Evaluating a Natural-Based Solution for Its Stimulation in Cucumis sativus Plants and Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants Used and Experimental Design
2.2. Preliminary Test
2.3. Main Experiment
2.3.1. Plant Growth and Physiological Parameters
2.3.2. Total Phenols and Antioxidant Activity
2.3.3. Hydrogen Peroxide, Lipid Peroxidation, and Antioxidative Enzyme Activities
2.3.4. Leaf Nutrient Content
2.3.5. Fruit Quality
2.3.6. Fresh Produce Storability
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lucini, L.; Colla, G.; Miras Moreno, M.B.; Bernardo, L.; Cardarelli, M.; Terzi, V.; Bonini, P.; Rouphael, Y. Inoculation of Rhizoglomus irregulare or Trichoderma atroviride differentially modulates metabolite profiling of wheat root exudates. Phytochemistry 2019, 157, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Ziogas, V.; Bravos, N.; Hussain, S.B. Preharvest Foliar Application of Si–Ca-Based Biostimulant Affects Postharvest Quality and Shelf-Life of Clementine Mandarin (Citrus clementina Hort. Ex Tan). Horticulturae 2022, 8, 996. [Google Scholar] [CrossRef]
- Giordano, F.S.; Reynolds, A.; Frias, J.M.; Foley, L. Impact of silicon-enriched plant biostimulant treatment on shelf-life of baby spinach (Spinacia oleracea) crops. J. Agric. Food Res. 2024, 15, 100924. [Google Scholar] [CrossRef]
- El-Shaieny, A.H.A.H.; Abd-Elkarim, N.A.A.; Taha, E.M.; Gebril, S. Bio-Stimulants Extend Shelf Life and Maintain Quality of Okra Pods. Agriculture 2022, 12, 1699. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Gąsecka, M.; Spiżewski, T. Influence of biostimulants on phenolic content in broccoli heads directly after harvest and after storage. Folia Hortic. 2017, 29, 221–230. [Google Scholar] [CrossRef]
