Aluminum Stress of Oriental Melon (Cucumis melo L.) Is Linked to the Dehydrin CmDHN3
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome-Wide Identification of the Dehydrin Gene Family in Six Cucurbit Crops
2.2. Plant Materials and Stress Treatments
2.3. Silencing of the CmDHN3 Gene in Melon
2.4. Gene Expression Analysis
2.5. Malondialdehyde (MDA) Content, Proline Content, and Relative Electrolyte Leakage
2.6. Subcellular Localization
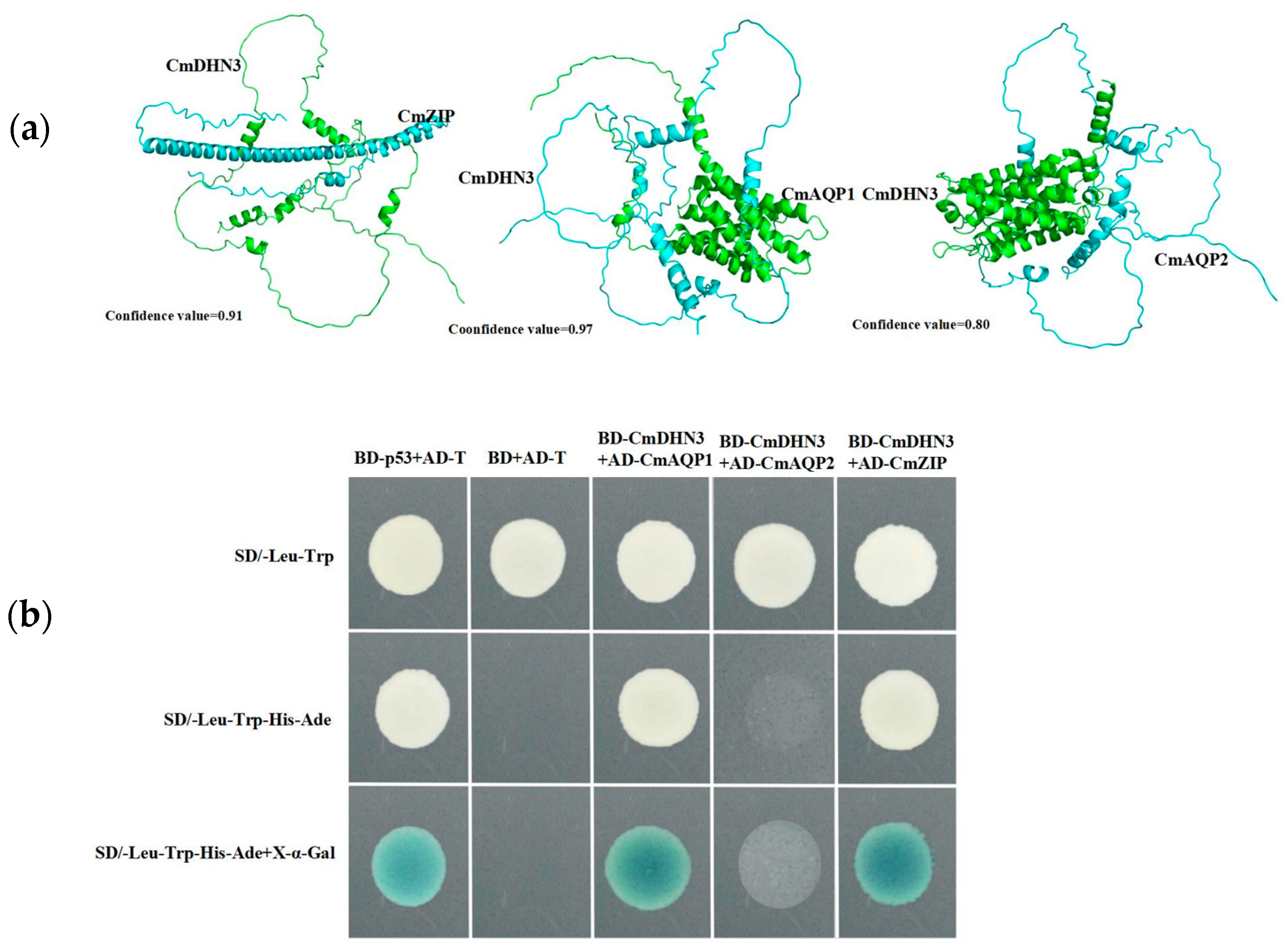
2.7. Yeast Two-Hybrid Validation Assay
2.8. Statistical Analysis
3. Results
3.1. DHN Gene Family Member Identification in Cucurbit Genomes
3.2. Analyses of Phylogenetic Relationships, Conserved Structural Domains, and Physicochemical Properties
3.3. Analyses of Gene Structure and Conserved Motifs
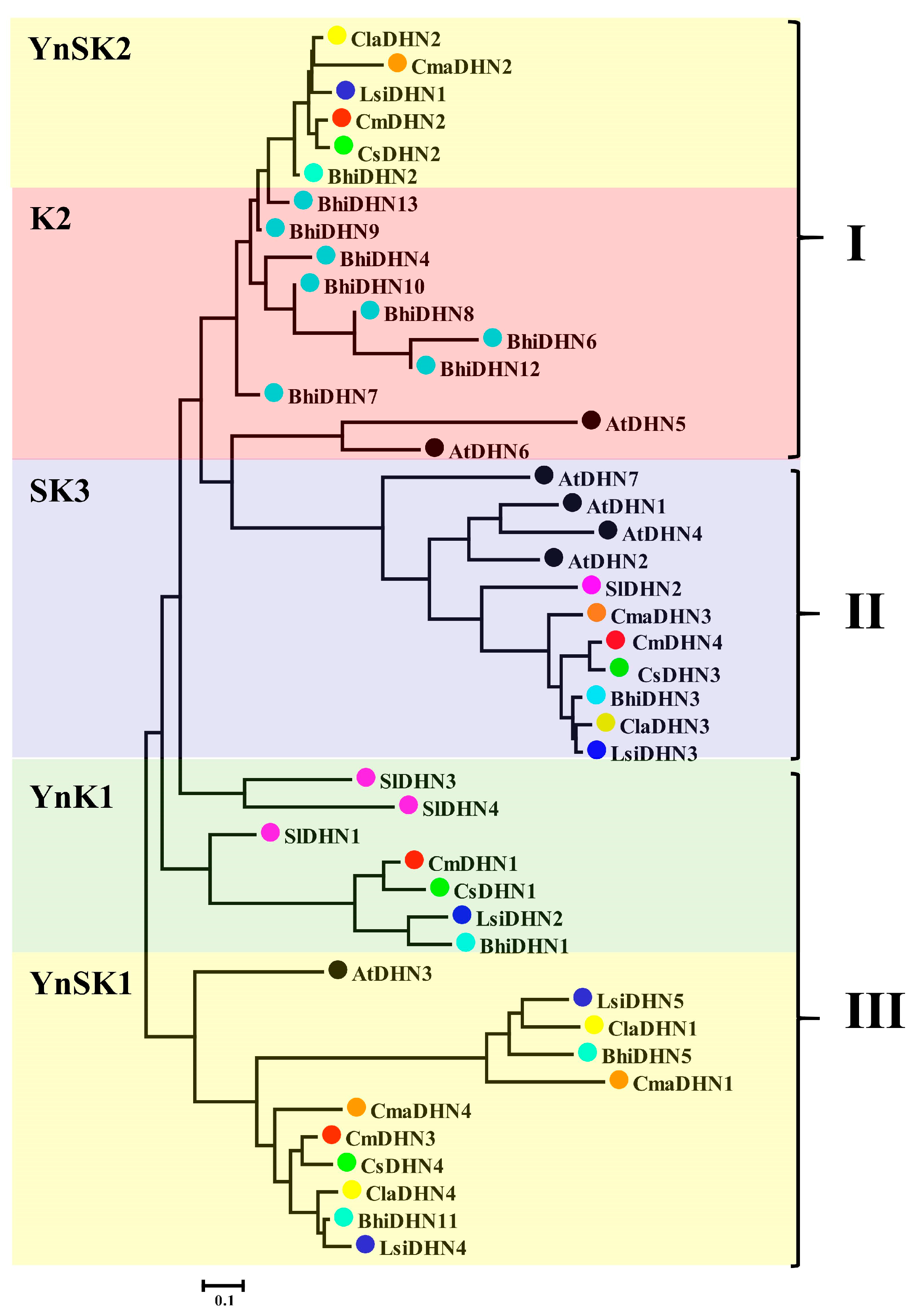
3.4. Evolutionary Relationships of DHN Gene Family Members in Cucurbitaceae
3.5. Expression Patterns of DHN Gene Family Members in Different Plant Tissues
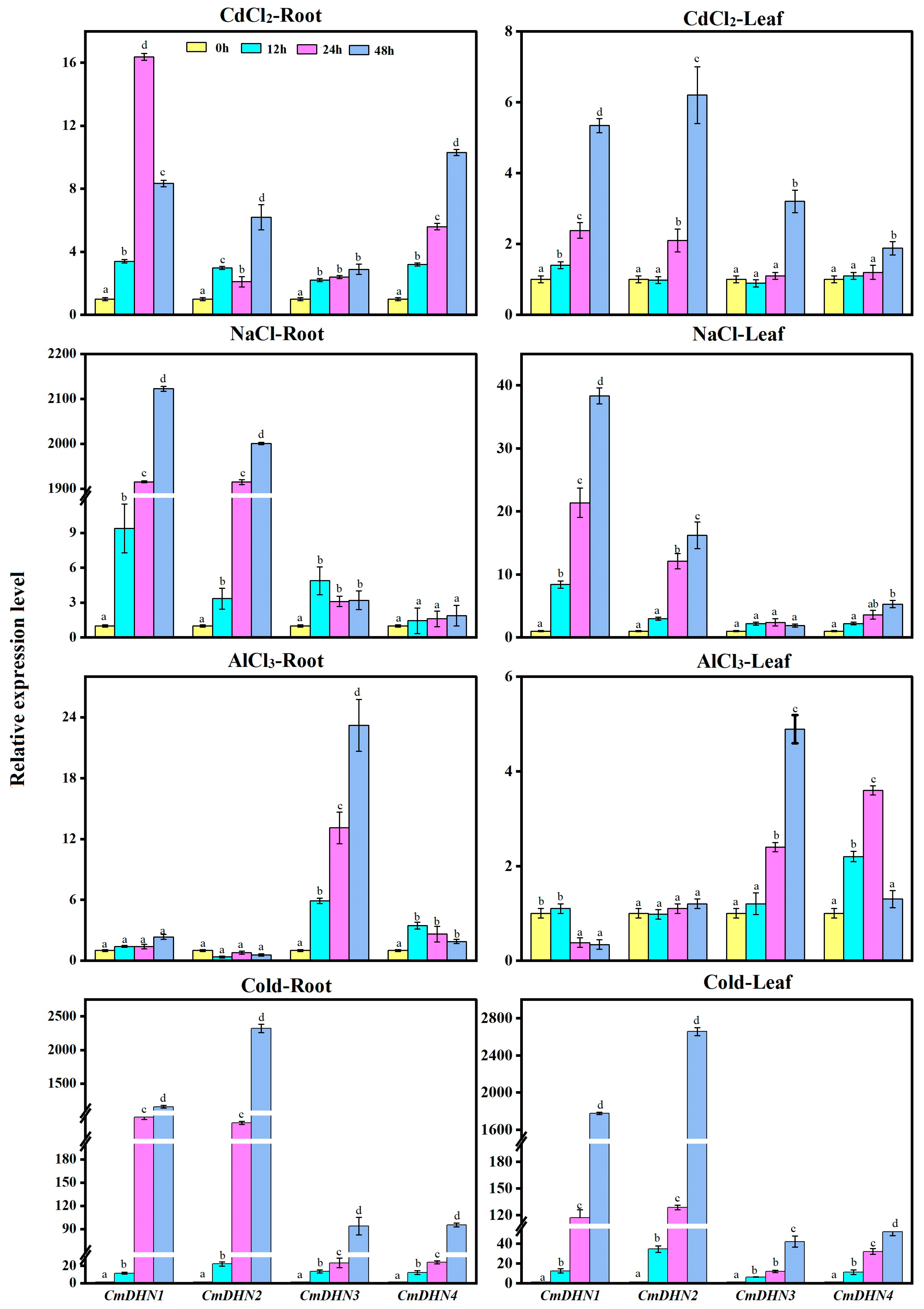
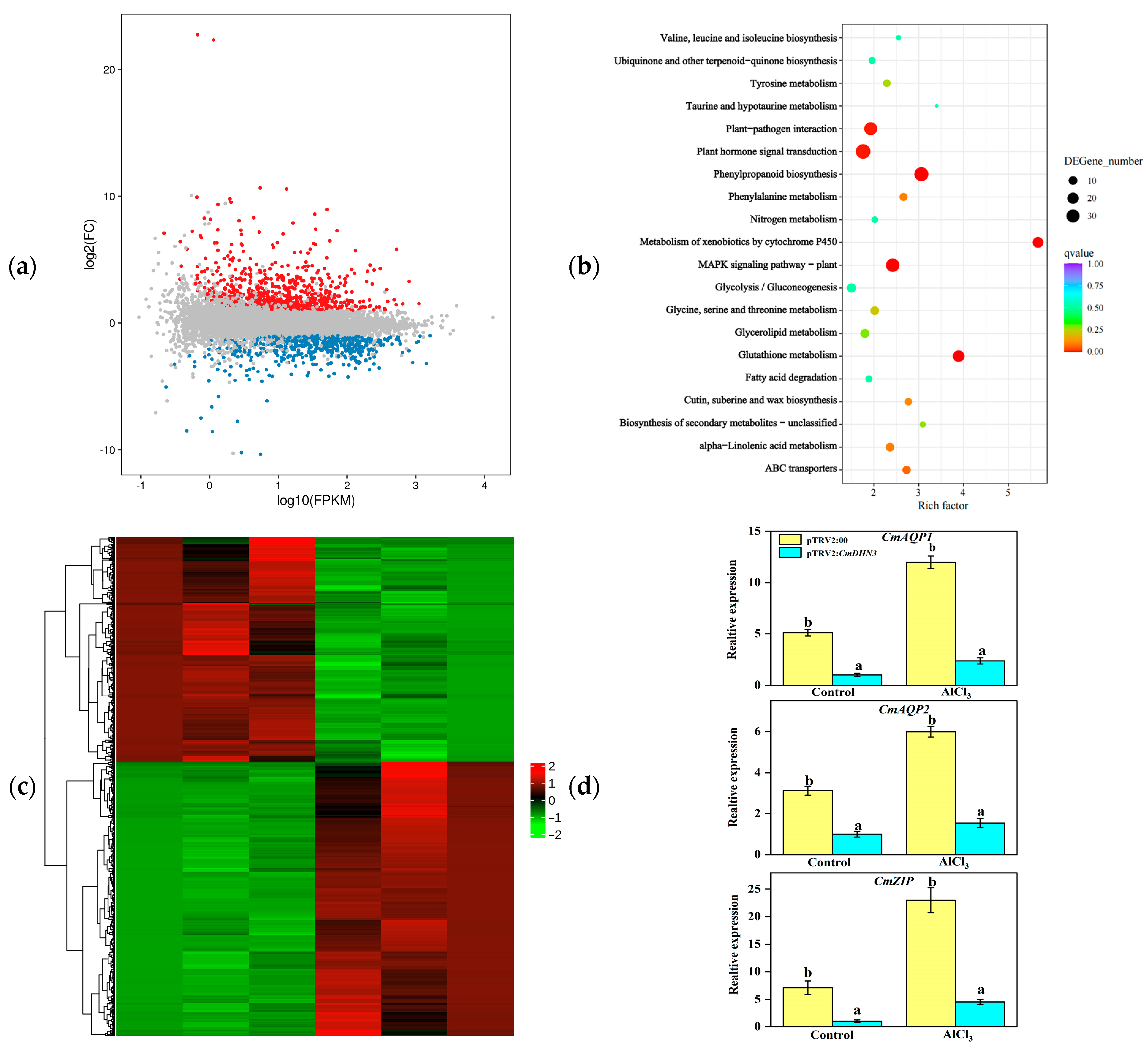
3.6. Expression Patterns of CmDHN Gene Family Members in Abiotic Stress Response
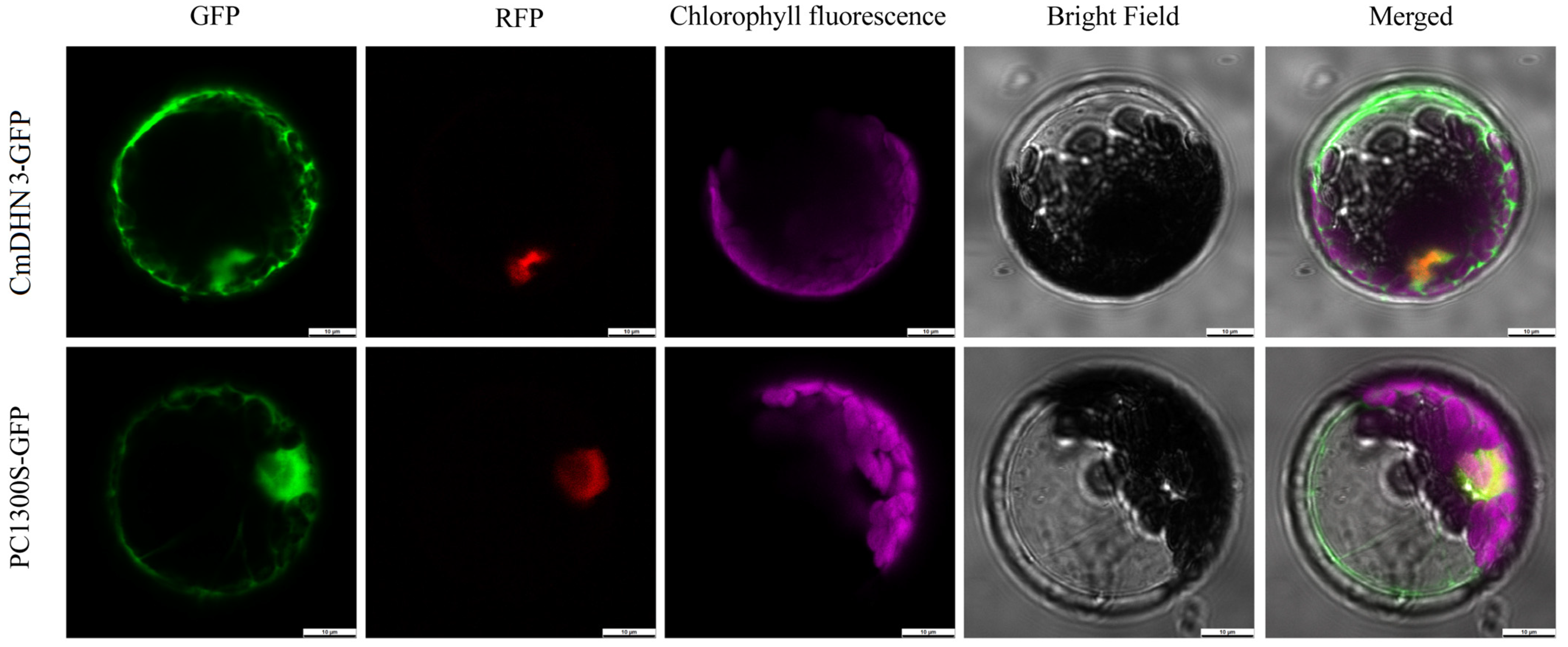
3.7. Subcellular Localization of CmDHN3 in Cucumis melo
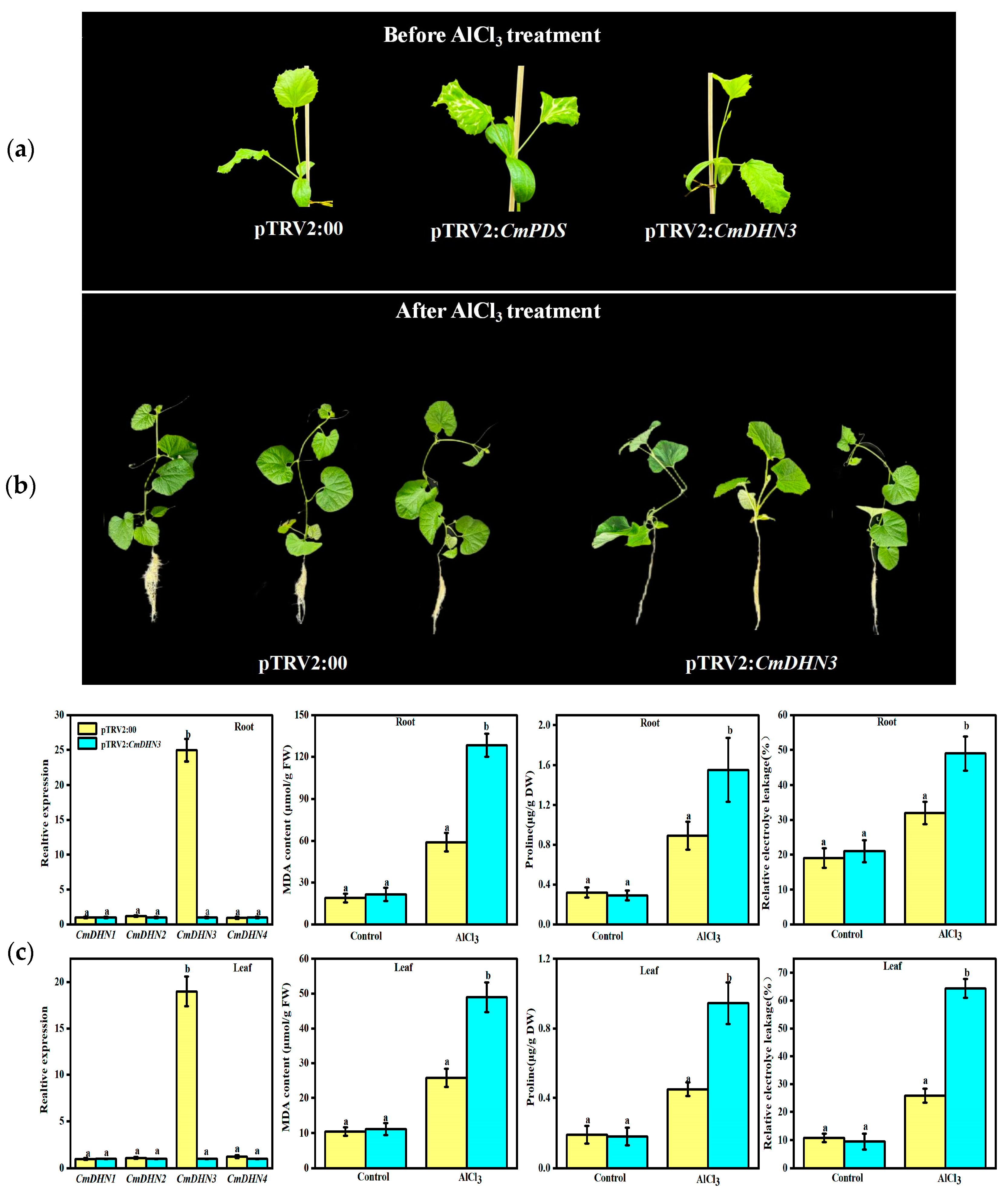
3.8. Effect of Cucumis melo CmDHN3 on Tolerance to Al Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, M.A.; Graether, S.P. The disordered dehydrin and its role in plant protection: A biochemical perspective. Biomolecules 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Nisha, N.; Singh, K.; Verma, R.; Gupta, R. Involvement of dehydrin proteins in mitigating the negative effects of drought stress in plants. Plant Cell Rep. 2022, 41, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Reyes, J.L.; Wei, H.; Yang, Y.; Karlson, D.; Covarrubias, A.A.; Krebs, S.L.; Fessehaie, A.; Arora, R. RcDhn5, a cold acclimation-responsive dehydrin from Rhododendron catawbiense rescues enzyme activity from dehydration effects in vitro and enhances freezing tolerance in RcDhn5-overexpressing Arabidopsis plants. Physiol. Plant. 