Comparative Global Metabolome Profile and Transcriptome Sequence Analysis of the Rough and Smooth Peel of the Orah Mandarin (Citrus reticulata)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Metabolome Profiling
2.2.1. Sample Preparation and Extraction
2.2.2. UPLC Conditions and ESI-Q TRAP-MS/MS Analysis
2.2.3. Metabolome Data Analysis
2.3. Transcriptome Sequencing
3. Results
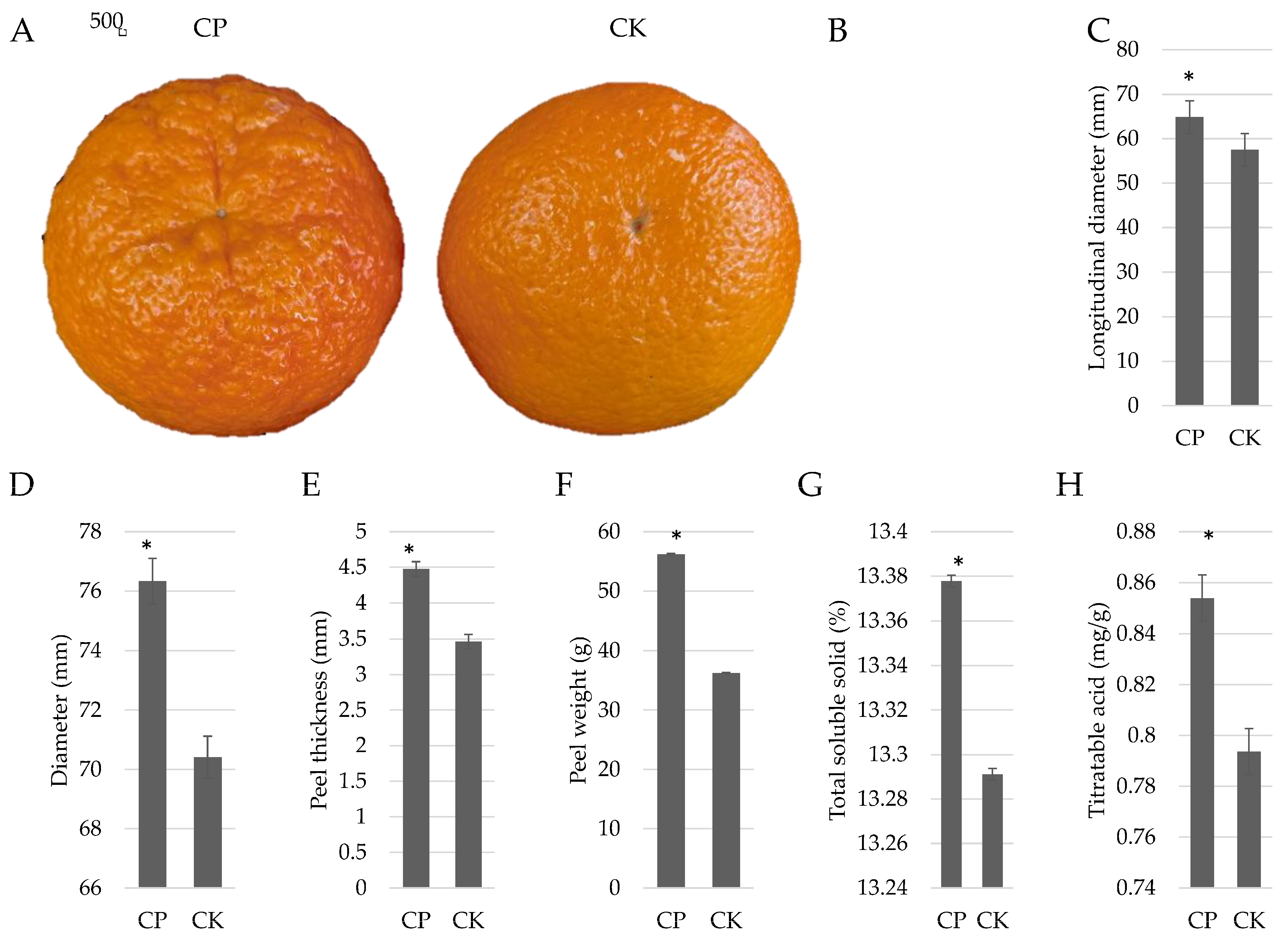
3.1. Physiological Indicators of Orah Mandarin
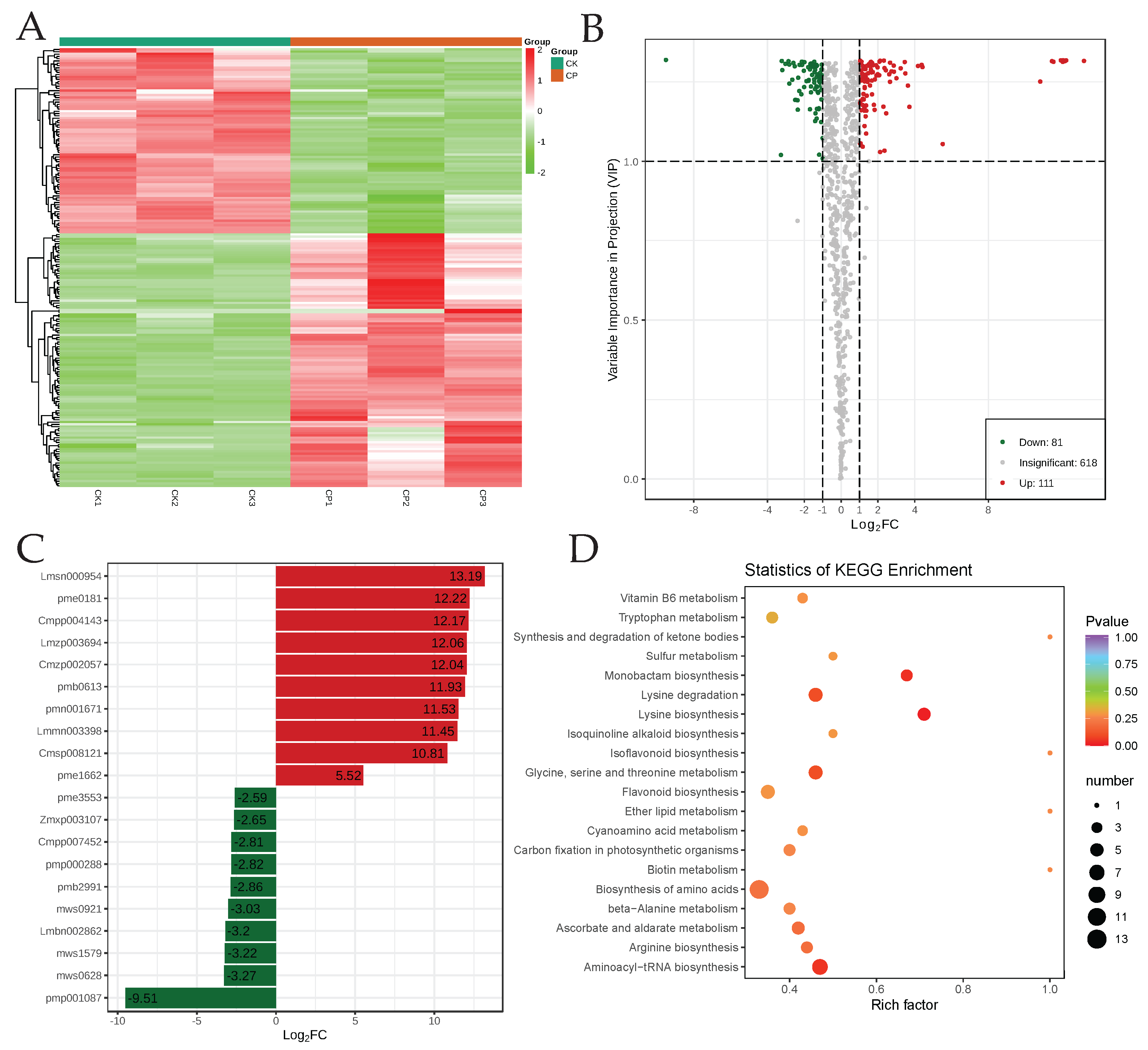
3.2. Global Metabolome Profiles of CP and CK Orah Mandarin Peels
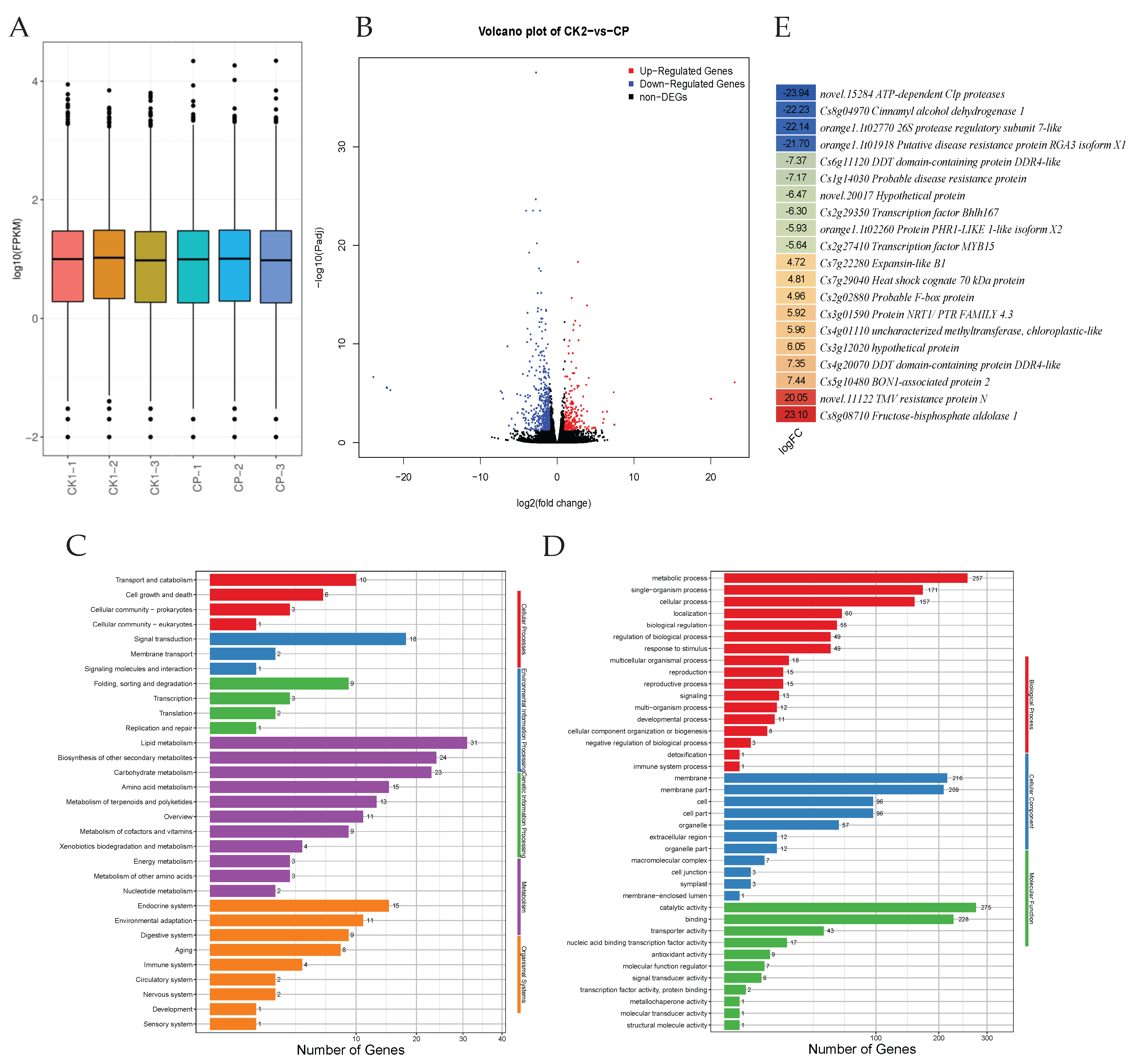
3.3. Comparative Transcriptome Profiles of Smooth and Rough Orah Mandarin Peels
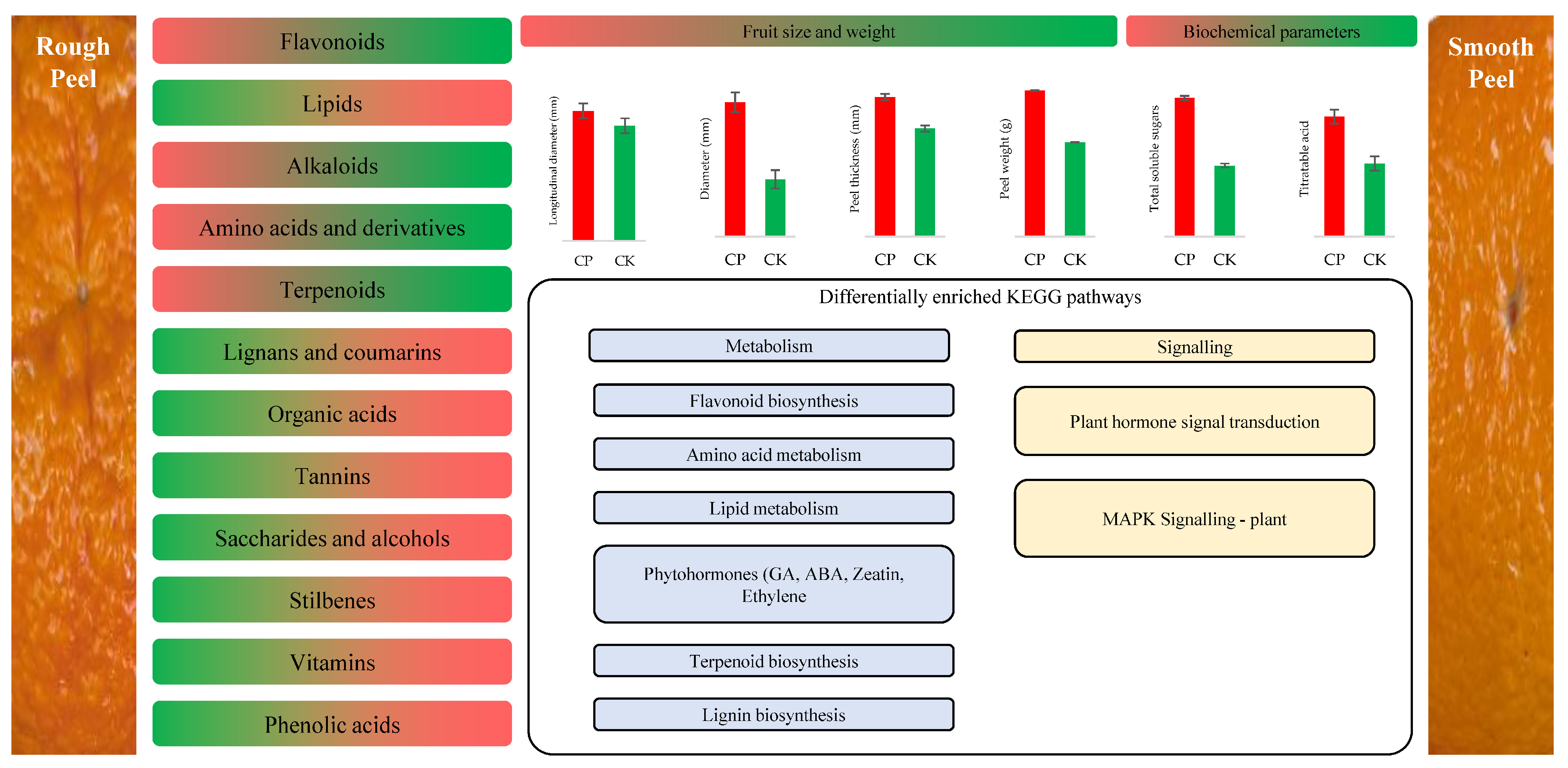
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, J.; Li, C.; Sun, D.; Guo, Q.; Liang, G. A tetraploid-dominated cytochimera developed from a natural bud mutant of the nonapomictic mandarin variety ‘Orah’. Mol. Breed. 2024, 44, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wei, P.; Tang, Y.; Li, J.; Yi, P.; Deng, Z.; He, X.; Ling, D.; Sun, J.; Zhang, L. Screening and Characteristics Analysis of Polysaccharides from Orah Mandarin (Citrus reticulata cv. Orah). Foods 2023, 13, 82. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Zhu, P.; Wang, M.; Qiu, J.; Sun, H.; Zhang, R.; Liu, P.; Ling, L.; Fu, X. Comparison between the vegetative and fruit characteristics of ‘Orah’ (Citrus reticulata Blanco) mandarin under different climatic conditions. Sci. Hortic. 2022, 300, 111064. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Zhu, H.; Qu, H.; You, S.; Duan, X.; Jiang, Y. Proteomic analysis of differentially expressed proteins involved in peel senescence in harvested mandarin fruit. Front. Plant Sci. 2016, 7, 725. [Google Scholar] [CrossRef]
- Erner, Y.; Monselise, S.; Goren, R. Rough fruit condition of the Shamouti orange-occurrence and patterns of development. Physiol. Veg. 1975, 13, 435–443. [Google Scholar]
- Erner, Y.; Goren, R.; Monselise, S. The rough fruit condition of the Shamouti orange—Connections with the endogenous hormonal balance. J. Hortic. Sci. 1976, 51, 367–374. [Google Scholar] [CrossRef]
- Liu, J.; Huang, C.; Chen, D. The effects of some plant growth regulators on fruit coloring and quality of Tankan (Citrus tankan Hayata). Guangdong Agric. Sci. 1987, 4, 3. [Google Scholar]
- Liu, F. Study on the Anatomical Morphology and Quality Changes of Rough of Satsuma. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2012. [Google Scholar]
- Kubo, T.; Hiratsuka, S. Effect of bearing angle of Satsuma mandarin fruit on rind roughness, pigmentation, and sugar and organic acid concentrations in the Juice. J. Jpn. Soc. Hortic. Sci. 1998, 67, 51–58. [Google Scholar] [CrossRef]
- Lu, X.-P.; Li, F.-F.; Xiong, J.; Cao, X.-J.; Ma, X.-C.; Zhang, Z.-M.; Cao, S.-Y.; Xie, S.-X. Transcriptome and metabolome analyses provide insights into the occurrence of peel roughing disorder on Satsuma Mandarin (Citrus unshiu Marc.) fruit. Front. Plant Sci. 2017, 8, 1907. [Google Scholar] [CrossRef]