- Elansary, H.O.; Mahmoud, E.A.; El-Ansary, D.O.; Mattar, M.A. Effects of water stress and modern biostimulants on growth and quality characteristics of mint. Agronomy 2020, 10, 6. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Dudaš, S.; Šola, I.; Sladonja, B.; Erhatić, R.; Ban, D.; Poljuha, D. The Effect of Biostimulant and Fertilizer on “Low Input” Lettuce Production. Acta Bot. Croat. 2016, 75, 253–259. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Dias, M.I.M.I.; Petropoulos, S.A.S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The effects of biostimulants, biofertilizers and water-stress on nutritional value and chemical composition of two spinach genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Zhang, J.; Kirby, C.W.; Ji, X.; Locke, S.J.; Critchley, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Rengasamy, K.R.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; Van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. N. Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and Nutritional Quality of Vesuvian Piennolo Tomato PDO as Affected by Farming System and Biostimulant Application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Paradiković, N.; Kukavica, B.; Parađiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Abou Jaoudé, R.; Ficca, A.G.; Ruzzi, M. Effect of microbial plant biostimulants on fruit and vegetable quality: Current research lines and future perspectives. Front. Plant Sci. 2023, 14, 1251544. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic plant biostimulants and fruit quality-a review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis®’ apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Figueiredo, C.; Barroso, J.; Pedro, L.; Scheffer, J. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic Substances Contribute to Plant Iron Nutrition Acting as Chelators and Biostimulants. Front. Plant Sci. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Sfara, V.; Zerba, E.N.; Alzogaray, R.A. Fumigant insecticidal activity and repellent effect of five essential oils and seven monoterpenes on first-instar nymphs of Rhodnius prolixus. J. Med. Entomol. 2009, 46, 511–515. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, X.; Cao, J.; Wu, Z.; Pan, D. Effects of 1,8-cineole on carbohydrate metabolism related cell structure changes of Salmonella. Front. Microbiol. 