Physiological Responses and Gene Expression Profiling of Drought Tolerance in Two Almond Tree Genotypes
Abstract
1. Introduction
2. Materials and Methods
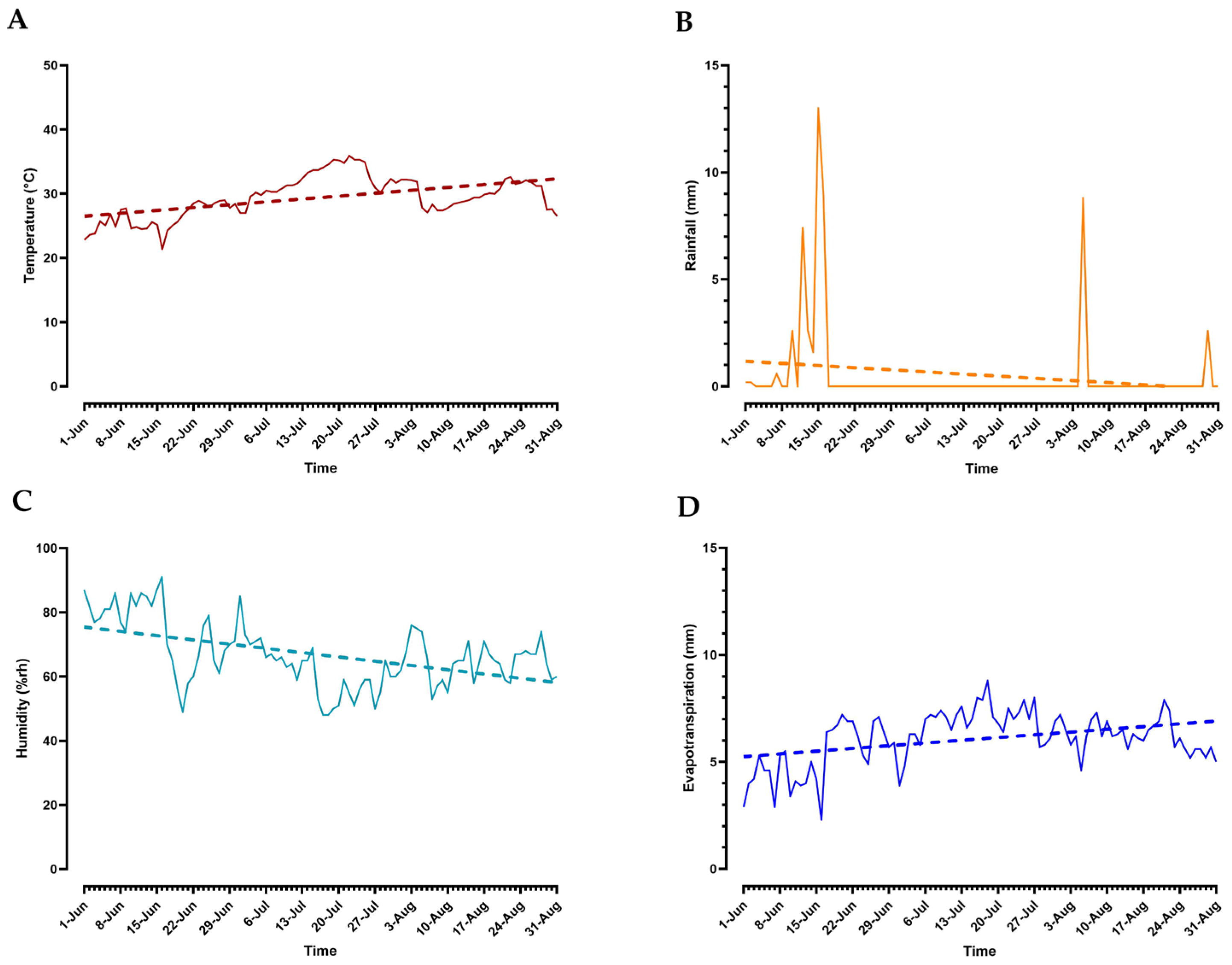
2.1. Site and Experimental Trial
2.2. Biometric Measurements
2.3. Physiological Index Determination
2.3.1. Relative Water Content (RWC)
2.3.2. Biomolecules Determination
2.4. Total RNA Isolation, cDNA Synthesis, and Real-Time PCR Analysis
2.5. Statistical Analysis
3. Results
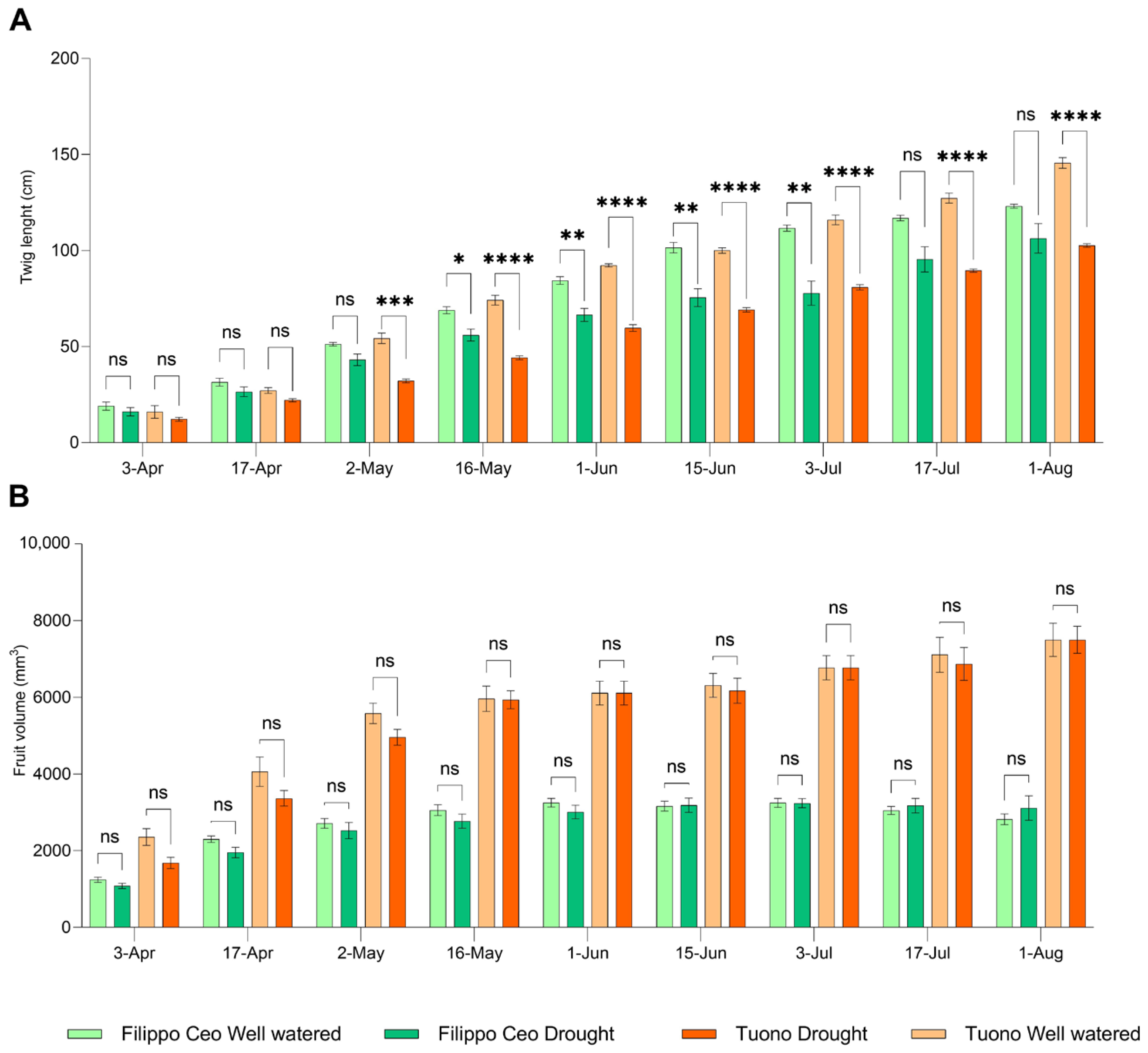
3.1. Biometric Data
3.2. Physiological Parameters
3.3. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Food and Agriculture—Statistical Pocketbook 2018; FAO: Rome, Italy, 2018; p. 254. [Google Scholar]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic Stress Responses in Plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Indoliya, Y.; Agrawal, L.; Awasthi, S.; Deeba, F.; Dwivedi, S.; Chakrabarty, D.; Shirke, P.A.; Pandey, V.; Singh, N.; et al. Genomic and Proteomic Responses to Drought Stress and Biotechnological Interventions for Enhanced Drought Tolerance in Plants. Curr. Plant Biol. 2022, 29, 100239. [Google Scholar] [CrossRef]