- Liu, H.-M.; Long, C.-R.; Wang, S.-H.; Fu, X.-M.; Zhou, X.-Y.; Mao, J.-M.; Yang, H.-X.; Du, Y.-X.; Li, J.-X.; Yue, J.-Q. Transcriptome and metabolome comparison of smooth and rough Citrus limon L. peels grown on same trees and harvested in different seasons. Front. Plant Sci. 2021, 12, 749803. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. KEGG bioinformatics resource for plant genomics and metabolomics. In Plant Bioinformatics: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2016; pp. 55–70. [Google Scholar]
- Pruitt, K.D.; Tatusova, T.; Maglott, D.R. NCBI reference sequences (RefSeq): A curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 2007, 35, D61–D65. [Google Scholar] [CrossRef]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, 1–28. [Google Scholar] [CrossRef]
- Apweiler, R. Functional information in SWISS-PROT: The basis for large-scale characterisation of protein sequences. Brief. Bioinform. 2001, 2, 9–18. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Arakawa, K.; Kono, N.; Yamada, Y.; Mori, H.; Tomita, M. KEGG-based pathway visualization tool for complex omics data. Silico Biol. 2005, 5, 419–423. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, H.; Kong, L.; Gao, G.; Luo, J. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014, 42, D1182–D1187. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Li, X.; Yin, T.; Chen, C.; Zi, Y.; Zhao, K.; Pu, J.; Yan, W.; Wang, X.; Zhou, X. Integrated transcriptome and metabolome analyses reveal the mechanism by which bagging treatment affects peel reddening in Orah mandarin. Postharvest Biol. Technol. 2025, 221, 113336. [Google Scholar] [CrossRef]