2018, 9, 1078. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Maintaining postharvest quality of fresh produce with volatile compounds. Innov. Food Sci. Emerg. Technol. 2007, 8, 111–116. [Google Scholar] [CrossRef]
- Bedoya-Pérez, M.A.; Isler, I.; Banks, P.B.; McArthur, C. Roles of the volatile terpene, 1,8-cineole, in plant-herbivore interactions: A foraging odor cue as well as a toxin? Oecologia 2014, 174, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Caldas, R.G.F.; Oliveira, A.R.D.S.; Araújo, A.V.; Lafayette, S.S.L.; Albuquerque, G.S.; Silva-Neto, J.D.C.; Costa-Silva, J.H.; Ferreira, F.; Da Costa, J.G.M.; Wanderley, A.G. Gastroprotective mechanisms of the monoterpene 1,8-cineole (eucalyptol). PLoS ONE 2015, 10, e0134558. [Google Scholar]
- Xylia, P.; Ioannou, I.; Chrysargyris, A.; Stavrinides, M.C.; Tzortzakis, N. Quality attributes and storage of tomato fruits as affected by an eco-friendly, essential oil-based product. Plants 2021, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Marinou, E.; Chrysargyris, A.; Tzortzakis, N. Use of sawdust, coco soil and pumice in hydroponically grown strawberry. Plant Soil Environ. 2013, 59, 452–459. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Nikou, A.; Tzortzakis, N. Effectiveness of Aloe vera gel coating for maintaining tomato fruit quality. N. Z. J. Crop Hortic. Sci. 2016, 44, 203–217. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Grzebisz, W. Effect of biofertilizers on nutrient uptake by vegetables grown in a short cropping sequence. J. Elem. 2018, 23, 807–823. [Google Scholar] [CrossRef]
- Souri, M.K.; Roemheld, V. Split daily application of ammonium cannot ameliorate ammonium toxicity in tomato plants. Hortic. Environ. Biotechnol. 2009, 50, 384–391. [Google Scholar]
