhancing drought resistance. Ind. Crops Prod. 2025, 229, 120984. [Google Scholar] [CrossRef]
- Wei, B.; Hou, K.; Zhang, H.H.; Wang, X.Y.; Wu, W. Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L. Under drought stress. Ind. Crops Prod. 2020, 151, 112465. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kan, L.; Liao, Q.C.; Chen, Z.P.; Wang, S.Y.; Ma, Y.F.; Su, Z.Y.; Zhang, L. Dynamic Transcriptomic and Metabolomic Analyses of Madhuca pasquieri (Dubard) H. J. Lam During the Post-germination Stages. Front. Plant Sci. 2021, 12, 731203. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. The signalling role of ROS in the regulation of seed germination and dormancy. Biochem. J. 2019, 476, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Aragão, V.P.M.; Navarro, B.V.; Passamani, L.Z.; Macedo, A.F.; Floh, E.I.S.; Silveira, V.; Santa-Catarina, C. Free amino acids, polyamines, soluble sugars and proteins during seed germination and early seedling growth of Cedrela fissilis Vellozo (Meliaceae), an endangered hardwood species from the Atlantic Forest in Brazil. Theor. Exp. Plant Physiol. 2015, 27, 157–169. [Google Scholar] [CrossRef]
- Li, W.J.; Niu, Y.Z.; Zheng, Y.Y.; Wang, Z.F. Advances in the Understanding of Reactive Oxygen Species-Dependent Regulation on Seed Dormancy, Germination, and Deterioration in Crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Stress effects on the reactive oxygen species-dependent regulation of plant growth and development. J. Exp. Bot. 2021, 72, 5795–5806. [Google Scholar] [CrossRef]
- Khanna, K.; Bhardwaj, R.; Alam, P.; Reiter, R.J.; Ahmad, P. Phytomelatonin: A master regulator for plant oxidative stress management. Plant Physiol. Biochem. 2023, 196, 260–269. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef]
- Xu, J.Y.; Yu, Y.L.; Shi, R.Y.; Xie, G.Y.; Zhu, Y.; Wu, G.; Qin, M.J. Organ-Specific Metabolic Shifts of Flavonoids in Scutellaria baicalensis at Different Growth and Development Stages. Molecules 2018, 23, 428. [Google Scholar] [CrossRef]
- Gan, R.Y.; Wang, M.F.; Lui, W.Y.; Wu, K.; Corke, H. Dynamic changes in phytochemical composition and antioxidant capacity in green and black mung bean (Vigna radiata) sprouts. Int. J. Food Sci. Technol. 2016, 51, 2090–2098. [Google Scholar] [CrossRef]
- Peng, W.P.; Wang, N.; Wang, S.M.; Wang, J.Z.; Dong, Y.L. Effects of Exogenous Caffeic Acid, L-Phenylalanine and NaCl Treatments on Main Active Components Content and In Vitro Digestion of Germinated Tartary Buckwheat. Foods 2022, 11, 3682. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Rosado, M.J.; Kim, H.; Timokhin, V.I.; Gutiérrez, A.; Bausch, F.; Rosenau, T.; Potthast, A.; Ralph, J.; Río, J.C.D. Flavonoids naringenin chalcone, naringenin, dihydrotricin, and tricin are lignin monomers in papyrus. Plant Physiol. 2022, 188, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lyu, G.Z.; Li, D.B.; Xiong, H.; Xiao, L.T.; Tong, J.H.; Ning, C.J.; Wang, P.; Li, S.S. Quantitative Proteomic Analyses Identify STO/BBX24 -Related Proteins Induced by UV-B. Int. J. Mol. Sci. 2020, 21, 2496. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.S.; Lv, M.Y.; Wang, J.P.; Shi, X.P.; Liang, X.X.; Liu, Y.W.; Yang, F.; Zhao, H.; Chai, J.F.; Zhou, S. Transcriptome analysis of activated charcoal-induced growth promotion of wheat seedlings in tissue culture. BMC Genet. 2020, 21, 69. [Google Scholar] [CrossRef]
- Liu, X.G.; Lin, C.L.; Ma, X.D.; Tan, Y.; Wang, J.Z.; Zeng, M. Functional Characterization of a Flavonoid Glycosyltransferase in Sweet Orange (Citrus sinensis). Front. Plant Sci. 2018, 9, 166. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; Serna-Guerrero, D.; Serna-Saldívar, S.O.; Jacobo-Velázquez, D.A. Effect of Germination and UV-C Radiation on the Accumulation of Flavonoids and Saponins in Black Bean Seed Coats. Cereal Chem. 2014, 91, 276–279. [Google Scholar] [CrossRef]