2008, 134, 583–597. [Google Scholar] [CrossRef]
- Cheng, Y.; He, J.; Feng, Y.; Zhao, J.; Guan, J. Low temperature conditioning reduced the chilling injury by regulating expression of the dehydrin genes in postharvest huangguan pear (pyrus bretschneideri rehd. Cv. Huangguan). Horticulturae 2022, 8, 1022. [Google Scholar] [CrossRef]
- Szlachtowska, Z.; Rurek, M. Plant dehydrins and dehydrin-like proteins: Characterization and participation in abiotic stress responses. Front. Plant Sci. 2023, 14, 1197469. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.P.; Liu, Y.; Kang, Y.; Liu, W.; Li, S.; Wang, Z.H.; Xia, X.Y.; Chen, X.Y.; Qian, L.W.; Xiong, X.H.; et al. Genome-wide characterization of LEA gene family reveals a positive role of BnaA.LEA6.a in freezing tolerance in rapeseed (Brassica napus L.). BMC Plant Biol. 2024, 24, 433. [Google Scholar] [CrossRef]
- Tiwari, P.; Chakrabarty, D. Dehydrin in the past four decades: From chaperones to transcription co-regulators in regulating abiotic stress responses. Curr. Res. Biotechnol. 2021, 3, 249–259. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, L.; Wang, Y.; Zhou, Y.; Gao, F. AmDHN4, a winter accumulated SKn-type dehydrin from Ammopiptanthus mongolicus, and regulated by AmWRKY45, enhances the tolerance of Arabidopsis to low temperature and osmotic stress. Int. J. Biol. Macromol. 2024, 266, 131020. [Google Scholar] [CrossRef]
- Koag, M.C.; Wilkens, S.; Fenton, R.D.; Resnik, J.; Vo, E.; Close, T.J. The K-segment of maize DHN1 mediates its binding to anionic phospholipid vesicles and the concomitant structural changes. Plant Physiol. 2009, 150, 1503–1514. [Google Scholar] [CrossRef]