- Denre, M.; Ghanti, S.; Sarkar, K. Effect of humic acid application on accumulation of mineral nutrition and pungency in garlic (Allium sativum L.). Int. J. Biotechnol. Mol. Biol. Res. 2014, 5, 7–12. [Google Scholar]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 37–89. [Google Scholar]
- El-Nemr, M.A.; El-Desuki, M.; El-Bassiony, A.M.; Fawzy, Z.F. Response of growth and yield of cucumber plants (Cucumis sativus L.) to different foliar applications of humic acid and bio-stimulators. Aust. J. Basic Appl. Sci. 2012, 6, 630–637. [Google Scholar]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal conductance increases with rising temperature. Plant Signal Behav 2017, 12, e1356534. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mou, B. Drench Application of Fish-derived Protein Hydrolysates Affects Lettuce Growth, Chlorophyll Content, and Gas Exchange. HortTechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous application of amino acids improves the growth and yield of lettuce by enhancing photosynthetic assimilation and nutrient availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Xu, C.; Mou, B. Chitosan as Soil Amendment Affects Lettuce Growth, Photochemical Efficiency, and Gas Exchange. HortTechnology 2018, 28, 476–480. [Google Scholar] [CrossRef]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant mode of action: Impact of biostimulant on cellular level. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 229–243. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-a review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Guillén, F.; Castillo, S.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Efficacy of 1-MCP treatment in tomato fruit. 1. Duration and concentration of 1-MCP treatment to gain an effective delay of postharvest ripening. Postharvest Biol. Technol. 2007, 43, 23–27. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Borland, A.; Singleton, I.; Barnes, J. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol. Technol. 2007, 45, 317–325. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
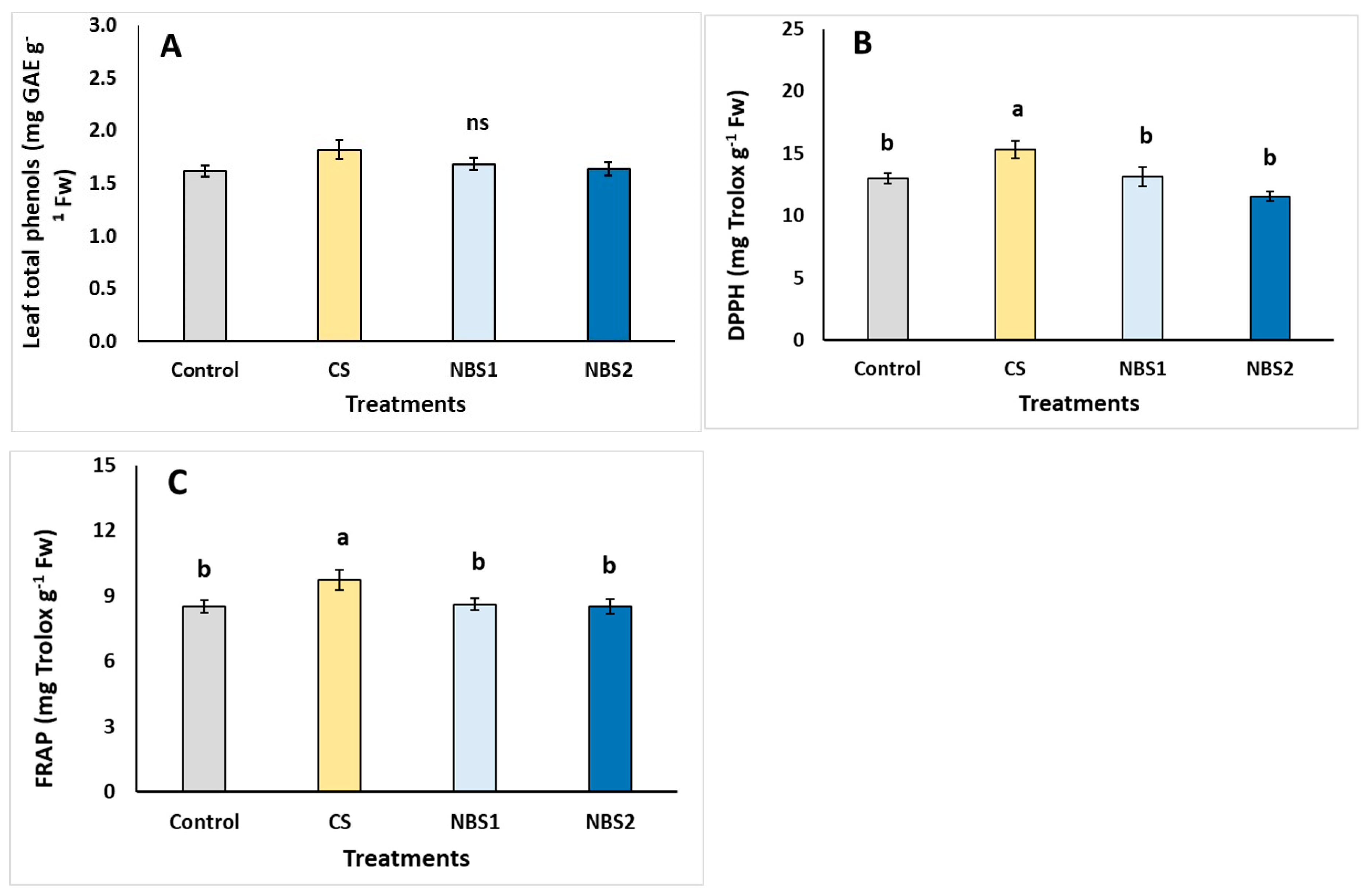
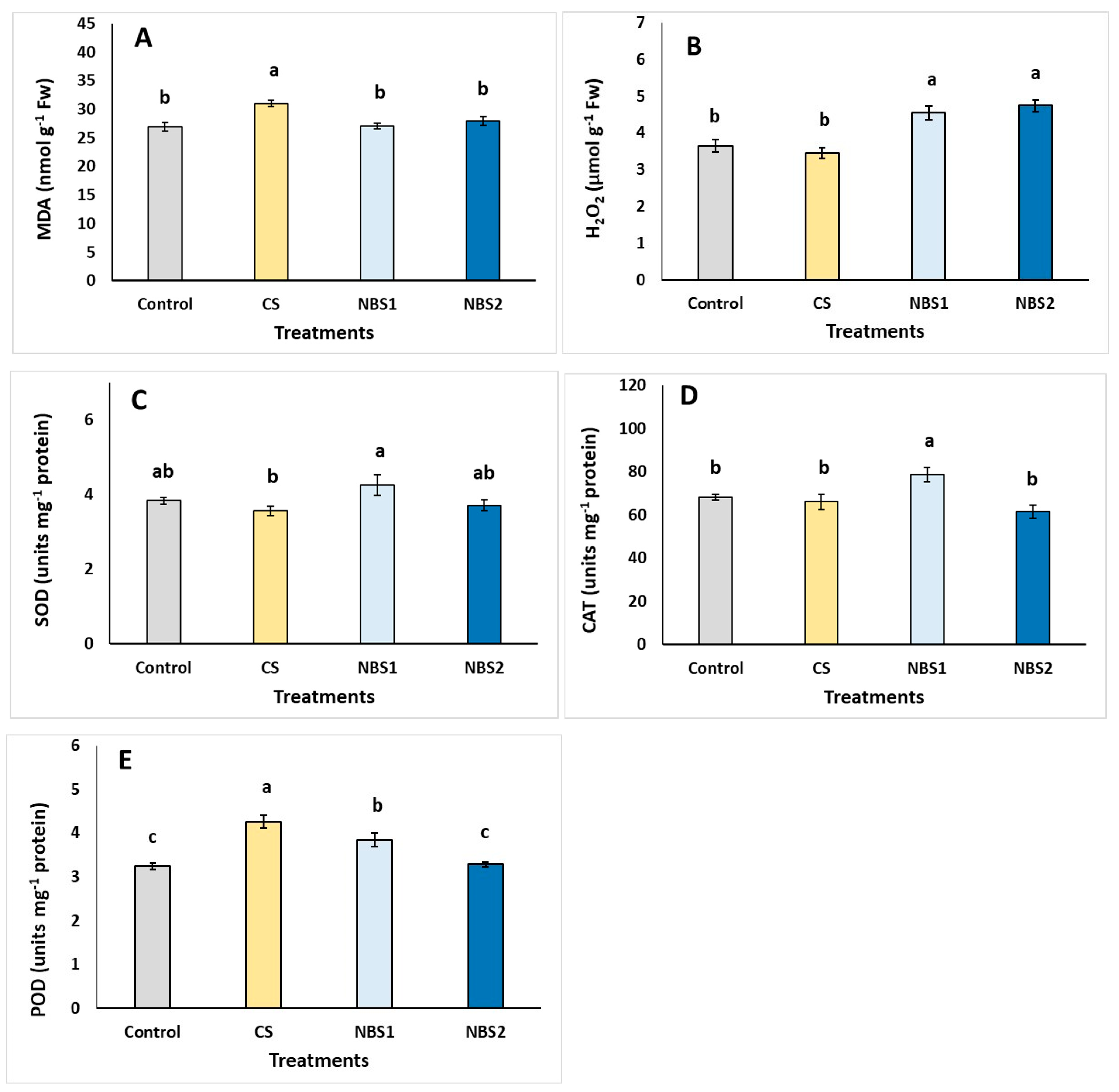
