Identification and Characterization of the BBX Gene Family in Pomegranate (Punica granatum L.) and Its Potential Role in Anthocyanin Accumulation During Fruit Ripening
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. BBX Family Gene Identification in Pomegranate
2.3. Phylogenetic Analysis and Group Classification
2.4. Gene Structure and Conserved Motif Analysis
2.5. Chromosomal Mapping and Collinearity Analysis of BBX Genes
2.6. Cis-Acting Elements Analysis of PgBBX Genes
2.7. Gene Ontology (GO) Annotation and Enrichment Analysis of PgBBX Proteins
2.8. Expression Pattern Analysis of PgBBX Genes in Pomegranate
2.9. Protein Sequence Alignment and Structure Analysis of PgBBX5 with Its Homologs
2.10. Anthocyanin Content Assay
2.11. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.12. Subcellular Localization
2.13. Statistical Analysis
3. Results
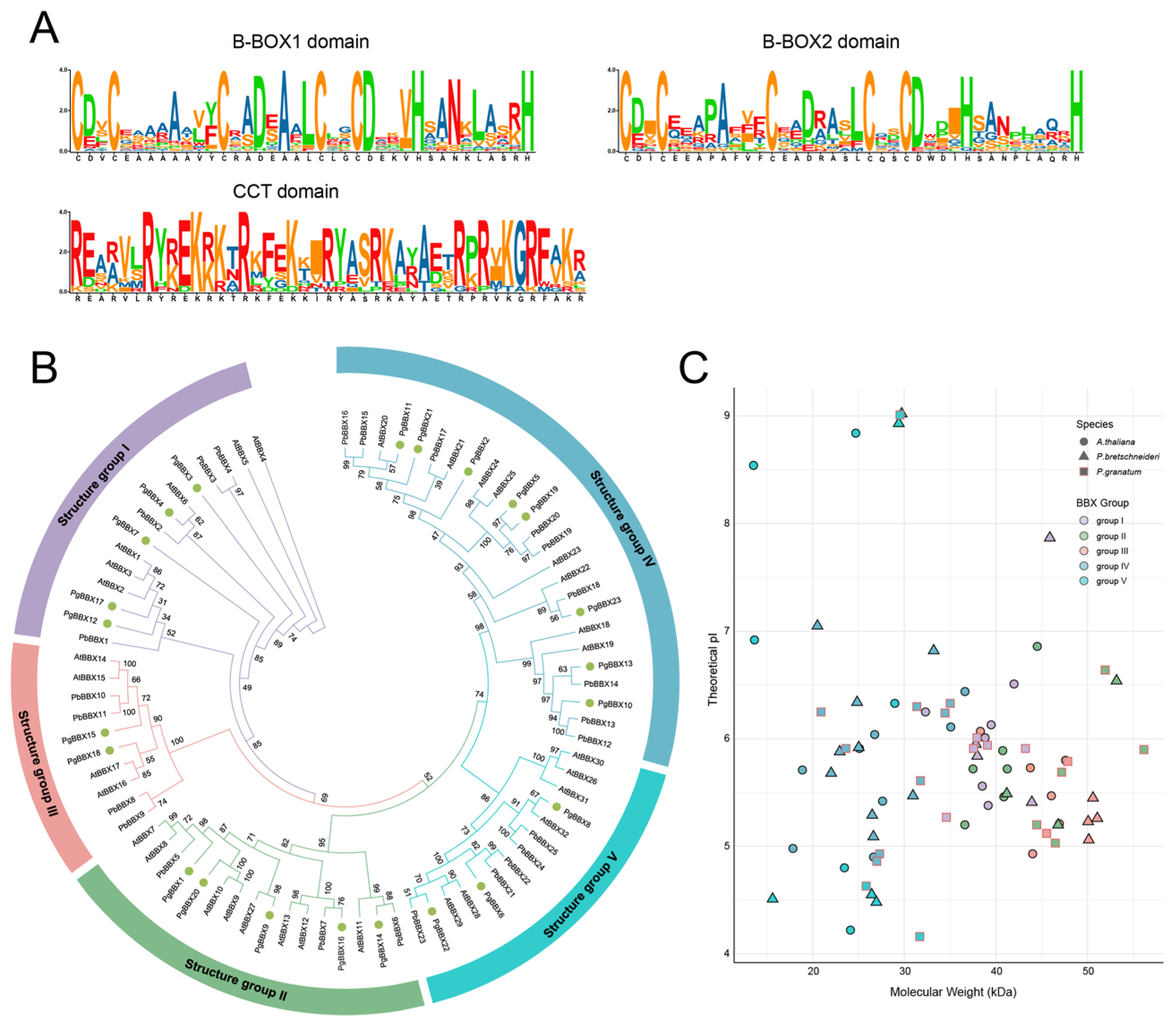
3.1. BBX Gene Family Members Identification and Phylogenetic Relationship Analysis in Pomegranate
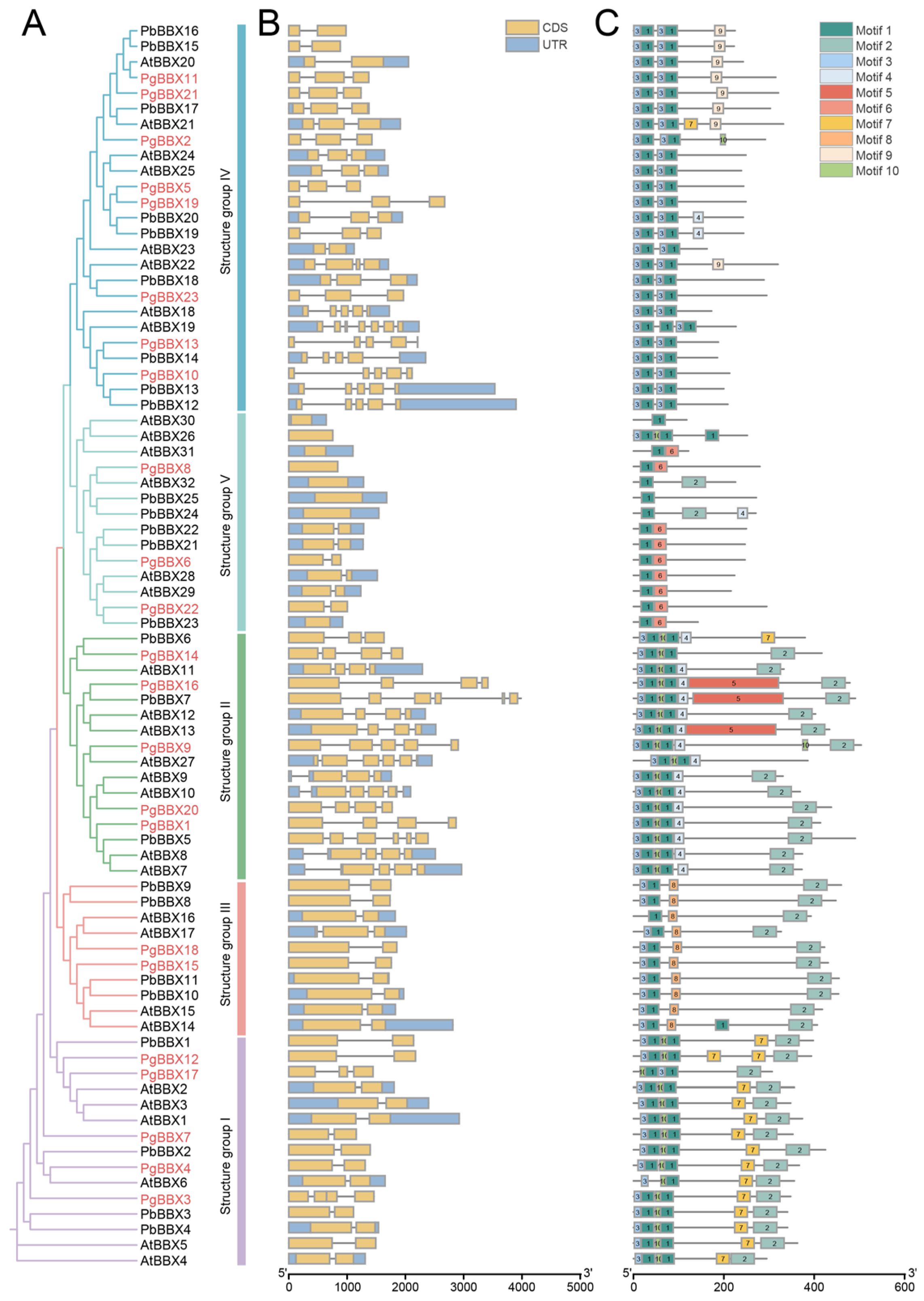
3.2. Phylogenetic Classification, Gene Structure, and Conserved Motif Composition of BBX Genes in Pomegranate, Arabidopsis, and Chinese White Pear
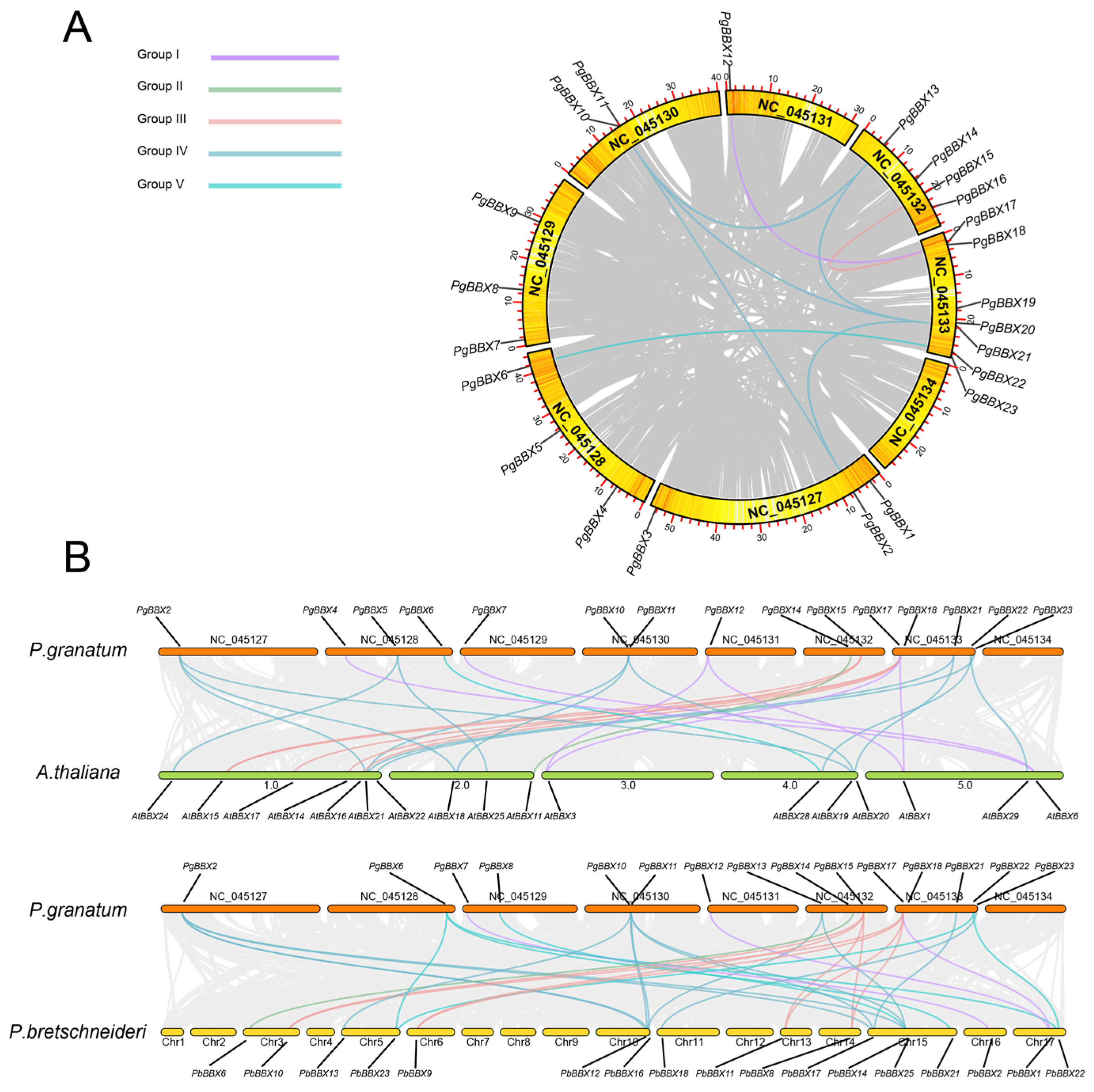
3.3. Collinearity Relationship of BBX Genes
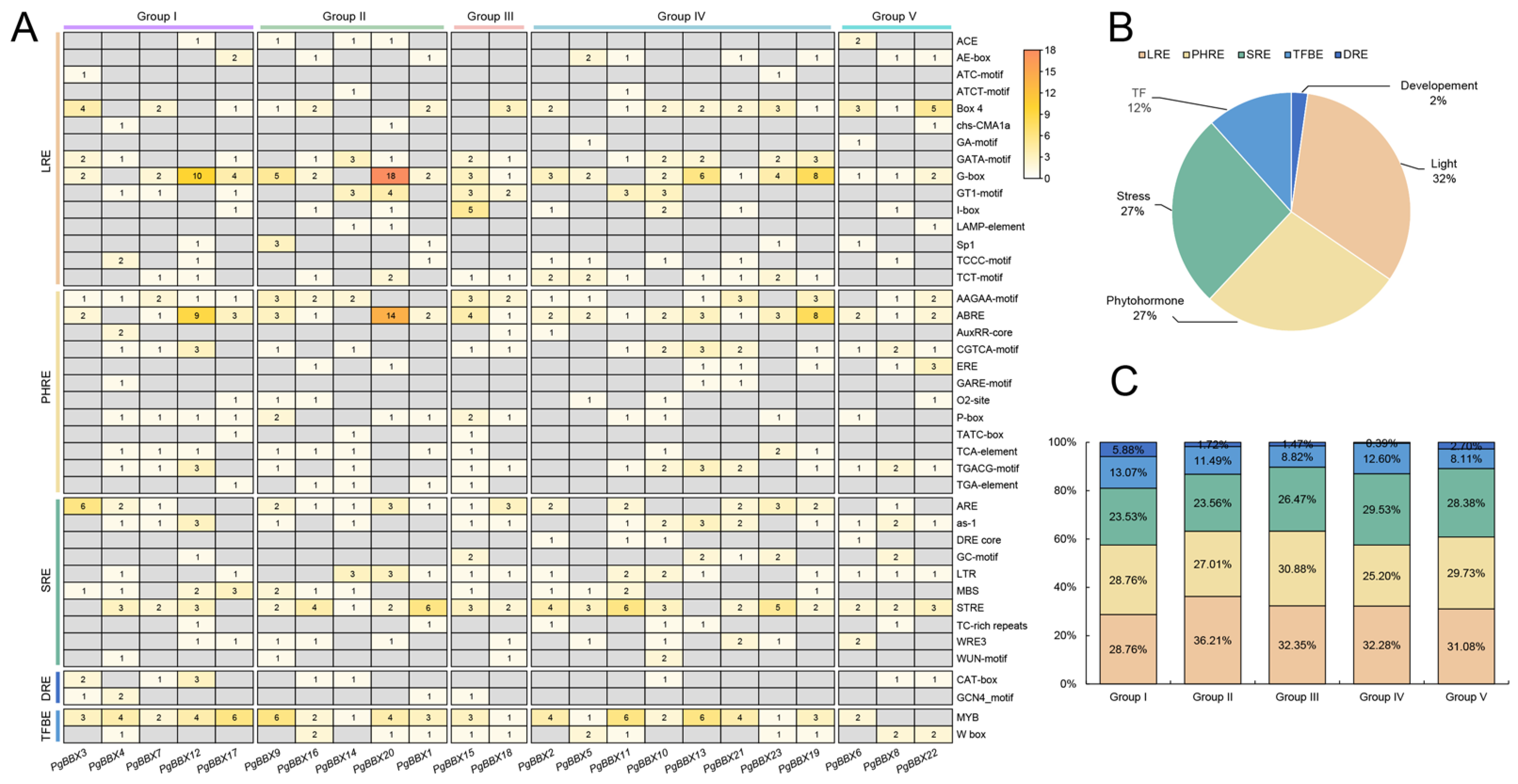
3.4. Cis-Acting Elements in the Promoter Regions of PgBBX Genes
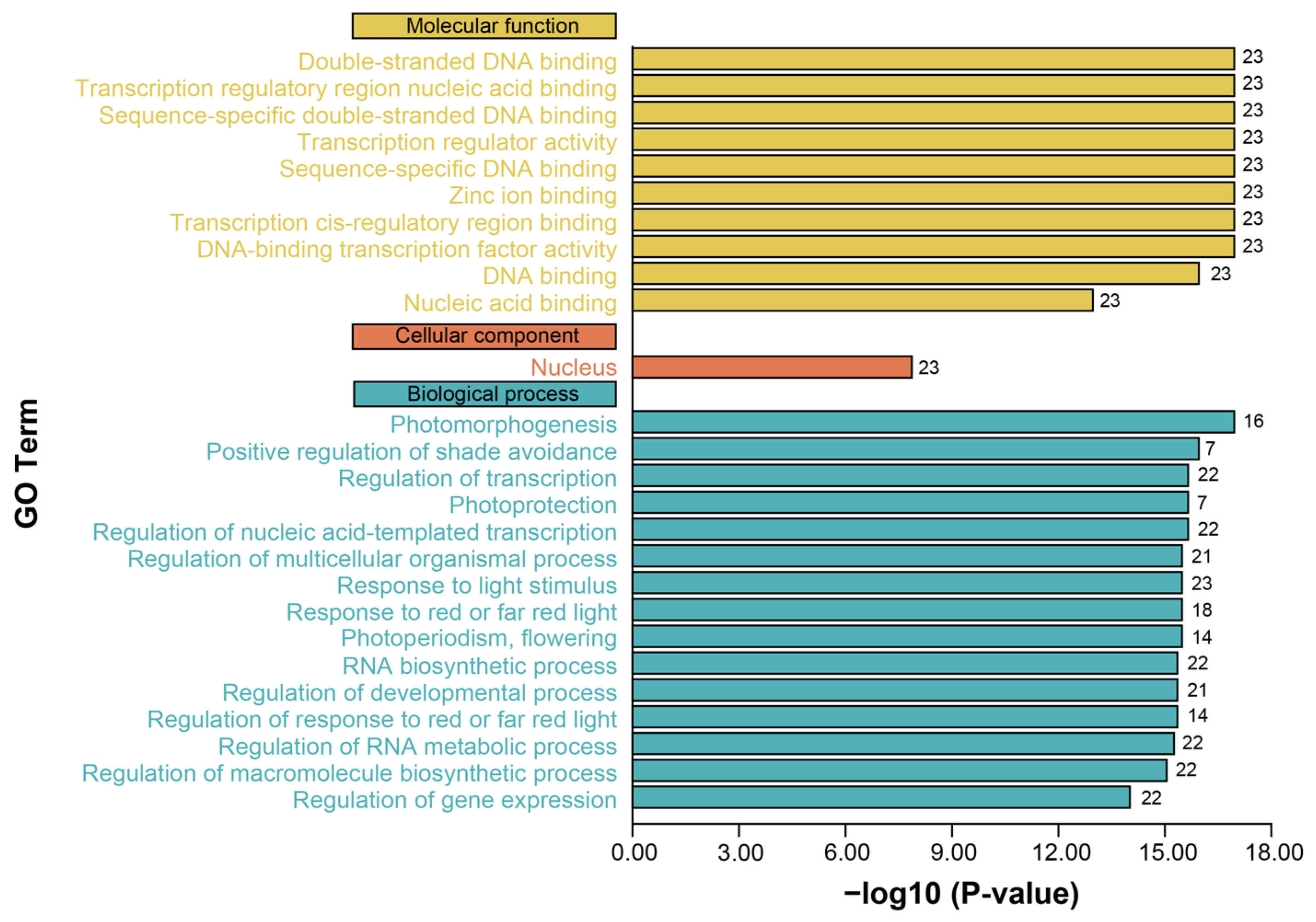
3.5. GO Term Enrichment of PgBBX Proteins
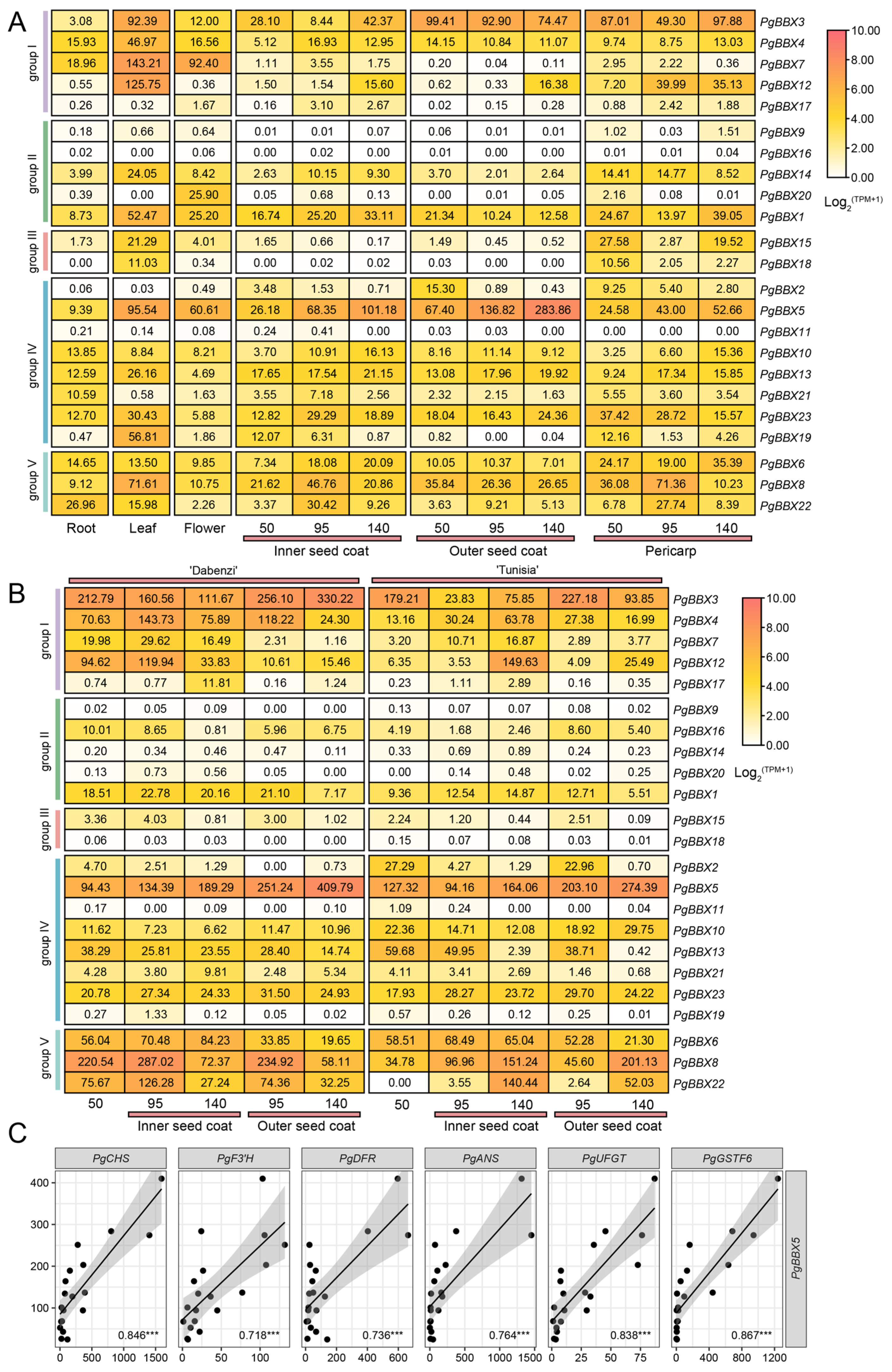
3.6. Expression Characteristic of PgBBX Genes in Diverse Organs and Fruit Tissues
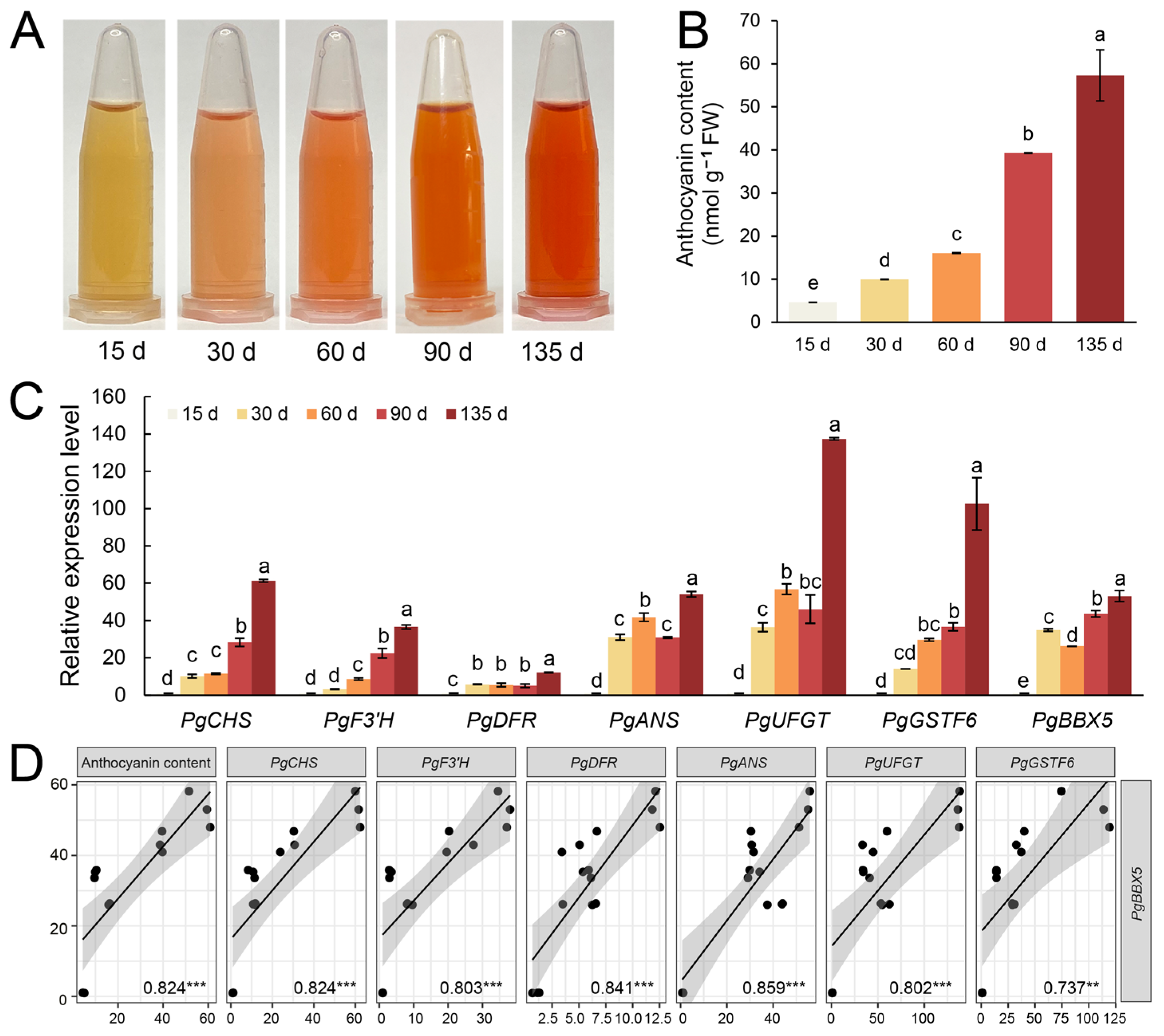
3.7. PgBBX5 May Play a Role in Anthocyanin Accumulation During Fruit Ripening
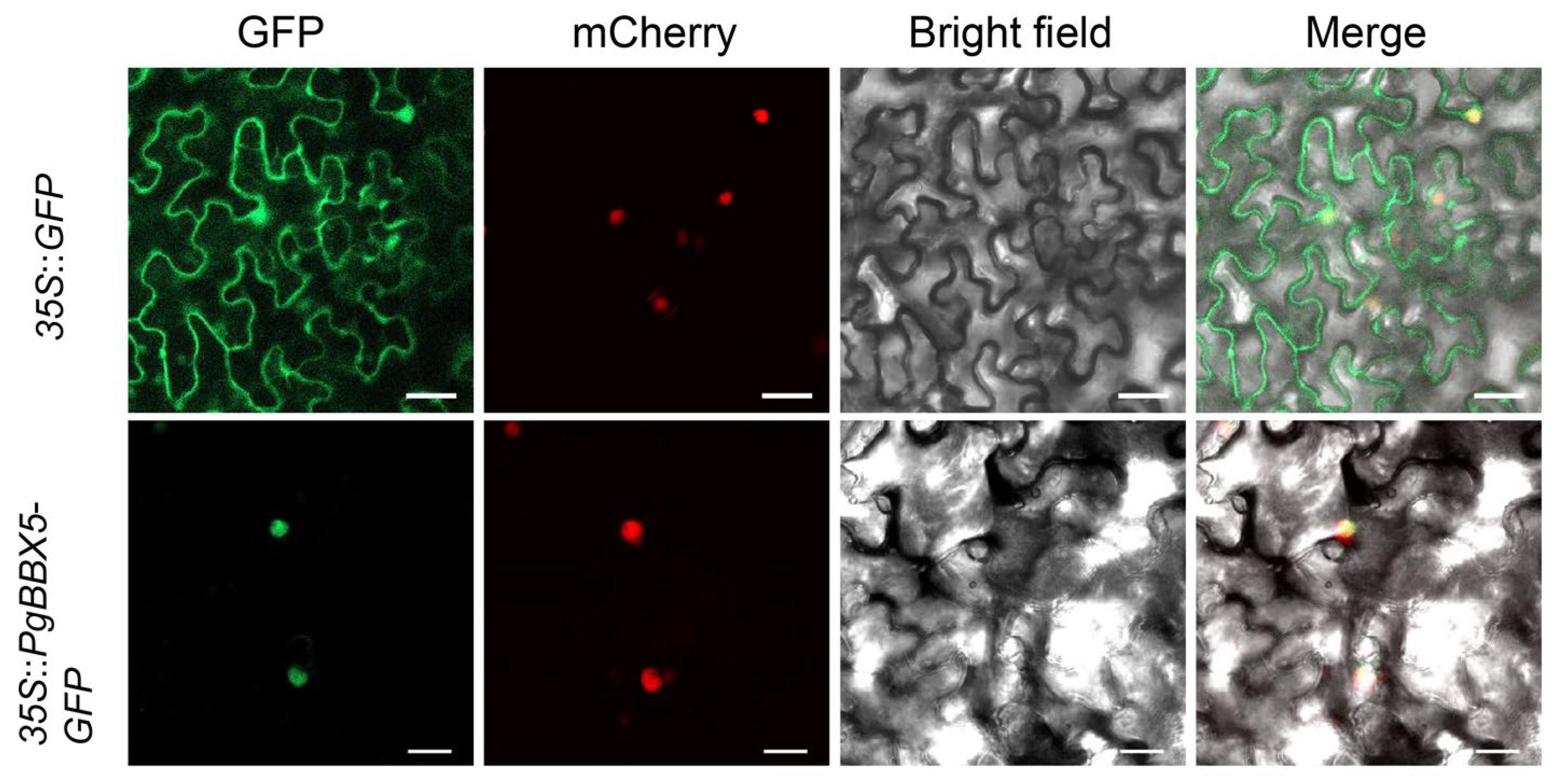
3.8. Subcellular Localization of PgBBX5
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.H. The Arabidopsis B-Box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef]
- Robson, F.; Costa, M.M.R.; Hepworth, S.R.; Vizir, I.; Piñeiro, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001, 28, 619–631. [Google Scholar] [CrossRef]
- Xiong, C.; Luo, D.; Lin, A.; Zhang, C.; Shan, L.; He, P.; Li, B.; Zhang, Q.; Hua, B.; Yuan, Z.; et al. A tomato B-Box protein SlBBX20 modulates carotenoid biosynthesis by directly activating Phytoene Synthase 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2018, 221, 279–294. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, H.; Mi, J.; Qin, X.; Zhao, J.; Zhang, D.; Guo, C.; He, X.; An, W.; Cao, Y.; et al. Genome-Wide identification and analysis of the BBX gene family and its role in carotenoid biosynthesis in wolfberry (Lycium barbarum L.). Int. J. Mol. Sci. 2022, 23, 8440. [Google Scholar] [CrossRef]
- Ma, J.; Dai, J.; Liu, X.; Lin, D. The transcription factor CaBBX20 regulates capsanthin accumulation in pepper (Capsicum annuum L.). Sci. Hortic. 2023, 314, 111907. [Google Scholar] [CrossRef]