- Alsheikh, M.K.; Svensson, J.T.; Randall, S.K. Phosphorylation-regulated ion binding is a property shared by the acidic subclass of dehydrins. Plant Cell Environ. 2005, 28, 1114–1122. [Google Scholar] [CrossRef]
- Hernández-Sánchez, I.E.; Maruri-López, I.; Ferrando, A.; Carbonell, J.; Graether, S.P.; Jiménez-Bremont, J.F. Nuclear localization of the dehydrin opsdhn1 is determined by a histidine-rich motif. Front. Plant Sci. 2015, 6, 702. [Google Scholar] [CrossRef]
- Jia, J.S.; Ge, N.; Wang, Q.Y.; Zhao, L.T.; Chen, C.; Chen, J.W. Genome-wide identification and characterization of LEA gene family members in Panax notoginseng and their transcriptional responses to dehydration in recalcitrant seeds. BMC Genom. 2023, 24, 126. [Google Scholar] [CrossRef]
- Chen, R.G.; Jing, H.; Guo, W.L.; Wang, S.B.; Ma, F.; Pan, B.G.; Gong, Z.H. Silencing of dehydrin CaDHN1 diminishes tolerance to multiple abiotic stresses in Capsicum annuum L. Plant Cell Rep. 2015, 34, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Lv, A.M.; Wen, W.W.; Fan, N.N.; Su, L.T.; Peng, Z.; Yuan, A. Dehydrin MsDHN1 improved aluminum tolerance in alfalfa (Medicago sativa L.) by affecting oxalate exudation from root tips. Plant J. 2021, 108, 441–458. [Google Scholar] [CrossRef]
- Jing, H.; Li, C.; Ma, F.; Ma, J.H.; Khan, A.; Wang, X.; Zhao, L.Y.; Gong, Z.H.; Chen, R.G. Genome-wide identification, expression divergence of the dehydrin gene family, and characterization of CaDHN3 in pepper (Capsicum annuum L.). PLoS ONE 2016, 11, e0161073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Liu, S.Y.; Ma, J.H.; Wang, X.K.; Haq, S.U.; Meng, Y.C.; Zhang, Y.M.; Chen, R.G. CaDHN4, a salt and cold stress-responsive dehydrin gene from pepper, decreases abscisic acid sensitivity in Arabidopsis. Int. J. Mol. Sci. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Hou, X.M.; Zhang, Y.M.; Meng, Y.C.; Zhang, H.F.; Liu, S.Y.; Wang, X.K.; Chen, R.G. CaDHN5, a pepper dehydrin gene, plays an important role in salt and osmotic stress responses. Int. J. Mol. Sci. 2019, 20, 1989. [Google Scholar] [CrossRef]
- Verma, G.; Dhar, Y.V.; Srivastava, D.; Kidwai, M.; Chauhan, P.S.; Bag, S.K.; Asif, M.H.; Chakrabarty, D. Genome-wide analysis of the rice dehydrin gene family: Evolutionary conservation and expression patterns in response to PEG-induced dehydration stress. PLoS ONE 2017, 12, e0176399. [Google Scholar] [CrossRef]
- Tiwari, P.; Indoliya, Y.; Singh, P.K.; Chauhan, P.S.; Pande, V.; Chakrabarty, D. Role of the dehydrin-FK506-binding protein complex in enhancing drought tolerance via the ABA-mediated signaling pathway. Environ. Exp. Bot. 2019, 158, 136–149. [Google Scholar] [CrossRef]
- Kanbar, A.; Weinert, C.H.; Kottutz, D.; Thinh, L.; Abuslima, E.; Kabil, F.; Hazman, M.; Egert, B.; Trierweiler, B.; Kulling, S.E.; et al. Cold tolerance of woodland strawberry (Fragaria vesca) is linked to Cold Box Factor 4 and the dehydrin Xero2. J. Exp. Bot. 2024, 75, 5857–5879. [Google Scholar] [CrossRef]
- Chauhan, D.K.; Vaishali, Y.; Marek, V.; Walter, G.; Sharon, P.; Namira, A.; Vijay, P.S.; Rupesh, D.; Shivendra, S.; Durgesh, K.T. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Panda, C.K.; Lastochkina, O.; Gavassi, M.A.; Habermann, G.; Pereira, J.F. Aluminum toxicity in plants: Present and future. J. Plant Growth Regul. 2023, 42, 3967–3999. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plants: Benefits, toxicity, and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Liu, J.; Yu, M.; Cuncang, J.; Jiang, C. The Aluminum tolerance and detoxification mechanisms in plants: Recent advances and prospects. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. ClustalW and ClustalX version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Ma, W.X.; Noble, W.S.; Bailey, T.L. Motif-based analysis of large nucleotide datasets using MEME-ChIP. Nat. Protoc. 2014, 9, 1428–1450. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: Upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef]
- Wang, Y.P.; Tang, H.B.; DeBarry, J.D.; Tan, X.; Li, J.P.; Wang, X.Y.; Lee, T.H.; Jin, H.Z.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for the detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Liao, J.J.; Wang, C.H.; Xing, Q.J.; Li, Y.P.; Liu, X.F.; Qi, H.Y. Overexpression and VIGS for functional gene validation in oriental melon (Cucumis melo var. makuwa Makino). Plant Cell Tissue Organ Cult. 2019, 137, 275–284. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, Q.; Cao, S.X.; Tang, Y.F.; Liu, J.Y.; Jin, Y.Z.; Qi, H.Y. Effects of postharvest treatments on expression of three lipoxygenase genes in oriental melon (Cucumis melo var. makuwa Makino). Postharvest Biol. Technol. 2015, 110, 229–238. [Google Scholar] [CrossRef]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions, and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence correlated with increased membrane permeability, lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline levels in water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 292–298. [Google Scholar]
- Zhang, C.; Cao, S.X.; Jin, Y.Z.; Ju, L.J.; Chen, Q.; Qi, H.Y. Melon13-lipoxygenase CmLOX18 may be involved in C6 volatile biosynthesis in fruits. Sci. Rep. 2017, 7, 2816. [Google Scholar] [CrossRef]
- Szabała, B.M. The cationic nature of lysine-rich segments modulates the structural and biochemical properties of wild potato FSK3 dehydrin. Plant Physiol. Biochem. 2023, 194, 480–488. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Rezanejad, F. Effect of low-temperature stress on dehydrin protein, GABA, endogenous proline and antioxidant enzymes in Citrus reticulata extract in postharvest conditions. J. Plant Process Funct. 2024, 13, 79–86. [Google Scholar]
