Effects of Utilizing Plasma-Activated Water as a Nitrate Source on Growth and Flowering of Vanda Orchids
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Effect of Nitrate Levels Produced by Plasma-Activated Water on the Growth of Vanda Orchids
2.1.1. Experimental Design and Plant Growing Conditions
2.1.2. Installing Pinhole Plasma Jet
2.1.3. Plasma-Activated Water Generation
2.1.4. Data Collection
2.2. Experiment 2: Study on the Effect of Plant Age and Application Frequency of Nitrate Produced by Plasma-Activated Water (PAW-NO3−) on Growth and Flowering of Vanda Orchids
2.3. Data Analysis
3. Results and Discussion
3.1. Experiment 1
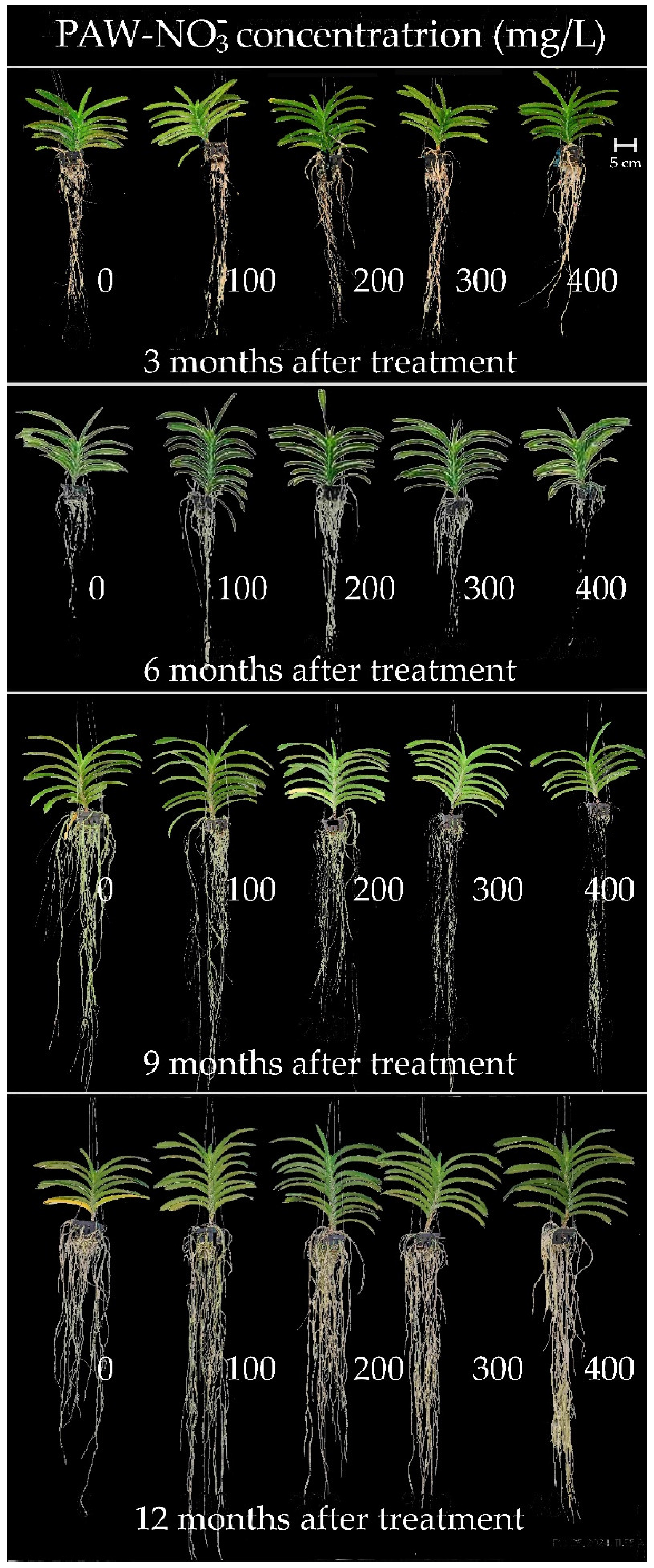
3.1.1. Plant Height
3.1.2. Number of Leaves
3.1.3. Fresh and Dry Weights, Leaf Nitrate, and Total Nitrogen Contents
3.1.4. Photosynthesis
3.1.5. Flowering and Flower Quality
3.2. Experiment 2: Study on the Effect of Plant Age and Application Frequency of Nitrate Produced by Plasma-Activated Water (PAW-NO3−) on Growth and Flowering of Vanda Orchids
3.2.1. Plant Height and Number of Leaves per Plant
3.2.2. Fresh Weight, Dry Weight, Leaf Nitrate, and Total Leaf Nitrogen Contents
3.2.3. Photosynthesis
3.2.4. Flowering and Flower Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setiaji, A.; Annisa, R.R.R.; Santoso, A.D.; Kinasih, A.; Riyadi, A.D.R. In vitro propagation of Vanda orchid: A review. Commun. Sci. 2021, 12, 3427. [Google Scholar]
- Neto, A.E.F.; Boldrin, K.V.F.; Mattson, N.S. Nutrition and quality in ornamental plants. Ornam. Hortic. 2015, 21, 139–150. [Google Scholar] [CrossRef]
- Wang, Y.-T. Effects of six fertilizers on vegetative growth and flowering of Phalaenopsis orchids. Sci. Hortic. 1996, 65, 191–197. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Konow, E.A. Fertilizer source and medium composition affect vegetative growth and mineral nutrition of a hybrid moth orchid. J. Am. Soc. Hortic. Sci. 2002, 127, 442–447. [Google Scholar] [CrossRef]
- Takane, R.; Yanagisawa, S.; Pivetta, K. Cultivo Moderno de Orquídeas: Cattleya e Seus Híbridos; UFC: Fortaleza, Brazil, 2010. [Google Scholar]
- Brun, P.; Bernabè, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef]
- Ruamrungsri, S.; Sawangrat, C.; Panjama, K.; Sojithamporn, P.; Jaipinta, S.; Srisuwan, W.; Intanoo, M.; Inkham, C.; Thanapornpoonpong, S. Effects of using plasma-activated water as a nitrate source on the growth and nutritional quality of hydroponically grown green oak lettuces. Horticulturae 2023, 9, 248. [Google Scholar] [CrossRef]