- Gao, X.R.; Zhang, H.; Li, X.; Bai, Y.W.; Peng, K.; Wang, Z.; Dai, Z.R.; Bian, X.F.; Zhang, Q.; Jia, L.C.; et al. The B-Box transcription factor IbBBX29 regulates leaf development and flavonoid biosynthesis in sweet potato. Plant Physiol. 2022, 191, 496–514. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, F.; Chu, L.; Lin, H.; Xiao, Y.; Fang, Z.; Wang, X.; Dong, J.; Lyu, X.; Yu, D.; et al. The GmSTF1/2-GmBBX4 negative feedback loop acts downstream of blue-light photoreceptors to regulate isoflavonoid biosynthesis in soybean. Plant Commun. 2024, 5, 100730. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Li, Y.; Wu, Z.; Xie, Y.; Yan, X.; Wang, X.; Miao, Q.; Chen, T.; Rahman, S.; et al. Genome-wide characterization of B-box gene family in Artemisia annua L. and its potential role in the regulation of artemisinin biosynthesis. Ind. Crop Prod. 2023, 199, 116736. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Wu, Z.; Miao, Q.; Hu, X.; Yan, X.; Wen, H.; Zhang, Y.; Fu, X.; Ren, L.; et al. The AaBBX21–AaHY5 module mediates light-regulated artemisinin biosynthesis in Artemisia annua L. J. Integr. Plant Biol. 2024, 66, 1735–1751. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Holm, M.; Deng, X.W. BBX21, an Arabidopsis B-Box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc. Natl. Acad. Sci. USA 2016, 113, 7655–7660. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.S.J.; Maloof, J.N.; Wu, S.H. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol. 2011, 156, 228–239. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Holm, M.; Botto, J.F. Molecular Interactions of BBX24 and BBX25 with HYH, HY5 HOMOLOG, to modulate Arabidopsis seedling development. Plant Signal. Behav. 2013, 8, e25208. [Google Scholar] [CrossRef]
- Plunkett, B.J.; Henry-Kirk, R.; Friend, A.; Diack, R.; Helbig, S.; Mouhu, K.; Tomes, S.; Dare, A.P.; Espley, R.V.; Putterill, J.; et al. Apple B-box factors regulate light-responsive anthocyanin biosynthesis genes. Sci. Rep. 2019, 9, 17762. [Google Scholar] [CrossRef]
- Bai, S.; Saito, T.; Honda, C.; Hatsuyama, Y.; Ito, A.; Moriguchi, T. An apple B-box protein, MdCOL11, is involved in UV-B- and temperature-induced anthocyanin biosynthesis. Planta 2014, 240, 1051–1062. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Yue, X.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; Zhang, J.; Zhang, Z.; et al. The B-box zinc finger protein MdBBX20 integrates anthocyanin accumulation in response to ultraviolet radiation and low temperature. Plant Cell Environ. 2019, 42, 2090–2104. [Google Scholar] [CrossRef]
- Fang, H.; Dong, Y.; Yue, X.; Chen, X.; He, N.; Hu, J.; Jiang, S.; Xu, H.; Wang, Y.; Su, M.; et al. MdCOL4 interaction mediates crosstalk between UV-B and high temperature to control Fruit coloration in apple. Plant Cell Physiol. 2019, 60, 1055–1066. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Z.Z.; Qu, D.; Wang, B.C.; Hao, N.N.; Yang, Y.Z.; Yang, H.J.; Zhao, Z.Y. MdBBX21, a B-Box protein, positively regulates light-induced anthocyanin accumulation in apple peel. Front. Plant Sci. 2021, 12, 774446. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Bi, S.Q.; You, C.X.; Hao, Y.J. MdBBX22 regulates UV-B-induced anthocyanin biosynthesis through regulating the function of MdHY5 and is targeted by MdBT2 for 26S proteasome-mediated degradation. Plant Biotechnol. J. 2019, 17, 2231–2233. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Espley, R.V.; Kui, L.W.; Bi, S.Q.; You, C.X.; Hao, Y.J. An apple B-Box protein MdBBX37 modulates anthocyanin biosynthesis and hypocotyl elongation synergistically with MdMYBs and MdHY5. Plant Cell Physiol. 2019, 61, 130–143. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Yin, L.; Ni, J.; Yang, Q.; Yan, X.; Yang, F.; Guo, X.; Li, H.; Teng, Y. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019, 100, 1208–1223. [Google Scholar] [CrossRef]