- Hundertmark, M.; Hincha, D.K. LEA (late embryogenesis abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef]
- Liu, H.; Yu, C.; Li, H.; Ouyang, B.; Wang, T.; Zhang, J.; Wang, X.; Ye, Z. Overexpression of ShDHN, a dehydrin gene from Solanum habrochaites enhances tolerance to multiple abiotic stresses in tomatoes. Plant Sci. 2015, 231, 198–211. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wu, J.F.; Fu, D.D.; Zhang, M.; Wang, L.J.; Gong, M.G. Prokaryotic expression, purification, and the in vitro and in vivo protection study of dehydrin WDHN2 from Triticum aestivum. Protoplasma 2024, 261, 771–781. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhu, H.B.; Jin, G.L.; Liu, H.L.; Wu, W.R.; Zhu, J. Genome-scale identification and analysis of LEA genes in rice (Oryza sativa L.). Plant Sci. 2007, 172, 414–420. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.F.; Xu, S.Y.; Jiang, L.W.; Liu, S.Q. Identification and transcriptional analysis of dehydrin gene family in cucumber (Cucumis sativus). Acta Physiol. Plant. 2018, 40, 144. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, S.; Bai, Y.; Sun, H.H.; Jiao, C.; Guo, S.G.; Zhao, K.; Blanca, J.; Zhang, Z.H.; Huang, S.; et al. Cucurbit Genomics Database (CuGenDB): A central portal for the comparative and functional genomics of cucurbit crops. Nucleic Acids Res. 2019, 47, D1128–D1136. [Google Scholar] [CrossRef]
- Eriksson, S.K.; Kutzer, M.; Procek, J.; Gröbner, G.; Harryson, P. The tunable membrane binding of the intrinsically disordered dehydrin Lti30, a cold-induced plant stress protein. Plant Cell 2011, 23, 2391–2404. [Google Scholar] [CrossRef]