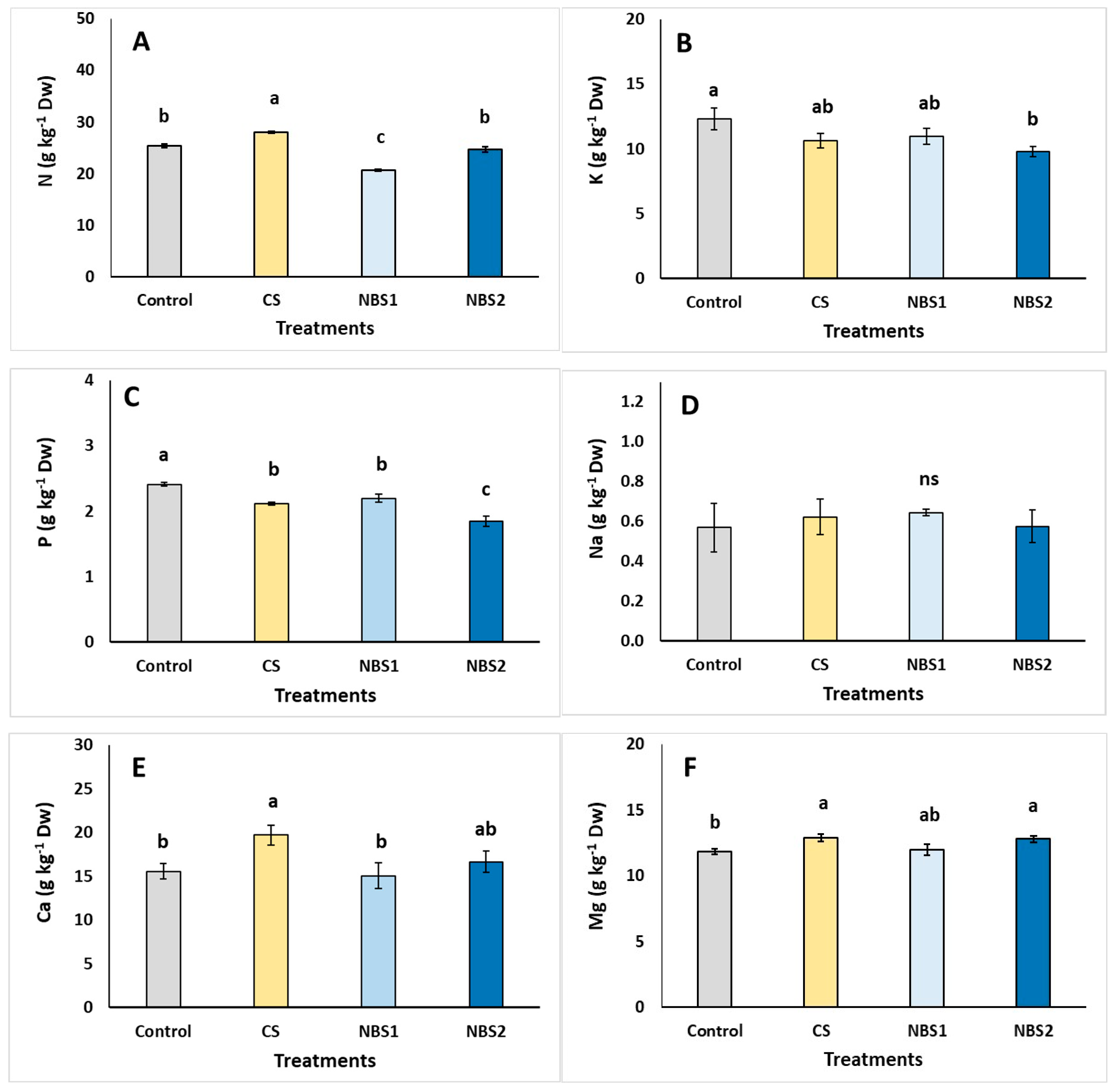
| Plant Development | Control | CS | NBS1 | NBS2 |
|---|---|---|---|---|
| Height (m) | 1.66 ± 0.01 a | 1.73 ± 0.04 a | 1.71 ± 0.03 a | 1.72 ± 0.04 a |
| Leaf number | 28.83 ± 0.31 a | 26.66 ± 0.49 a | 29.66 ± 0.76 a | 29.50 ± 0.71 a |
| Plant biomass (g) | 556.60 ± 38.52 a | 585.08 ± 17.19 a | 622.85 ± 42.02 a | 623.18 ± 20.00 a |
| Plant dry weight (g) | 67.88 ± 3.16 a | 67.36 ± 2.38 a | 69.38 ± 4.27 a | 71.63 ± 1.65 a |
| Yield (kg plant−1) | 2.74 ± 0.15 ab | 2.87 ± 1.14 a | 2.37 ± 0.09 b | 2.51 ± 0.12 ab |
| Fruit number | 11.76 ± 0.65 ab | 12.33 ± 0.71 a | 10.15 ± 0.52 b | 10.90 ± 0.69 ab |
| Plant Physiology | Control | CS | NBS1 | NBS2 |
|---|---|---|---|---|
| Stomatal conductance (mmol m−2s−1) | 315.75 ± 16.75 b | 428.33 ± 38.09 a | 340.66 ± 11.75 ab | 340.91 ± 37.21 ab |
| Chlorophyll fluorescence (Fv/Fm) | 0.79 ± 0.00 a | 0.79 ± 0.00 a | 0.78 ± 0.00 a | 0.78 ± 0.00 a |
| Chlorophyll a (mg g−1 Fw) | 0.81 ± 0.03 a | 0.69 ± 0.05 ab | 0.66 ± 0.04 b | 0.72 ± 0.02 ab |
| Chlorophyll b (mg g−1 Fw) | 0.22 ± 0.01 a | 0.17 ± 0.02 ab | 0.15 ± 0.01 b | 0.18 ± 0.00 ab |
| Total Chlorophylls (mg g−1 Fw) | 1.03 ± 0.04 a | 0.86 ± 0.07 ab | 0.81 ± 0.05 b | 0.91 ± 0.03 ab |
| Quality Attributes | Control | CS | NBS1 | NBS2 |
|---|---|---|---|---|
| Fruit fresh weight (Fw; g) | 233.31 ± 4.98 a | 235.09 ± 3.67 a | 236.81 ± 5.96 a | 236.85 ± 7.36 a |
| Firmness (in Newtons) | 33.53 ± 2.25 a | 33.63 ± 1.76 a | 31.30 ± 2.13 a | 31.76 ± 1.62 a |
| Respiration rates (mL CO2 kg−1 h−1) | 22.68 ± 1.29 a | 22.15 ± 2.27 a | 21.47 ± 1.05 a | 25.29 ± 2.41 a |
| Ethylene production (μL kg−1 h−1) | 3.81 ± 0.37 a | 3.99 ± 0.64 a | 4.22 ± 0.42 a | 4.73 ± 0.27 a |
| Lightness (L* value) | 34.04 ± 0.61 b | 34.35 ± 0.76 ab | 36.73 ± 0.73 a | 34.06 ± 1.08 b |
| Redness (a* value) | −9.38 ± 0.91 ab | −9.44 ± 0.56 ab | −11.70 ± 0.85 b | −9.05 ± 0.87 a |
| Yellowness (b* value) | 11.95 ± 1.30 b | 11.86 ± 0.84 b | 15.82 ± 1.41 a | 11.45 ± 1.31 b |