- Chalise, R.; Shrestha, P.; Sharma, S.; Basnet, S.; Mishra, L.N.; Khanal, R. Enhancing seed germination and growth parameters of cauliflower (Brassica oleracea, variety Botrytis) using plasma-activated water. J. Phys. D 2023, 56, 588. [Google Scholar] [CrossRef]
- Laurita, R.; Contaldo, N.; Zambon, Y.; Bisag, A.; Canel, A.; Gherardi, M.; Laghi, G.; Bertaccini, A.; Colombo, V. The use of plasma-activated water in viticulture: Induction of resistance and agronomic performance in greenhouse and open field. Plasma Process. Polym. 2021, 18, 206. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Shrestha, B.; Guragain, D.P.; Subedi, D.P. Improvements in germination and growth of sprouts irrigated using plasma-activated water (PAW). Water 2023, 15, 744. [Google Scholar] [CrossRef]
- Than, H.A.Q.; Pham, T.H.; Nguyen, D.K.V.; Pham, T.H.; Khacef, A. Non-thermal plasma-activated water for increasing germination and plant growth of Lactuca sativa L. Plasma Chem. Plasma Process. 2022, 42, 73–89. [Google Scholar] [CrossRef]
- Chuea-uan, S.; Boonyawan, D.; Sawangrat, C.; Thanapornpoonpong, S. Using plasma-activated water generated by an air gliding arc as a nitrogen source for rice seed germination. Agronomy 2024, 14, 15. [Google Scholar] [CrossRef]
- Stoleru, V.; Cojocaru, A.; Burducea, M.; Burducea, I.; Gherasim, O. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Sabbadini, S.; Gamliel, A.; Maffei, M.E.; Spadaro, D. Non-thermal plasma-activated water enhances nursery production of horticultural crops. Agronomy 2025, 15, 209. [Google Scholar] [CrossRef]
- Rathore, V.; Nema, S.K. A nitrogen alternative: Use of plasma activated water as nitrogen source in hydroponic solution for radish growth. arXiv 2024, arXiv:2404.16910. Available online: https://arxiv.org/abs/2404.16910 (accessed on 25 April 2025). [CrossRef]
- Ohyama, T. Analytical procedures of N, P, K concentrations in plant and manure materials using H2SO4–H2O2 Kjeldahl digestion method. Jpn. Bull. Fac. Agric. Niigata Univ. 1991, 43, 110–120. [Google Scholar]
- Cataldo, D.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Boyd, C.; Petersen, S.; Gilbert, W.; Rodgers, R.; Fuhlendorf, S.; Larsen, R.; Wolfe, D.; Jensen, K.; Gonzales, P.; Nenneman, M. Statistix 9; Analytical Software: Tallahassee, FL, USA, 2009. [Google Scholar]
- Sze, C.; Wang, B.; Xu, J.; Rivas-Davila, J.; Cappelli, M.A. Plasma-fixated nitrogen as fertilizer for turf grass. RSC Adv. 2021, 11, 37886–37895. [Google Scholar] [CrossRef]
- Wang, T.C.; Hsu, S.Y.; Lai, Y.T.; Duh, J.G. Improving the growth rate of Lactuca sativa young plants via plasma-activated water generated by multitubular dielectric barrier discharge cold plasma system. IEEE Trans. Plasma Sci. 2022, 50, 2104–2109. [Google Scholar] [CrossRef]