- Ou, C.; Zhang, X.; Wang, F.; Zhang, L.; Zhang, Y.; Fang, M.; Wang, J.; Wang, J.; Jiang, S.; Zhang, Z. A 14 nucleotide deletion mutation in the coding region of the PpBBX24 gene is associated with the red skin of "Zaosu Red" pear (Pyrus pyrifolia White Pear Group): A deletion in the PpBBX24 gene is associated with the red skin of pear. Hortic. Res. 2020, 7, 39. [Google Scholar] [CrossRef]
- Yang, G.; Sun, M.; Brewer, L.; Tang, Z.; Nieuwenhuizen, N.; Cooney, J.; Xu, S.; Sheng, J.; Andre, C.; Xue, C.; et al. Allelic variation of BBX24 is a dominant determinant controlling red coloration and dwarfism in pear. Plant Biotech. J. 2024, 22, 1468–1490. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Sun, Y.; Zhang, X.; Du, B.; Turupu, M.; Yao, Q.; Gai, S.; Tong, S.; Huang, J.; et al. Two B-Box proteins, PavBBX6/9, positively regulate light-induced anthocyanin accumulation in sweet cherry. Plant Physiol. 2023, 192, 2030–2048. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.; Wang, Y.; Jiang, L.; Yue, M.; Tang, L.; Jin, M.; Zhang, Y.; Lin, Y.; Tang, H. B-Box transcription factor FaBBX22 promotes light-induced anthocyanin accumulation in strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2022, 23, 7757. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yue, M.; Jiang, L.; Zhang, N.; Luo, Y.; Chen, Q.; Zhang, Y.; Wang, Y.; Li, M.; et al. FaMYB5 interacts with FaBBX24 to regulate anthocyanin and proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa). Int. J. Mol. Sci. 2023, 24, 12185. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, G.; Chen, J.; Ying, Y.; Yao, L.; Li, X.; Teixeira, J.A.; Yu, Z. Role of Rubus chingii BBX gene family in anthocyanin accumulation during fruit ripening. Front. Plant Sci. 2024, 15, 1427359. [Google Scholar] [CrossRef]
- Liu, W.; Mu, H.; Yuan, L.; Li, Y.; Li, Y.; Li, S.; Ren, C.; Duan, W.; Fan, P.; Dai, Z.; et al. VvBBX44 and VvMYBA1 form a regulatory feedback loop to balance anthocyanin biosynthesis in grape. Hortic. Res. 2023, 10, uhad176. [Google Scholar] [CrossRef]
- Li, C.; Pei, J.; Yan, X.; Cui, X.; Tsuruta, M.; Liu, Y.; Lian, C. A poplar B-box protein PtrBBX23 modulates the accumulation of anthocyanins and proanthocyanidins in response to high light. Plant Cell Environ. 2021, 44, 3015–3033. [Google Scholar] [CrossRef]