| Hue value | 128.29 ± 0.37 a | 128.63 ± 0.31 a | 126.67 ± 0.45 b | 128.60 ± 0.55 a |
| Chroma value | 15.19 ± 1.58 ab | 15.16 ± 1.01 ab | 19.68 ± 1.64 a | 14.60 ± 1.57 b |
| Color Index | −23.26 ± 0.72 b | −23.35 ± 0.74 b | −20.35 ± 0.72 a | −23.64 ± 1.88 b |
| TSS (%) | 3.40 ± 0.10 a | 3.07 ± 0.08 a | 3.07 ± 0.28 a | 3.15 ± 0.09 a |
| TA (malic acid g L−1) | 1.51 ± 0.13 a | 2.07 ± 0.32 a | 1.75 ± 0.03 a | 1.64 ± 0.02 a |
| AA (mg 100 g−1 Fw) | 6.92 ± 0.70 a | 6.65 ± 0.66 a | 6.70 ± 0.48 a | 7.88 ± 0.94 a |
| Phenols (mg GAE g−1 Fw) | 0.21 ± 0.02 a | 0.19 ± 0.01 a | 0.20 ± 0.01 a | 0.22 ± 0.01 a |
| DPPH (mg trolox g−1 Fw) | 1.03 ± 0.07 a | 1.08 ± 0.12 a | 0.82 ± 0.07 a | 0.96 ± 0.06 a |
| FRAP (mg trolox g−1 Fw) | 0.32 ± 0.02 a | 0.32 ± 0.00 a | 0.30 ± 0.02 a | 0.32 ± 0.03 a |
| Marketability (1–10) | 8.72 ± 0.30 a | 8.55 ± 0.20 a | 8.61 ± 0.16 a | 9.11 ± 0.14 a |
| Aroma (1–10) | 9.66 ± 0.17 a | 9.38 ± 0.26 a | 9.44 ± 0.11 a | 9.83 ± 0.07 a |
| Appearance (1–10) | 8.78 ± 0.07 ab | 8.89 ± 0.69 a | 8.61 ± 0.05 bc | 8.50 ± 0.07 c |
| Decay (1–10) | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a |
| Quality Attributes | Control | CS | NBS1 | NBS2 |
|---|---|---|---|---|
| Fruit weight loss (%) | 1.61 ± 0.06 a | 1.75 ± 0.06 a | 1.64 ± 0.07 a | 1.59 ± 0.08 a |
| Firmness (in Newtons) | 35.13 ± 1.97 a | 35.82 ± 1.20 a | 35.64 ± 1.75 a | 34.69 ± 1.53 a |
| Respiration rates (mL CO2 kg−1 h−1) | 13.69 ± 2.38 a | 18.73 ± 0.62 a | 12.95 ± 1.58 a | 18.69 ± 2.49 a |
| Ethylene production (μL kg−1 h−1) | 4.51 ± 0.66 ab | 2.97 ± 0.47 b | 5.93 ± 0.41 a | 4.80 ± 0.51 a |
| Lightness (L* value) | 35.27 ± 1.04 a | 33.78 ± 0.77 a | 35.25 ± 0.68 a | 34.59 ± 1.25 a |
| Redness (a* value) | −11.91 ± 0.19 ab | −10.61 ± 0.49 a | −11.54 ± 0.76 ab | −12.78 ± 0.54 b |
| Yellowness (b* value) | 15.93 ± 0.31 ab | 14.12 ± 0.83 b | 15.32 ± 1.21 ab | 17.33 ± 0.96 a |
| Hue value | 126.79 ± 0.13 a | 127.01 ± 0.34 a | 127.13 ± 0.40 a | 126.52 ± 0.42 a |
| Chroma value | 19.90 ± 0.36 ab | 17.67 ± 0.96 b | 19.19 ± 1.43 ab | 21.54 ± 1.10 a |