- Du, K.; Zhang, J.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Effects of varying NO3−:NH4⁺ ratios on lettuce (Lactuca sativa L.) nitrogen metabolism. Pak. J. Bot. 2022, 54, 2081–2088. [Google Scholar] [CrossRef]
- Rutkowski, K.; Łysiak, G.P. Effect of nitrogen fertilization on tree growth and nutrient content in soil and cherry leaves (Prunus cerasus L.). Agriculture 2023, 13, 578. [Google Scholar] [CrossRef]
- Jibia, S.S.; Panjama, K.; Inkham, C.; Sato, T.; Ohtake, N.; Ruamrungsri, S. Effects of source on the nitrogen uptake, allocation patterns, and performance of strawberry (Fragaria × ananassa Duch.): A 15N-tracer study. Plants 2025, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Zhang, J.; Wang, C.; Yang, Y.; Chang, Y.; Gao, Y.; Liu, Y.; Xie, J. Changes in growth, physiology, and photosynthetic capacity of spinach (Spinacia oleracea L.) under different nitrate levels. PLoS ONE 2023, 18, e0283787. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Fernández, I.; De Diego, N.; Brzobohatý, B.; Muñoz-Rueda, A.; Lacuesta, M. The imbalance between C and N metabolism during high nitrate supply inhibits photosynthesis and overall growth in maize (Zea mays L.). Plant Physiol. Biochem. 2017, 120, 213–222. [Google Scholar] [CrossRef]
- Kowalczyk, W.; Wrona, D.; Przybyłko, S. Effect of nitrogen fertilization of apple orchard on soil mineral nitrogen content, yielding of the apple trees and nutritional status of leaves and fruits. Agriculture 2022, 12, 2169. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Kumar, A. Effect of nitrogen, phosphorus, and their interaction on flower quality and vase life of snapdragon. Plant Arch. 2018, 18, 62–64. [Google Scholar]
- Zhang, S.; Liu, Y.; Du, M.; Shou, G.; Wang, Z.; Xu, G. Nitrogen as a regulator for flowering time in plants. Plant Soil 2022, 480, 1–29. [Google Scholar] [CrossRef]
- Sharma, N.; Bala, M.; Singh, P. Effect of different nitrogen levels on growth and flowering of chrysanthemum (Chrysanthemum morifolium Ramat.) cv. ‘White Star’. Agric. Res. J. 2023, 60, 712–716. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Kumar, A. Effect of nitrogen, planting distance, and bulb size on vase life of tuberose (Polianthes tuberosa L.) cv. Hyderabad Double. Plant Arch. 2018, 18, 512–514. [Google Scholar]
- Suárez, N. Effect of nitrogen on the dynamics of leaf population demography in whole plants of Wedelia trilobata. Int. J. Plant Sci. 2016, 177, 694–705. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–218. [Google Scholar]