- Luo, D.; Xiong, C.; Lin, A.; Zhang, C.; Sun, W.; Zhang, J.; Yang, C.; Lu, Y.; Li, H.; Ye, Z.; et al. SlBBX20 interacts with the COP9 signalosome subunit SlCSN5-2 to regulate anthocyanin biosynthesis by activating SlDFR expression in tomato. Hortic. Res. 2021, 8, 163. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Tian, S.; Hao, W.; Du, L. Two B-Box proteins, MaBBX20 and MaBBX51, coordinate light-induced anthocyanin biosynthesis in grape hyacinth. Int. J. Mol. Sci. 2022, 23, 5678. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, S.; Lee, J.Y.; Ha, S.H.; Lee, J.G.; Lim, S.H. A rice B-Box protein, OsBBX14, finely regulates anthocyanin biosynthesis in rice. Int. J. Mol. Sci. 2018, 19, 2190. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, L.; Wang, L.; Zhao, J.; Yang, C.; Li, H.; Huang, J.; Shi, T.; Zhu, L.; Damaris, R.N.; et al. The complex FtBBX22 and FtHY5 positively regulates light-induced anthocyanin accumulation by activating FtMYB42 in Tartary buckwheat sprouts. Int. J. Mol. Sci. 2024, 25, 8376. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, Y.; Zhu, Z.; Ni, N.; Sun, S.; Nie, M.; Du, W.; Irfan, M.; Chen, L.; Zhang, L. Transcription factors LvBBX24 and LvbZIP44 coordinated anthocyanin accumulation in response to light in lily petals. Hortic. Res. 2024, 11, uhae211. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.; Qu, D.; Zhu, Z.; Yang, Y.; Zhao, Z. The MdBBX22–miR858–MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel. Plant Biotechnol. J. 2022, 20, 1683–1700. [Google Scholar] [CrossRef]
- Song, H.; Ding, G.; Zhao, C.; Li, Y. Genome-wide identification of B-Box gene family and expression analysis suggest its roles in responses to cercospora leaf spot in sugar beet (Beta vulgaris L.). Genes 2023, 14, 1248. [Google Scholar] [CrossRef]
- Yu, L.; Wang, D.; Huang, R.; Cao, F.; Guo, C.; Zhang, J. Genome-wide identification, characterization and expression profile analysis of BBX gene family in Chinese chestnut (Castanea mollissima). Plant Biotechnol. Rep. 2023, 18, 129–142. [Google Scholar] [CrossRef]
- Ouyang, Y.; Pan, X.; Wei, Y.; Wang, J.; Xu, X.; He, Y.; Zhang, X.; Li, Z.; Zhang, H. Genome-wide identification and characterization of the BBX gene family in pineapple reveals that candidate genes are involved in floral induction and flowering. Genomics 2022, 114, 110397. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Feng, X.; Ma, Y.; Huang, Y.; Wang, Y.; Ding, J.; Chen, H.; Wu, H. Genome-wide identification, phylogenetic and expression analysis of the B-BOX gene family in the woodland strawberry (Fragaria vesca). Horticulturae 2023, 9, 842. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Chen, Y.; Dai, Y.; Yuan, Q.; Shan, Q.; Pan, L.; Dai, L.; Zou, X.; Liu, F.; et al. Genome-wide characterization and anthocyanin-related expression analysis of the B-BOX gene family in Capsicum annuum L. Front. Genet. 2022, 13, 847328. [Google Scholar] [CrossRef]
- Liu, X.; Sun, W.; Ma, B.; Song, Y.; Guo, Q.; Zhou, L.; Wu, K.; Zhang, X.; Zhang, C. Genome-wide analysis of blueberry B-box family genes and identification of members activated by abiotic stress. BMC Genom. 2023, 24, 584. [Google Scholar] [CrossRef]
- Wei, H.; Wang, P.; Chen, J.; Li, C.; Wang, Y.; Yuan, Y.; Fang, J.; Leng, X. Genome-wide identification and analysis of B-BOX gene family in grapevine reveal its potential functions in berry development. BMC Plant Biol. 2020, 20, 72. [Google Scholar] [CrossRef]