| Color Index | −21.30 ± 0.64 a | −22.40 ± 0.70 a | −21.54 ± 0.70 a | −21.53 ± 0.81 a |
| TSS (%) | 3.62 ± 0.13 a | 3.07 ± 0.75 b | 3.10 ± 0.10 b | 3.10 ± 0.12 b |
| TA (malic acid g L−1) | 1.76 ± 0.05 a | 1.90 ± 0.05 a | 1.74 ± 0.06 a | 1.72 ± 0.04 a |
| AA (mg 100 g−1 Fw) | 7.19 ± 0.48 a | 6.91 ± 0.55 a | 6.75 ± 0.75 a | 7.27 ± 0.28 a |
| Phenols (mg GAE g−1 Fw) | 0.23 ± 0.01 a | 0.24 ± 0.00 a | 0.22 ± 0.00 a | 0.22 ± 0.00 a |
| DPPH (mg trolox g−1 Fw) | 1.07 ± 0.06 a | 1.21 ± 0.04 a | 1.13 ± 0.06 a | 1.25 ± 0.08 a |
| FRAP (mg trolox g−1 Fw) | 0.32 ± 0.01 a | 0.28 ± 0.01 b | 0.26 ± 0.01 b | 0.26 ± 0.01 b |
| Marketability (1–10) | 7.94 ± 0.33 ab | 8.1600.23 a | 7.22 ± 0.33 b | 7.77 ± 0.18 ab |
| Aroma (1–10) | 9.05 ± 0.20 a | 8.44 ± 0.22 b | 7.66 ± 0.22 c | 8.61 ± 0.10 ab |
| Appearance (1–10) | 7.78 ± 0.07 b | 7.78 ± 0.07 b | 7.67 ± 0.00 b | 8.16 ± 0.07 a |
| Decay (1–10) | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a | 1.00 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Xylia, P.; Stavrinides, M.; Tzortzakis, N. Evaluating a Natural-Based Solution for Its Stimulation in Cucumis sativus Plants and Fruits. Horticulturae 2025, 11, 499. https://doi.org/10.3390/horticulturae11050499
Chrysargyris A, Xylia P, Stavrinides M, Tzortzakis N. Evaluating a Natural-Based Solution for Its Stimulation in Cucumis sativus Plants and Fruits. Horticulturae. 2025; 11(5):499. https://doi.org/10.3390/horticulturae11050499
Chicago/Turabian StyleChrysargyris, Antonios, Panayiota Xylia, Menelaos Stavrinides, and Nikolaos Tzortzakis. 2025. "Evaluating a Natural-Based Solution for Its Stimulation in Cucumis sativus Plants and Fruits" Horticulturae 11, no. 5: 499. https://doi.org/10.3390/horticulturae11050499
APA StyleChrysargyris, A., Xylia, P., Stavrinides, M., & Tzortzakis, N. (2025). Evaluating a Natural-Based Solution for Its Stimulation in Cucumis sativus Plants and Fruits. Horticulturae, 11(5), 499. https://doi.org/10.3390/horticulturae11050499