- Shalom, L.; Samuels, S.; Zur, N.; Shlizerman, L.; Doron-Faigenboim, A.; Blumwald, E.; Sadka, A. Fruit load induces changes in global gene expression and in abscisic acid (ABA) and indole acetic acid (IAA) homeostasis in citrus buds. J. Exp. Bot. 2014, 65, 3029–3044. [Google Scholar] [CrossRef]
- Wang, J.-G.; Gao, X.-M.; Ma, Z.-L.; Chen, J.; Liu, Y.-N.; Shi, W.-Q. Metabolomic and transcriptomic profiling of three types of litchi pericarps reveals that changes in the hormone balance constitute the molecular basis of the fruit cracking susceptibility of Litchi chinensis cv. Baitangying. Mol. Biol. Rep. 2019, 46, 5295–5308. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Zhao, X.; Zhao, Y.; Hao, Z.; Luo, H.; Yuan, Z. Advances in mechanisms and omics pertaining to fruit cracking in horticultural plants. Agronomy 2021, 11, 1045. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Liu, Y.; Wang, Z.; Zhao, J. JAZ proteins: Key regulators of plant growth and stress response. Crop J. 2024, 12, 1505–1516. [Google Scholar] [CrossRef]
- Zi, J.; Mafu, S.; Peters, R.J. To gibberellins and beyond! Surveying the evolution of (di) terpenoid metabolism. Annu. Rev. Plant Biol. 2014, 65, 259–286. [Google Scholar] [CrossRef]
- Sharma, V.; Gupta, P.; Kagolla, P.; Hangargi, B.; Veershetty, A.; Ramrao, D.P.; Suresh, S.; Narasanna, R.; Naik, G.R.; Kumar, A. Metabolomics intervention towards better understanding of plant traits. Cells 2021, 10, 346. [Google Scholar] [CrossRef]