- Cao, Y.; Han, Y.; Meng, D.; Li, D.; Jiao, C.; Jin, Q.; Lin, Y.; Cai, Y. B-BOX genes: Genome-wide identification, evolution and their contribution to pollen growth in pear (Pyrus bretschneideri Rehd.). BMC Plant Biol. 2017, 17, 156. [Google Scholar] [CrossRef]
- Obel, H.O.; Cheng, C.; Li, Y.; Tian, Z.; Njogu, M.K.; Li, J.; Lou, Q.; Yu, X.; Yang, Z.; Ogweno, J.O.; et al. Genome-wide identification of the B-Box gene family and expression analysis suggests their potential role in photoperiod-mediated β-carotene accumulation in the endocarp of cucumber (Cucumis sativus L.) fruit. Genesis 2022, 13, 658. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.; Huang, B.; Huang, Z.; Zhang, Z. The BBX gene family in Moso bamboo (Phyllostachys edulis): Identification, characterization and expression profiles. BMC Genom. 2021, 22, 535. [Google Scholar] [CrossRef]
- Li, Y.; Tong, Y.; Ye, J.; Zhang, C.; Li, B.; Hu, S.; Xue, X.; Tian, Q.; Wang, Y.; Li, L.; et al. Genome-wide characterization of B-Box gene family in Salvia miltiorrhiza. Int. J. Mol. Sci. 2023, 24, 2146. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Huang, J.; Shi, T.; Meng, Z.; Chen, Q.; Deng, J. Genome-wide analysis of BBX gene family in Tartary buckwheat (Fagopyrum tataricum). PeerJ 2021, 9, e1193. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Weng, X.; Wang, L.; Xie, W. The rice B-Box zinc finger gene family: Genomic identification, characterization, expression profiling and diurnal analysis. PLoS ONE 2012, 7, e48242. [Google Scholar] [CrossRef]
- Bu, X.; Wang, X.; Yan, J.; Zhang, Y.; Zhou, S.; Sun, X.; Yang, Y.; Ahammed, G.J.; Liu, Y.; Qi, M.; et al. Genome-wide characterization of B-Box gene family and its roles in responses to light quality and cold stress in tomato. Front. Plant Sci. 2021, 12, 698525. [Google Scholar] [CrossRef]
- Hou, W.; Ren, L.; Zhang, Y.; Sun, H.; Shi, T.; Gu, Y.; Wang, A.; Ma, D.; Li, Z.; Zhang, L. characterization of BBX family genes and their expression profiles under various stresses in the sweet potato wild ancestor Ipomoea trifida. Sci. Hortic. 2021, 288, 110374. [Google Scholar] [CrossRef]
- Xu, X.; Li, W.; Yang, S.; Zhu, X.; Sun, H.; Li, F.; Lu, X.; Cui, J. Identification, evolution, expression and protein interaction analysis of genes encoding B-box zinc-finger proteins in maize. J. Integr. Agr. 2023, 22, 371–388. [Google Scholar] [CrossRef]
- Liu, S.; Lyu, S.; Yang, Z.; Xu, G.; Zhang, Y.; Liu, Y.; Jin, J.; Deng, S. Genome-wide characterization of tobacco B-BOX gene family identified two close members play contrast roles under cold stress. Environ. Exp. Bot. 2023, 216, 105533. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Li, X.; Wang, G.; Zhou, Q.; Chen, Q.; Li, J.; Wang, X.; Tang, H. An evolutionary analysis of B-Box transcription factors in strawberry reveals the role of FaBBx28c1 in the regulation of flowering time. Int. J. Mol. Sci. 2021, 22, 11766. [Google Scholar] [CrossRef]
- Sui, C.; Cheng, S.; Wang, D.; Lv, L.; Meng, H.; Du, M.; Li, J.; Su, P.; Guo, S. Systematic identification and characterization of the soybean (Glycine max) B-Box transcription factor family. Biotechnol. Biotec. Eq. 2023, 37, 104–116. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Dai, Y.; Chen, X.; Wang, X. Genome-wide identification and expression analysis of the B-box gene family in the apple (Malus domestica Borkh.) genome. Mol. Genet. Genom. 2017, 293, 303–315. [Google Scholar] [CrossRef]