- Trono, D.; Pecchioni, N. Candidate Genes Associated with Abiotic Stress Response in Plants as Tools to Engineer Tolerance to Drought, Salinity and Extreme Temperatures in Wheat: An Overview. Plants 2022, 11, 3358. [Google Scholar] [CrossRef]
- Shivaraj, S.M.; Sharma, Y.; Chaudhary, J.; Rajora, N.; Sharma, S.; Thakral, V.; Ram, H.; Sonah, H.; Singla-Pareek, S.L.; Sharma, T.R.; et al. Dynamic Role of Aquaporin Transport System under Drought Stress in Plants. Environ. Exp. Bot. 2021, 184, 104367. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Prgomet, I.; Pascual-Seva, N.; Morais, M.C.; Aires, A.; Barreales, D.; Castro Ribeiro, A.; Silva, A.P.; Barros, A.I.R.N.A.; Gonçalves, B. Physiological and Biochemical Performance of Almond Trees under Deficit Irrigation. Sci. Hortic. 2020, 261, 108990. [Google Scholar] [CrossRef]
- ISTAT. Available online: https://esploradati.istat.it/databrowser/#/it/dw/categories/IT1,Z1000AGR,1.0/AGR_CRP/DCSP_COLTIVAZIONI/IT1,101_1015_DF_DCSP_COLTIVAZIONI_1,1.0 (accessed on 6 March 2025).