- Pathare, P.B.; Koteyeva, N.; Cousins, A.B. The velamen radicum of orchids: A special porous structure for water absorption and gas exchange. Ann. Bot. 2020, 126, 1001–1013. [Google Scholar]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-Y.; Wong, S.-L.; Weng, J.-H. Photosynthesis in a Vanda sp. orchid with photosynthetic roots. Photosynthetica 2020, 58, 411–419. [Google Scholar] [CrossRef]
- Watthanasrisong, K.; Panjama, N.; Talee, W. Effects of Short Day Cycles on Flowering Time and Nutritional Status of Vanda ‘Manuvadee’. Hortic. J. 2021, 90, 22–31. [Google Scholar]
- Ruamrungsri, S.; Suksathan, P.; Charoenpakdee, S. Nitrogen Uptake and Translocation in Vanda Orchid after Roots and Leaves Application of Different Forms of 15N Tracer. Horticulturae 2022, 8, 902. [Google Scholar] [CrossRef]




| Macronutrients | Concentration (mg/L) | Micronutrients | Concentration (mg/L) |
|---|---|---|---|
| NH4+ | 58 | B | 0.24 |
| P | 100 | Mn | 0.226 |
| K | 200 | Zn | 0.11 |
| Ca | 100 | Mo | 0.005 |
| Mg | 100 | Cu | 0.01 |
| S | 212 | FeEDTA | 0.30 |
| Time (min) | Temperature (°C) | pH | Conductivity | ORP (mV) | NO3− Content (mg/L) |
|---|---|---|---|---|---|
| 0 | 24.0 ± 0.00 | 6.98 ± 0.25 | 191.4 ± 1.44 | 247.0 ± 1.25 | 0.00 ± 0.00 |
| 30 | 29.2 ± 1.39 | 7.84 ± 0.14 | 197.4 ± 4.89 | 216.0 ± 3.86 | 100.75 ± 15.52 |
| 60 | 32.0 ± 1.08 | 7.05 ± 0.26 | 203.5 ± 4.49 | 220.0 ± 8.06 | 200.28 ± 22.56 |
| 90 | 33.0 ± 0.47 | 7.85 ± 0.01 | 209.4 ± 3.19 | 218.0 ± 6.70 | 300.59 ± 8.39 |
| 120 | 33.5 ± 0.71 | 7.68 ± 0.19 | 222.4 ± 6.28 | 229.0 ± 8.60 | 400.38 ± 45.67 |
| PAW-NO3− (mg/L) | Plant Height (cm) | Number of Leaves per Plant | ||||||
|---|---|---|---|---|---|---|---|---|
| Months After Treatments (MAT) | ||||||||
| 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | |
| 0 | 18.25 ± 2.08 | 18.70 ± 1.94 b | 20.30 ± 2.33 c | 20.82 ± 1.83 b | 13.67 ± 2.23 b | 14.27 ± 1.80 b | 16.00 ± 1.92 b | 14.33 ± 2.13 |
| 100 | 20.41 ± 2.20 | 20.92 ± 1.98 a | 23.23 ± 2.41 ab | 24.03 ± 2.56 a | 15.40 ± 1.64 a | 16.27 ± 2.13 a | 18.20 ± 2.26 a | 16.53 ± 2.13 |
| 200 | 20.53 ± 2.14 | 21.50 ± 1.96 a | 23.64 ± 2.10 ab | 22.90 ± 3.01 a | 14.67 ± 1.53 ab | 16.40 ± 1.73 a | 18.13 ± 1.92 a | 16.47 ± 2.53 |
| 300 | 20.23 ± 2.22 | 19.95 ± 1.91 ab | 21.82 ± 2.35 bc | 23.75 ± 3.00 a | 13.93 ± 1.37 b | 15.07 ± 2.45 ab | 16.67 ± 2.18 ab | 16.86 ± 3.83 |
| 400 | 19.71 ± 2.19 | 19.85 ± 1.61 ab | 21.40 ± 2.54 c | 24.25 ± 2.16 a | 13.87 ± 1.31 b | 14.80 ± 1.83 b | 16.40 ± 1.98 ab | 16.27 ± 1.83 |
| LSD0.05 | ns | * | * | * | * | * | * | ns |
| PAW-NO3− (mg/L) | Fresh Weight (g) | Dry Weight (g) | Nitrate (mg/Kg DW) | Total Nitrogen (%) |