- Tang, H.; Yuan, C.; Shi, H.; Liu, F.; Shan, S.; Wang, Z.; Sun, Q.; Sun, J. Genome-wide identification of peanut B-BOXs and functional characterization of AhBBX6 in salt and drought stresses. Plants 2024, 13, 955. [Google Scholar] [CrossRef]
- Faria, G.M.L.; Silva, E.K. Pulsed electric field, ultrasound and microwave heating based extraction techniques for valorization of pomegranate peel byproducts: A review. J. Environ. Chem. Eng. 2024, 12, 113078. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, Y.; Ke, D.; Teng, Y.; Yuan, Z. Comparative transcriptomic and metabolomic profiles reveal fruit peel color variation in two red pomegranate cultivars. Plant Mol. Biol. 2024, 114, 51. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Z.; Feng, L.; Fang, Y. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015, 128, 687–696. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Wang, C.; Feng, L.; Yin, Y.; Li, J. PgMYB1 positively regulates anthocyanin accumulation by activating PgGSTF6 in pomegranate. Int. J. Mol. Sci. 2023, 24, 6366. [Google Scholar] [CrossRef]
- Arlotta, C.; Puglia, G.D.; Genovese, C.; Toscano, V.; Karlova, R.; Beekwilder, J.; De, C.H.; Raccuia, S.A. MYB5-like and bHLH influence flavonoid composition in pomegranate. Plant Sci. 2020, 298, 110563. [Google Scholar] [CrossRef]
- Ben-Simhon, Z.; Judeinstein, S.; Nadler-Hassar, T.; Trainin, T.; Bar-Ya’akov, I.; Borochov-Neori, H.; Holland, D. A pomegranate (Punica granatum L.) WD40-repeat gene is a functional homologue of Arabidopsis TTG1 and is involved in the regulation of anthocyanin biosynthesis during pomegranate fruit development. Planta 2011, 234, 865–881. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Li, B.; Zhao, X.; Shen, Y.; Yuan, Z. genome-wide identification and characterization of BZIP gene family and cloning of candidate genes for anthocyanin biosynthesis in pomegranate (Punica granatum). BMC Plant Biol. 2022, 22, 170. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.X.; Wu, Z.K.; Yao, W.; Zhao, P.; Cao, D.; Yu, H.Y.; Li, K.D.; Poudel, K.; Zhao, D.G.; et al. The pomegranate (Punica granatum L.) draft genome dissects genetic divergence between soft- and hard-seeded cultivars. Plant Biotechnol. J. 2020, 18, 955–968. [Google Scholar] [CrossRef]
- Hu, J.; Huang, B.; Yin, H.; Qi, K.; Jia, Y.; Xie, Z.; Gao, Y.; Li, H.; Li, Q.; Wang, Z.; et al. PearMODB: A multiomics database for pear (Pyrus) genomics, genetics and breeding study. Database 2023, 2023, baad050. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A "one for all, all for one" bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. EggNOG-mapper V2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Qin, G.H.; Xu, C.Y.; Ming, R.; Tang, H.B.; Guyot, R.; Kramer, E.M.; Hu, Y.D.; Yi, X.K.; Qi, Y.J.; Xu, X.Y.; et al. The pomegranate (Punica granatum L.) genome and the genomics of punicalagin biosynthesis. Plant J. 2017, 91, 1108–1128. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Yu, Q.; Cao, Z.; Yang, Y.; Jia, B.; Su, Y.; Li, G.; Qin, G. Identification of sugar transporter (SWEET) genes involved in pomegranate seed coat sugar accumulation. 3 Biotech 2022, 12, 181. [Google Scholar] [CrossRef]