- De Pascali, M.; Vergine, M.; Sabella, E.; Aprile, A.; Nutricati, E.; Nicolì, F.; Buja, I.; Negro, C.; Miceli, A.; Rampino, P.; et al. Molecular Effects of Xylella fastidiosa and Drought Combined Stress in Olive Trees. Plants 2019, 8, 437. [Google Scholar] [CrossRef]
- Vergine, M.; Vita, F.; Casati, P.; Passera, A.; Ricciardi, L.; Pavan, S.; Aprile, A.; Sabella, E.; De Bellis, L.; Luvisi, A. Characterization of the Olive Endophytic Community in Genotypes Displaying a Contrasting Response to Xylella fastidiosa. BMC Plant Biol. 2024, 24, 337. [Google Scholar] [CrossRef]
- De Pascali, M.; Greco, D.; Vergine, M.; Carluccio, G.; De Bellis, L.; Luvisi, A. A Physiological and Molecular Focus on the Resistance of “Filippo Ceo” Almond Tree to Xylella fastidiosa. Plants 2024, 13, 576. [Google Scholar] [CrossRef]
- De Giorgio, D.; Polnigano, G.B. Evaluating the Biodiversity of Almond Cultivars From a Germplasm Collection Field in Southern Italy. Sustain. Glob. Farm 2001, 56, 305–311. [Google Scholar]
- Barrs, H.D.; Weatherlea, P.E. Re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The Rainbow Protocol: A Sequential Method for Quantifying Pigments, Sugars, Free Amino Acids, Phenolics, Flavonoids, and MDA from a Small Amount of Sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A Rapid and Effective Method for RNA Extraction from Different Tissues of Grapevine and Other Woody Plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Quackenbush, J. Microarray Data Normalization and Transformation. Nat. Genet. 2002, 32, 496–501. [Google Scholar] [CrossRef]
- Fernandes de Oliveira, A.; Mameli, M.G.; De Pau, L.; Satta, D. Almond Tree Adaptation to Water Stress: Differences in Physiological Performance and Yield Responses among Four Cultivar Grown in Mediterranean Environment. Plants 2023, 12, 1131. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Akkurak, H.; Güldür, M.E.; Dikilitas, M.; Karakas, S.; Alfaifi, M.Y.; Shati, A.A.; Sayyed, R.Z. Molecular Characterization of ‘Candidatus Phytoplasma phoenicium’ Infecting Almond (Prunus dulcis) and Evaluation of Biochemical Defenses Produced in the plants. J. Phytopathol. 2024, 172, e13260. [Google Scholar] [CrossRef]
- Chandrasekaran, U.; Byeon, S.; Kim, K.; Kim, S.H.; Park, C.O.; reum Han, A.; Lee, Y.S.; Kim, H.S. Short-Term Severe Drought Influences Root Volatile Biosynthesis in Eastern White Pine (Pinus strobus L). Front. Plant Sci. 2022, 13, 1030140. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Havaux, M. Carotenoid Oxidation Products as Stress Signals in Plants. Plant J. 2014, 79, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and Its Actions during the Drought Stress in Plants. Physiol. Plant. 2021, 172, 1321–1335. [Google Scholar] [CrossRef]
- Galović, V.; Kebert, M.; Popović, B.M.; Kovačević, B.; Vasić, V.; Joseph, M.P.; Orlović, S.; Szabados, L. Biochemical and Gene Expression Analyses in Different Poplar Clones: The Selection Tools for Afforestation of Halomorphic Environments. Forests 2021, 12, 636. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in Physiological and Biochemical Traits of Oak Seedlings Grown under Drought and Ozone Stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef]
- Shvaleva, A.L.; Costa, F.; Breia, E.; Jouve, L.; Hausman, J.F.; Almeida, M.H.; Maroco, J.P.; Rodrigues, M.L.; Pereira, J.S.; Chaves, M.M. Metabolic Responses to Water Deficit in Two Eucalyptus Globulus Clones with Contrasting Drought Sensitivity. Tree Physiol. 2006, 26, 239–248. [Google Scholar] [CrossRef]