- Pervaiz, T.; Park, S.; Rezk, A.; Hur, M.; Obenland, D.; Arpaia, M.L.; El-Kereamy, A. Metabolomic analyses provide insights into the preharvest rind disorder in Satsuma Owari Mandarin. Front. Plant Sci. 2023, 14, 1263354. [Google Scholar] [CrossRef]
- Bennett, R.D. Acidic limonoids of grapefruit seeds. Phytochemistry 1971, 10, 3065–3068. [Google Scholar] [CrossRef]
- Ibrahim, M.; Oksanen, E.; Holopainen, J. Effects of limonene on the growth and physiology of cabbage (Brassica oleracea L.) and carrot (Daucus carota L.) plants. J. Sci. Food Agric. 2004, 84, 1319–1326. [Google Scholar] [CrossRef]
- Shi, Y.-S.; Zhang, Y.; Li, H.-T.; Wu, C.-H.; El-Seedi, H.R.; Ye, W.-K.; Wang, Z.-W.; Li, C.-B.; Zhang, X.-F.; Kai, G.-Y. Limonoids from Citrus: Chemistry, anti-tumor potential, and other bioactivities. J. Funct. Foods 2020, 75, 104213. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Li, W.; Lin, Y.-C.J.; Chen, Y.-L.; Zhou, C.; Li, S.; De Ridder, N.; Oliveira, D.M.; Zhang, L.; Zhang, B.; Wang, J.P. Woody plant cell walls: Fundamentals and utilization. Mol. Plant 2024, 17, 112–140. [Google Scholar] [CrossRef]
- Stasolla, C.; Scott, J.; Egertsdotter, U.; Kadla, J.; O’Malley, D.; Sederoff, R.; van Zyl, L. Analysis of lignin produced by cinnamyl alcohol dehydrogenase-deficient Pinus taeda cultured cells. Plant Physiol. Biochem. 2003, 41, 439–445. [Google Scholar] [CrossRef]