- Liu, L.B.; Zheng, J. Identification and expression analysis of the sucrose synthase gene family in pomegranate (Punica granatum L.). PeerJ 2022, 10, e12814. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, L.; Tao, H.; An, Y.; Wang, L. Transcriptomic profiling of apple calli with a focus on the key genes for ALA-induced anthocyanin accumulation. Front. Plant Sci. 2021, 12, 640606. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fu, Z.; Shang, H.; Jiang, H.; Gao, J.; Dong, X.; Wang, H.; Li, Y.; Wang, L.; Zhang, J.; Shu, Q.; et al. Systematic identification of the light-quality responding anthocyanin synthesis-related transcripts in Petunia petals. Hortic. Plant J. 2020, 6, 428–438. [Google Scholar] [CrossRef]
- Crocco, C.D.; Botto, J.F. BBX proteins in green plants: Insights into their evolution, structure, feature and functional diversification. Gene 2013, 531, 44–52. [Google Scholar] [CrossRef]
- Moore, R.C.; Purugganan, M.D. The Early Stages of Duplicate Gene Evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef]
- Yadav, A.; Ravindran, N.; Singh, D.; Rahul, P.V.; Datta, S. Role of Arabidopsis BBX proteins in light signaling. J. Plant Biochem. Biotechnol. 2020, 29, 623–635. [Google Scholar] [CrossRef]
- Fu, J.L.; Liao, L.; Jin, J.; Lu, Z.; Sun, J.; Song, L.; Huang, Y.; Liu, S.; Huang, D.; Xu, Y.; et al. A transcriptional cascade involving BBX22 and HY5 finely regulates both plant height and fruit pigmentation in citrus. J. Integr. Plant Biol. 2024, 66, 1752–1768. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, L.; Wang, H.; Chen, X.; Wang, Y.; Yang, H.; Wei, C.; Wan, X.; Xia, T. The R2R3-MYB, BHLH, WD40, and related transcription factors in flavonoid biosynthesis. Funct. Integr. Genom. 2013, 13, 75–98. [Google Scholar] [CrossRef]
- Chang, C.S.J.; Li, Y.; Chen, L.T.; Chen, W.C.; Hsieh, W.P.; Shin, J.; Jane, W.N.; Chou, S.J.; Choi, G.; Hu, J.M.; et al. LZF1, a HY5-Regulated Transcriptional Factor, Functions in Arabidopsis De-Etiolation. Plant J. 2008, 54, 205–219. [Google Scholar] [CrossRef]
- Job, N.; Yadukrishnan, P.; Bursch, K.; Datta, S.; Johansson, H. Two B-Box proteins regulate photomorphogenesis by oppositely modulating HY5 through their diverse C-terminal domains. Plant Physiol. 2018, 176, 2963–2976. [Google Scholar] [CrossRef]

| Gene Name | Gene ID | Group Classification | Number of Amino Acids (aa) | Molecular Weight (KDa) | Theoretical pI | Subcellular Localization | NCBI Annotation |
|---|---|---|---|---|---|---|---|
| PgBBX3 | LOC116192820 | group I | 347 | 37.56 | 5.91 | nucleus | zinc finger protein CONSTANS-LIKE 4-like |
| PgBBX4 | LOC116197790 | group I | 366 | 39.09 | 5.94 | nucleus | zinc finger protein CONSTANS-LIKE 5-like |
| PgBBX7 | LOC116198472 | group I | 352 | 37.93 | 6.01 | nucleus | zinc finger protein CONSTANS-LIKE 5-like |
| PgBBX12 | LOC116208207 | group I | 393 | 43.23 | 5.91 | nucleus | zinc finger protein CONSTANS-LIKE 2 |