- Godoy, J.A.; Lunar, R.; Torres-Schumann, S.; Moreno, J.; Rodrigo, R.M.; Pintor-Toro, J.A. Expression, tissue distribution, and subcellular localization of dehydrin TAS14 in salt-stressed tomato plants. Plant Mol. Biol. 1994, 26, 1921–1934. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.D.; Wang, Y. Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef]
- Liang, D.; Xia, H.; Wu, S.; Ma, F.W. Genome-wide identification and expression profiling of the dehydrin gene family in Malus domestica. Mol. Biol. Rep. 2012, 39, 10759–10768. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, F.; Ouellet, F.; Vazquez-Tello, A. The wheat WCS120 gene family. A useful model to understand the molecular genetics of freezing tolerance in cereals. Physiol. Plant. 1997, 101, 439–445. [Google Scholar] [CrossRef]
- Welin, B.V.; Olson, Å.; Nylander, M.; Palva, E.T. Characterization and differential expression of Dhn/lea/rab-like genes during cold acclimation and drought stress in Arabidopsis thaliana. Plant Mol. Biol. 1994, 26, 131–144. [Google Scholar] [CrossRef]



| Name | ID | Protein Length (bp) | CD Length (aa) | Molecular Weight (kDa) | PI | Chromosome Location | Predicted Location |
|---|---|---|---|---|---|---|---|
| Citrullus lanatus | |||||||
| ClaDHN1 | Cla97C02G031760 | 212 | 639 | 22.17 | 6.07 | chr02:4613149 4615148 (−) | Nucleus |
| ClaDHN2 | Cla97C07G135750 | 181 | 546 | 19.29 | 6.87 | chr07:21540677 21541876 (+) | Nucleus |
| ClaDHN3 | Cla97C08G152370 | 228 | 687 | 25.58 | 5.2 | chr08:20736178 20737049 (+) | Nucleus |
| ClaDHN4 | Cla97C11G210870 | 180 | 543 | 19.39 | 8.05 | chr11:4212633 4215064 (−) | Nucleus |
| Lagenaria siceraria | |||||||
| LsiDHN1 | Lsi07G002410 | 165 | 498 | 18.04 | 9.00 | chr07:2648912 2650391 (−) | Nucleus |
| LsiDHN2 | Lsi07G002420 | 111 | 336 | 12.83 | 6.28 | chr07:2652684 2653807 (−) | Peroxisome |