- Malik, A.U.; Hasan, M.U.; Khalid, S.; Mazhar, M.S.; Shafique Khalid, M.; Khan, M.N.; Saleem, B.A.; Khan, A.S.; Anwar, R. Biotic and abiotic factors causing rind blemishes in citrus and management strategies to improve the cosmetic quality of fruits. Int. J. Agric. Biol. 2021, 25, 298–318. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Long, C.; Fu, X.; Wang, S.; Lou, Y.; Dong, J.; Dong, M.; Mao, J.; Yang, J.; Yang, H.; et al. Comparative Global Metabolome Profile and Transcriptome Sequence Analysis of the Rough and Smooth Peel of the Orah Mandarin (Citrus reticulata). Horticulturae 2025, 11, 496. https://doi.org/10.3390/horticulturae11050496
Liu H, Long C, Fu X, Wang S, Lou Y, Dong J, Dong M, Mao J, Yang J, Yang H, et al. Comparative Global Metabolome Profile and Transcriptome Sequence Analysis of the Rough and Smooth Peel of the Orah Mandarin (Citrus reticulata). Horticulturae. 2025; 11(5):496. https://doi.org/10.3390/horticulturae11050496
Chicago/Turabian StyleLiu, Hongming, Chunrui Long, Xiaomeng Fu, Shaohua Wang, Yuqiang Lou, Jianmei Dong, Meichao Dong, Jiamei Mao, Jiandong Yang, Hongxia Yang, and et al. 2025. "Comparative Global Metabolome Profile and Transcriptome Sequence Analysis of the Rough and Smooth Peel of the Orah Mandarin (Citrus reticulata)" Horticulturae 11, no. 5: 496. https://doi.org/10.3390/horticulturae11050496
APA StyleLiu, H., Long, C., Fu, X., Wang, S., Lou, Y., Dong, J., Dong, M., Mao, J., Yang, J., Yang, H., Du, Y., & Zhou, X. (2025). Comparative Global Metabolome Profile and Transcriptome Sequence Analysis of the Rough and Smooth Peel of the Orah Mandarin (Citrus reticulata). Horticulturae, 11(5), 496. https://doi.org/10.3390/horticulturae11050496