- Taïbi, K.; Del Campo, A.D.; Vilagrosa, A.; Bellés, J.M.; López-Gresa, M.P.; Pla, D.; Calvete, J.J.; López-Nicolás, J.M.; Mulet, J.M. Drought Tolerance in Pinus Halepensis Seed Sources as Identified by Distinctive Physiological and Molecular Markers. Front. Plant Sci. 2017, 8, 1202. [Google Scholar] [CrossRef]
- Huang, J.; Sun, S.J.; Xu, D.Q.; Yang, X.; Bao, Y.M.; Wang, Z.F.; Tang, H.J.; Zhang, H. Increased Tolerance of Rice to Cold, Drought and Oxidative Stresses Mediated by the Overexpression of a Gene That Encodes the Zinc Finger Protein ZFP245. Biochem. Biophys. Res. Commun. 2009, 389, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Mollenhauer, H.H. Rickettsia-like Bacterium Associated with Pierce’s Disease of Grapes. Science 1973, 179, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Szlachtowska, Z.; Rurek, M. Plant dehydrins and dehydrin-like proteins: Characterization and participation in abiotic stress response. Front. Plant Sci. 2023, 14, 1213188. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, A.; Dzinyela, R.; Aghaei-Dargiri, S.; Alhassan, A.R.; Yang, L.; Xu, C. Advanced Study of Drought-Responsive Protein Pathways in Plants. Agronomy 2023, 13, 849. [Google Scholar] [CrossRef]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription Factors and Plants Response to Drought Stress: Current Understanding and Future Directions. Front. Plant Sci. 2016, 7, 1029. [Google Scholar] [CrossRef]
- Hrmova, M.; Hussain, S.S. Plant Transcription Factors Involved in Drought and Associated Stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef]
- Morsy, S.M.; Elbasyoni, I.S.; Abdallah, A.M.; Baenziger, P.S. Imposing Water Deficit on Modern and Wild Wheat Collections to Identify Drought-Resilient Genotypes. J. Agron. Crop Sci. 2022, 208, 427–440. [Google Scholar] [CrossRef]
- Bianchi, V.J.; Rubio, M.; Trainotti, L.; Verde, I.; Bonghi, C.; Martínez-Gómez, P. Prunus Transcription Factors: Breeding Perspectives. Front. Plant Sci. 2015, 6, 443. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to Drought and Salt Stress in Plants: Unraveling the Signaling Networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef]
- Wu, X.; Shiroto, Y.; Kishitani, S.; Ito, Y.; Toriyama, K. Enhanced Heat and Drought Tolerance in Transgenic Rice Seedlings Overexpressing OsWRKY11 under the Control of HSP101 Promoter. Plant Cell Rep. 2009, 28, 21–30. [Google Scholar] [CrossRef]
- Li, S.; Khoso, M.A.; Xu, H.; Zhang, C.; Liu, Z.; Wagan, S.; Dinislam, K.; Liu, L. WRKY Transcription Factors (TFs) as Key Regulators of Plant Resilience to Environmental Stresses: Current Perspective. Agronomy 2024, 14, 2421. [Google Scholar] [CrossRef]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-642-07768-5. [Google Scholar]
- Moulick, D.; Bhutia, K.L.; Sarkar, S.; Roy, A.; Mishra, U.N.; Pramanick, B.; Maitra, S.; Shankar, T.; Hazra, S.; Skalicky, M.; et al. The Intertwining of Zn-Finger Motifs and Abiotic Stress Tolerance in Plants: Current Status and Future Prospects. Front. Plant Sci. 2023, 13, 1083960. [Google Scholar] [CrossRef]