| PgBBX17 | LOC116213171 | group I | 306 | 34.6 | 5.27 | nucleus | zinc finger protein CONSTANS-like |
| PgBBX1 | LOC116214276 | group II | 413 | 44.46 | 5.2 | nucleus | zinc finger protein CONSTANS-LIKE 9-like |
| PgBBX9 | LOC116201937 | group II | 503 | 56.16 | 5.9 | nucleus | putative zinc finger protein At1g68190 |
| PgBBX14 | LOC116211445 | group II | 416 | 46.49 | 5.03 | nucleus | zinc finger protein CONSTANS-LIKE 13 |
| PgBBX16 | LOC116211053 | group II | 478 | 51.92 | 6.64 | nucleus | zinc finger protein CONSTANS-LIKE 14 |
| PgBBX20 | LOC116213697 | group II | 437 | 47.18 | 5.69 | nucleus | putative zinc finger protein CONSTANS-LIKE 11 |
| PgBBX15 | LOC116212500 | group III | 430 | 47.84 | 5.79 | nucleus | zinc finger protein CONSTANS-LIKE 16-like |
| PgBBX18 | LOC116215624 | group III | 422 | 45.54 | 5.12 | nucleus | zinc finger protein CONSTANS-LIKE 8-like |
| PgBBX2 | LOC116193972 | group IV | 291 | 31.78 | 5.61 | nucleus | B-box zinc finger protein 21-like |
| PgBBX5 | LOC116195293 | group IV | 243 | 27.01 | 4.86 | nucleus | B-box zinc finger protein 24-like |
| PgBBX10 | LOC116205582 | group IV | 212 | 23.63 | 5.91 | extracellular space | B-box zinc finger protein 19-like |
| PgBBX11 | LOC116205350 | group IV | 314 | 35.02 | 6.33 | nucleus | B-box zinc finger protein 20 |
| PgBBX13 | LOC116210784 | group IV | 187 | 20.92 | 6.25 | extracellular space | B-box zinc finger protein 18-like |
| PgBBX19 | LOC116213752 | group IV | 248 | 27.31 | 4.93 | nucleus | B-box zinc finger protein 25-like |
| PgBBX21 | LOC116212812 | group IV | 320 | 34.46 | 6.24 | nucleus | B-box zinc finger protein 21-like |
| PgBBX23 | LOC116213334 | group IV | 294 | 31.38 | 6.3 | nucleus | B-box zinc finger protein 22 |
| PgBBX6 | LOC116196796 | group V | 246 | 25.85 | 4.63 | nucleus | dentin sialophosphoprotein-like |
| PgBBX8 | LOC116201908 | group V | 279 | 29.54 | 9.01 | nucleus | B-box zinc finger protein 32 |
| PgBBX22 | LOC116214036 | group V | 294 | 31.69 | 4.16 | nucleus | nonsense-mediated mRNA decay protein 2-like |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zheng, J. Identification and Characterization of the BBX Gene Family in Pomegranate (Punica granatum L.) and Its Potential Role in Anthocyanin Accumulation During Fruit Ripening. Horticulturae 2025, 11, 507. https://doi.org/10.3390/horticulturae11050507
Liu L, Zheng J. Identification and Characterization of the BBX Gene Family in Pomegranate (Punica granatum L.) and Its Potential Role in Anthocyanin Accumulation During Fruit Ripening. Horticulturae. 2025; 11(5):507. https://doi.org/10.3390/horticulturae11050507
Chicago/Turabian StyleLiu, Longbo, and Jie Zheng. 2025. "Identification and Characterization of the BBX Gene Family in Pomegranate (Punica granatum L.) and Its Potential Role in Anthocyanin Accumulation During Fruit Ripening" Horticulturae 11, no. 5: 507. https://doi.org/10.3390/horticulturae11050507
APA StyleLiu, L., & Zheng, J. (2025). Identification and Characterization of the BBX Gene Family in Pomegranate (Punica granatum L.) and Its Potential Role in Anthocyanin Accumulation During Fruit Ripening. Horticulturae, 11(5), 507. https://doi.org/10.3390/horticulturae11050507