|---|---|---|---|---|
| 0 | 181.58 ± 7.42 d | 37.75 ± 1.54 b | 231.25 ± 10.59 d | 0.66 ± 0.01 c |
| 100 | 256.08 ± 7.70 a | 45.25 ± 5.28 a | 330.69 ± 5.18 b | 0.77 ± 0.05 a |
| 200 | 227.82 ± 5.76 b | 41.30 ± 2.23 ab | 309.22 ± 13.45 bc | 0.71 ± 0.01 b |
| 300 | 212.10 ± 7.22 c | 45.38 ± 3.90 a | 321.08 ± 16.17 c | 0.75 ± 0.00 ab |
| 400 | 218.74 ± 9.70 bc | 43.38 ± 2.30 ab | 352.72 ± 6.85 a | 0.71 ± 0.00 b |
| LSD0.05 | * | * | * | * |
| PAW-NO3− (mg/L) | Transpiration Rate (mol/m2/s1) | Stomatal Conductance (mol/m2/s1) | Photosynthetic Rate (µmol/m2/s1) |
|---|---|---|---|
| 0 | 0.532 ± 0.065 | 0.0480 ± 0.0064 | 1.1340 ± 0.63 |
| 100 | 0.538 ± 0.061 | 0.0460 ± 0.0062 | 2.4200 ± 0.61 |
| 200 | 0.640 ± 0.062 | 0.0540 ± 0.0059 | 2.2880 ± 0.58 |
| 300 | 0.682 ± 0.062 | 0.0620 ± 0.0053 | 1.6820 ± 0.63 |
| 400 | 0.610 ± 0.060 | 0.0560 ± 0.0057 | 1.0560 ± 0.65 |
| LSD0.05 | ns | ns | ns |
| PAW-NO3− (mg/L) | Days to Bloom (Cycle) | Flower Stalk Length (cm) | Florets per Inflorescence | Floret Diameter (cm) | Vase Life (Days) | ||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||||
| 0 | 233.67 ± 6.53 a | 297.88 ± 5.34 a | 326.50 ± 6.66 a | 29.66 ± 5.23 | 4.13 ± 0.35 c | 9.94 ± 1.52 | 7.13 ± 1.36 c |
| 100 | 208.22 ± 10.93 d | 284.00 ± 8.35 b | 304.75 ± 5.56 d | 30.59 ± 5.80 | 5.38 ± 0.52 a | 10.54 ± 1.29 | 12.63 ± 1.77 a |
| 200 | 226.11 ± 3.67 bc | 290.88 ± 6.00 ab | 312.88 ± 2.12 bc | 29.78 ± 1.86 | 5.25 ± 0.46 ab | 9.93 ± 0.84 | 11.00 ± 2.45 ab |
| 300 | 222.11 ± 5.00 c | 285.88 ± 6.03 b | 307.88 ± 1.41 cd | 27.64 ± 5.58 | 4.75 ± 0.46 b | 10.00 ± 1.20 | 11.88 ± 1.36 ab |
| 400 | 232.00 ± 4.36 ab | 293.88 ± 3.95 a | 315.88 ± 1.73 b | 30.20 ± 2.43 | 3.88 ± 0.83 c | 9.74 ± 1.38 | 10.50 ± 1.51 b |
| LSD0.05 | * | * | * | ns | * | ns | * |
| Factors | Plant Height (cm) | Number of Leaves | ||||||
|---|---|---|---|---|---|---|---|---|
| Months After Treatment (MAT) | Months After Treatment (MAT) | |||||||
| 3 | 6 | 9 | 12 | 3 | 6 | 9 | 12 | |
| Plant age(years) | ||||||||
| 1 | 0.15 ± 0.10 b | 0.12 ± 0.09 b | 0.33 ± 0.15 | 0.78 ± 0.67 | 0.44 ± 0.63 | 0.94 ± 0.70 | −1.1 ± 0.80 b | 0.86 ± 0.65 |
| 2 | 0.57 ± 0.12 a | 0.17 ± 0.11 b | 0.34 ± 0.10 | 0.79 ± 0.33 | 0.83 ± 0.65 | 0.83 ± 0.68 | 0.58 ± 0.45 a | 0.79 ± 0.60 |
| 3 | 0.37 ± 0.09 ab | 0.40 ± 0.14 a | 0.34 ± 0.13 | 0.92 ± 0.66 | 0.99 ± 0.36 | 0.62 ± 0.50 | 0.59 ± 0.46 a | 0.86 ± 0.62 |
| LSD0.05 | * | * | ns | ns | ns | ns | * | ns |
| Fertilizing frequency | ||||||||
| Weekly | 0.28 ± 0.11 | 0.25 ± 0.10 | 1.83 ± 0.73 | 1.01 ± 1.20 | 0.63 ± 0.58 | 0.74 ± 0.59 | 0.58 ± 0.46 a | 1.19 ± 0.95 a |