- Fu, Y.; Ma, H.; Chen, S.; Gu, T.; Gong, J. Control of Proline Accumulation under Drought via a Novel Pathway Comprising the Histone Methylase CAU1 and the Transcription Factor ANAC055. J. Exp. Bot. 2018, 69, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, G.; Yu, D. Activated Expression of WRKY57 Confers Drought Tolerance in Arabidopsis. Mol. Plant 2012, 5, 1375–1388. [Google Scholar] [CrossRef]
- Jae, H.Y.; Chan, Y.P.; Jong, C.K.; Won, D.H.; Mi, S.C.; Hyeong, C.P.; Min, C.K.; Byeong, C.M.; Man, S.C.; Yun, H.K.; et al. Direct Interaction of a Divergent CaM Isoform and the Transcription Factor, MYB2, Enhances Salt Tolerance in Arabidopsis. J. Biol. Chem. 2005, 280, 3697–3706. [Google Scholar] [CrossRef]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.; Ali, J.; Li, Z. Overexpression of OsMYB48-1, a Novel MYB-Related Transcription Factor, Enhances Drought and Salinity Tolerance in Rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef]
- Yildirim, A.N.; Şan, B.; Yildirim, F.; Çelik, C.; Bayar, B.; Karakurt, Y. Physiological and Biochemical Responses of Almond Rootstocks to Drought Stress. Turk. J. Agric. For. 2021, 45, 522–532. [Google Scholar] [CrossRef]
- Dogan, M.; Bolat, I.; Turan, M.; Kaya, O. Elucidating Stress Responses in Prunus Rootstocks through Comprehensive Evaluation under Drought, Heat Shock and Combined Stress Conditions. Sci. Hortic. 2025, 339, 113882. [Google Scholar] [CrossRef]
- del Pozo, A.; Brunel-Saldias, N.; Engler, A.; Ortega-Farias, S.; Acevedo-Opazo, C.; Lobos, G.A.; Jara-Rojas, R.; Molina-Montenegro, M.A. Climate Change Impacts and Adaptation Strategies of Agriculture in Mediterranean-Climate Regions (MCRs). Sustainability 2019, 11, 2769. [Google Scholar] [CrossRef]

| Conditions | Leaf RWC (%) | Chla (mg/g FW) | Chlb (mg/g FW) | Chla/b Ratio | Carotenoid (mg/g FW) | Proline (µmol/g FW) | MDA (µmol/g FW) |
|---|---|---|---|---|---|---|---|
| Filippo Ceo Well watered | 95.83 ± 0.70 a | 6.85 ± 0.04 a | 2.23 ± 0.09 a | 3.07 ± 0.11 c | 1.48 ± 0.40 c | 0.56 ± 0.03 b | 9.93 ± 0.43 c |
| Filippo Ceo Drought | 93.75 ± 1.05 b | 5.59 ± 0.12 b | 1.44 ± 0.45 b | 3.89 ± 1.14 bc | 2.62 ± 0.13 b | 1.93 ± 0.35 a | 11.40 ± 0.45 b |
| Tuono Well watered | 92.60 ± 2.28 b | 6.79 ± 0.19 a | 1.54 ± 0.11 b | 4.42 ± 0.35 ab | 3.40 ± 0.05 a | 0.93 ± 0.03 b | 9.92 ± 0.14 d |
| Tuono Drought | 87.14 ± 2.94 c | 5.18 ± 0.09 c | 0.93 ± 0.09 c | 5.57 ± 0.67 a | 3.62 ± 0.16 a | 1.98 ± 0.67 a | 12.44 ± 0.39 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pascali, M.; Vergine, M.; De Bellis, L.; Luvisi, A. Physiological Responses and Gene Expression Profiling of Drought Tolerance in Two Almond Tree Genotypes. Horticulturae 2025, 11, 515. https://doi.org/10.3390/horticulturae11050515
De Pascali M, Vergine M, De Bellis L, Luvisi A. Physiological Responses and Gene Expression Profiling of Drought Tolerance in Two Almond Tree Genotypes. Horticulturae. 2025; 11(5):515. https://doi.org/10.3390/horticulturae11050515
Chicago/Turabian StyleDe Pascali, Mariarosaria, Marzia Vergine, Luigi De Bellis, and Andrea Luvisi. 2025. "Physiological Responses and Gene Expression Profiling of Drought Tolerance in Two Almond Tree Genotypes" Horticulturae 11, no. 5: 515. https://doi.org/10.3390/horticulturae11050515
APA StyleDe Pascali, M., Vergine, M., De Bellis, L., & Luvisi, A. (2025). Physiological Responses and Gene Expression Profiling of Drought Tolerance in Two Almond Tree Genotypes. Horticulturae, 11(5), 515. https://doi.org/10.3390/horticulturae11050515