- Qi, K.J.; Wu, X.; Li, L.F.; Xie, Z.H.; Gu, C.; Zhang, S.L. Metabolome and transcriptome analyses unravel the inhibition of embryo germination by abscisic acid in pear. Sci. Hortic. 2022, 292, 110652. [Google Scholar] [CrossRef]
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [PubMed]
- Angel, A.; Song, J.; Dean, C.; Howard, M. A Polycomb-based switch underlying quantitative epigenetic memory. Nature 2011, 476, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.K.; Steber, C.M. Gibberellin hormone signal perception: Down-regulating DELLA repressors of plant growth and development. Annu. Plant Rev. 2016, 49, 153–188. [Google Scholar] [CrossRef]
- Sohn, S.I.; Pandian, S.; Kumar, T.S.; Zoclanclounon, Y.A.B.; Muthuramalingam, P.; Shilpha, J.; Satish, L.; Ramesh, M. Seed Dormancy and Pre-Harvest Sprouting in Rice—An Updated Overview. Int. J. Mol. Sci. 2021, 22, 11804. [Google Scholar] [CrossRef] [PubMed]





| Samples | Total Reads | Total Bases | Total Giga Bases | Q20 Bases | Q30 Bases | ≥Q20 | ≥Q30 | GC Content | Overall Mapping Rate |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 45,520,268 | 6,510,660,961 | 6.51G | 6,337,002,374 | 6,009,671,178 | 97.33 | 92.31 | 46.39 | 88.60 |
| A2 | 42,951,348 | 6,175,252,834 | 6.18G | 6,005,514,770 | 5,683,994,100 | 97.25 | 92.04 | 46.62 | 94.50 |
| A3 | 44,325,770 | 6,372,141,271 | 6.37G | 6,193,402,849 | 5,859,890,336 | 97.20 | 91.96 | 46.48 | 93.00 |
| B1 | 42,742,486 | 6,103,108,844 | 6.1G | 5,909,917,116 | 5,565,103,847 | 96.83 | 91.18 | 44.99 | 93.30 |
| B2 | 41,129,718 | 5,906,477,135 | 5.91G | 5,746,213,843 | 5,441,603,324 | 97.29 | 92.13 | 45.23 | 93.90 |
| B3 | 40,059,900 | 5,742,782,850 | 5.74G | 5,583,191,334 | 5,284,845,518 | 97.22 | 92.03 | 45.18 | 93.40 |
| C1 | 44,810,116 | 6,427,791,195 | 6.43G | 6,258,228,027 | 5,934,616,660 | 97.36 | 92.33 | 45.30 | 92.40 |
| C2 | 45,406,086 | 6,458,987,744 | 6.46G | 6,306,975,661 | 6,003,060,958 | 97.65 | 92.94 | 45.70 | 93.80 |
| C3 | 44,976,270 | 6,452,155,543 | 6.45G | 6,264,697,085 | 5,920,047,155 | 97.09 | 91.75 | 45.67 | 94.10 |
| D1 | 40,941,002 | 5,894,673,392 | 5.89G | 5,733,578,960 | 5,427,643,576 | 97.27 | 92.08 | 45.14 | 94.10 |
| D2 | 42,233,226 | 6,080,621,842 | 6.08G | 5,904,789,451 | 5,580,113,613 | 97.11 | 91.77 | 45.23 | 92.50 |
| D3 | 45,575,694 | 6,549,629,900 | 6.55G | 6,385,459,496 | 6,064,971,533 | 97.49 | 92.60 | 45.39 | 93.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Z.; Tao, L.; Guo, G.; Geng, J.; Zeng, H.; Song, X.; Tu, X.; Wang, W. Metabolomic and Transcriptomic Analyses Reveal the Response Mechanism of Seed Germination in Macadamia. Horticulturae 2025, 11, 519. https://doi.org/10.3390/horticulturae11050519
Kang Z, Tao L, Guo G, Geng J, Zeng H, Song X, Tu X, Wang W. Metabolomic and Transcriptomic Analyses Reveal the Response Mechanism of Seed Germination in Macadamia. Horticulturae. 2025; 11(5):519. https://doi.org/10.3390/horticulturae11050519
Chicago/Turabian StyleKang, Zhuanmiao, Liang Tao, Guangzheng Guo, Jianjian Geng, Hui Zeng, Ximei Song, Xinghao Tu, and Wenlin Wang. 2025. "Metabolomic and Transcriptomic Analyses Reveal the Response Mechanism of Seed Germination in Macadamia" Horticulturae 11, no. 5: 519. https://doi.org/10.3390/horticulturae11050519
APA StyleKang, Z., Tao, L., Guo, G., Geng, J., Zeng, H., Song, X., Tu, X., & Wang, W. (2025). Metabolomic and Transcriptomic Analyses Reveal the Response Mechanism of Seed Germination in Macadamia. Horticulturae, 11(5), 519. https://doi.org/10.3390/horticulturae11050519