| Biweekly | 0.44 ± 0.18 | 0.21 ± 0.08 | 1.64 ± 0.66 | 0.65 ± 0.43 | 0.88 ± 0.56 | 0.86 ± 0.56 | 0.20 ± 0.13 b | 0.48 ± 0.31 b |
| LSD0.05 | ns | ns | ns | ns | ns | ns | * | * |
| Plant age × frequency of fertilizing | ||||||||
| 1 year × weekly | 0.14 ± 0.09 | 0.18 ± 0.11 | 0.47 ± 0.19 | 0.96 ± 0.98 | 0.25 ± 0.52 | 1.00 ± 0.70 | 0.13 ± 0.15 | 1.29 ± 0.90 |
| 1 year × biweekly | 0.16 ± 0.10 | 0.06 ± 0.03 | 0.47 ± 0.19 | 0.66 ± 0.30 | 0.63 ± 0.74 | 0.88 ± 0.62 | −0.13 ± 0.13 | 1.14 ± 0.80 |
| 2 years × weekly | 0.32 ± 0.13 | 0.22 ± 0.09 | 0.47 ± 0.19 | 1.40 ± 1.51 | 0.50 ± 0.53 | 0.83 ± 0.58 | 0.88 ± 0.62 | 1.14 ± 0.80 |
| 2 years × biweekly | 0.82 ± 0.33 | 0.12 ± 0.06 | 0.50 ± 0.20 | 0.60 ± 0.62 | 1.17 ± 0.79 | 0.83 ± 0.58 | 0.30 ± 0.21 | 0.43 ± 0.30 |
| 3 years × weekly | 0.40 ± 0.16 | 0.36 ± 0.14 | 0.47 ± 0.19 | 0.91 ± 1.33 | 1.13 ± 0.00 | 0.38 ± 0.27 | 0.80 ± 0.56 | 0.43 ± 0.31 |
| 3 years × biweekly | 0.34 ± 0.14 | 0.44 ± 0.18 | 0.50 ± 0.20 | 0.44 ± 0.28 | 0.86 ± 0.49 | 0.86 ± 0.60 | 0.43 ± 0.30 | 0.57 ± 0.40 |
| LSD0.05 | ns | ns | ns | ns | ns | ns | ns | ns |
| Factor | Fresh Weight (g) | Dry Weight (g) | NO3− Content (mg/Kg DW) | Total N Content (%) |
|---|---|---|---|---|
| Plant age (years) | ||||
| 1 | 70.98 ± 16.03 c | 15.38 ± 3.15 c | 251.59 ± 13.35 | 0.52 ± 0.03 c |
| 2 | 247.59 ± 31.94 b | 47.41 ± 4.57 b | 246.51 ± 8.23 | 0.54 ± 0.02 b |
| 3 | 393.39 ± 53.23 a | 76.73 ± 9.61 a | 257.81 ± 18.80 | 0.62 ± 0.02 a |
| LSD0.05 | * | * | ns | * |
| Fertilizing frequency | ||||
| Weekly | 228.59 ± 129.04 | 44.55 ± 24.23 | 256.68 ± 12.05 | 0.57 ± 0.05 a |
| Biweekly | 246.05 ± 151.87 | 48.46 ± 28.79 | 247.26 ± 15.09 | 0.55 ± 0.05 b |
| LSD0.05 | ns | ns | ns | * |
| Plant age × Frequency of fertilizing | ||||
| 1 year × weekly | 77.50 ± 14.38 d | 15.89 ± 2.55 d | 260.07 ± 9.44 | 0.54 ± 0.006 cd |
| 1 year × biweekly | 64.46 ± 16.28 d | 14.86 ± 3.90 d | 243.12 ± 11.87 | 0.50 ± 0.021 c |
| 2 years × weekly | 242.12 ± 45.95 c | 45.68 ± 4.55 c | 245.94 ± 11.54 | 0.52 ± 0.021 d |
| 2 years × biweekly | 253.06 ± 10.50 c | 49.14 ± 4.33 c | 247.07 ± 5.95 | 0.55 ± 0.010 c |
| 3 years × weekly | 420.62 ± 31.62 a | 81.39 ± 10.02 a | 264.02 ± 9.33 | 0.63 ± 0.006 a |
| 3 years × biweekly | 366.1659.51 ± b | 72.08 ± 7.29 b | 251.59 ± 26.10 | 0.60 ± 0.006 b |
| LSD0.05 | * | * | ns | * |
| Factors | Transpiration Rate (mol/m2/s1) | Stomatal Conductance (mol/m2/s1) | Photosynthetic Rate (µmol/m2/s1) |
|---|---|---|---|
| Plant age (years) | |||
| 1 | 0.6663 ± 0.070 | 0.0575 ± 0.006 | 2.1387 ± 0.32 |
| 2 | 0.6238 ± 0.062 | 0.0600 ± 0.006 | 2.5087 ± 0.38 |
| 3 | 0.7275 ± 0.073 | 0.0800 ± 0.007 | 1.9913 ± 0.30 |
| LSD0.05 | ns | ns | ns |
| Fertilizing frequency | |||
| Weekly | 0.6450 ± 0.07 | 0.06090 ± 0.006 | 2.7750 ± 0.42 |
| Biweekly | 0.7000 ± 0.068 | 0.07080 ± 0.005 | 2.1483 ± 0.33 |
| LSD0.05 | ns | ns | ns |
| Plant age x Frequency of fertilizing | |||
| 1 year x weekly | 0.6375 ± 0.06 | 0.0525 ± 0.0053 | 1.5125 ± 0.23 |
| 1 year x biweekly | 0.6200 ± 0.05 | 0.0600 ± 0.0050 | 3.0550 ± 0.46 |
| 2 years x weekly | 0.6775 ± 0.07 | 0.0700 ± 0.0071 | 2.2650 ± 0.34 |
| 2 years x biweekly | 0.6950 ± 0.07 | 0.0625 ± 0.0067 | 2.7650 ± 0.41 |
| 3 years x weekly | 0.6275 ± 0.06 | 0.0600 ± 0.0058 | 1.9625 ± 0.29 |
| 3 years x biweekly | 0.7775 ± 0.08 | 0.0900 ± 0.0092 | 1.7175 ± 0.26 |
| LSD0.05 | ns | ns | ns |
| Treatments | Days to Bloom (Cycle) | Flower Stalk Length (cm) | Florets per Inflorescence | Floret Diameter (cm) | Vase Life (Days) | ||
|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||||
| Plant age × Frequency of fertilizing | |||||||
| 1 year × weekly | NF | NF | NF | NF | NF | NF | NF |
| 1 year × biweekly | NF | NF | NF | NF | NF | NF | NF |
| 2 years × weekly | NF | NF | NF | NF | NF | NF | NF |
| 2 years × biweekly | NF | NF | NF | NF | NF | NF | NF |
| 3 years × weekly | 185.16 ± 2.3 b | 276.00 ± 9.20 | 296.00 ± 1.00 b | 24.80 ± 0.23 | 5.71 ± 1.10 | 12.00 ± 0.01 | 13.57 ± 0.79 a |
| 3 years × biweekly | 193.25 ± 4.3 a | 279.29 ± 9.34 | 306.56 ± 9.34 a | 27.79 ± 1.39 | 5.85 ± 0.24 | 10.87 ± 1.70 | 9.86 ± 4.10 b |
| t-test 0.05 | * | ns | * | ns | ns | ns | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inkham, C.; Salisu Jibia, S.; Jaipinta, S.; Ruamrungsri, S.; Panjama, K. Effects of Utilizing Plasma-Activated Water as a Nitrate Source on Growth and Flowering of Vanda Orchids. Horticulturae 2025, 11, 491. https://doi.org/10.3390/horticulturae11050491
Inkham C, Salisu Jibia S, Jaipinta S, Ruamrungsri S, Panjama K. Effects of Utilizing Plasma-Activated Water as a Nitrate Source on Growth and Flowering of Vanda Orchids. Horticulturae. 2025; 11(5):491. https://doi.org/10.3390/horticulturae11050491
Chicago/Turabian StyleInkham, Chaiartid, Sirajo Salisu Jibia, Suchanuch Jaipinta, Soraya Ruamrungsri, and Kanokwan Panjama. 2025. "Effects of Utilizing Plasma-Activated Water as a Nitrate Source on Growth and Flowering of Vanda Orchids" Horticulturae 11, no. 5: 491. https://doi.org/10.3390/horticulturae11050491
APA StyleInkham, C., Salisu Jibia, S., Jaipinta, S., Ruamrungsri, S., & Panjama, K. (2025). Effects of Utilizing Plasma-Activated Water as a Nitrate Source on Growth and Flowering of Vanda Orchids. Horticulturae, 11(5), 491. https://doi.org/10.3390/horticulturae11050491