| LsiDHN3 | Lsi08G008060 | 228 | 687 | 25.67 | 5.17 | chr08:16443433 16444990 (+) | Nucleus |
| LsiDHN4 | Lsi10G003970 | 171 | 516 | 18.21 | 6.8 | chr10:6057706 6060658 (+) | Nucleus |
| LsiDHN5 | Lsi11G011220 | 202 | 609 | 20.85 | 6.8 | chr11:19658787 19661161 (+) | Nucleus |
| Benincasa hispida | |||||||
| BhiDHN1 | Bhi01G001874 | 105 | 318 | 12.12 | 6.43 | chr1:58655060 58655377 (+) | Nucleus |
| BhiDHN2 | Bhi01G001875 | 171 | 516 | 18.69 | 6.93 | chr1:58672054 58673439 (+) | Nucleus |
| BhiDHN3 | Bhi04G001222 | 228 | 687 | 25.81 | 5.43 | chr4:39155443 39156748 (−) | Nucleus |
| BhiDHN4 | Bhi04G001643 | 55 | 168 | 6.2 | 9.22 | chr4:55256231 55256398 (−) | Nucleus |
| BhiDHN5 | Bhi05G001121 | 172 | 519 | 18.38 | 6.06 | chr5:45299333 45301282 (+) | Nucleus |
| BhiDHN6 | Bhi06G000833 | 77 | 234 | 8.9 | 5.76 | chr6:25580125 25580635 (+) | Cytoplasm |
| BhiDHN7 | Bhi09G000580 | 69 | 210 | 7.9 | 8.76 | chr9:15488834 15489043 (+) | Nucleus |
| BhiDHN8 | Bhi09G000581 | 49 | 150 | 5.5 | 10.27 | chr9:15569525 15569674 (−) | Nucleus |
| BhiDHN9 | Bhi09G001205 | 71 | 213 | 7.8 | 9.72 | chr9:37216318 37216530 (+) | Nucleus |
| BhiDHN10 | Bhi10G001401 | 70 | 213 | 8.1 | 9.67 | chr10:44590139 44590351 (+) | Nucleus |
| BhiDHN11 | Bhi10G001578 | 154 | 465 | 16.54 | 7.21 | chr10:49044633 49046400(−) | Nucleus |
| BhiDHN12 | BhiUN737G11 | 126 | 381 | 14.54 | 6.59 | contig737:226811 227501(+) | Cytoplasm |
| BhiDHN13 | BhiUN737G17 | 70 | 213 | 8.0 | 9.84 | contig737:325991 326203 (+) | Nucleus |
| Cucumis sativus | |||||||
| CsaDHN1 | Csa016162 | 177 | 534 | 19.18 | 7.26 | chr4:3543549 3544599 (−) | Nucleus |
| CsaDHN2 | Csa016163 | 236 | 711 | 26.63 | 5.17 | chr6:16107424 16109149 (+) | Nucleus |
| CsaDHN3 | Csa013074 | 162 | 489 | 17.47 | 8.11 | chr2:12194592 12196235 (+) | Nucleus |
| CsaDHN4 | Csa020767 | 100 | 303 | 11.41 | 6.13 | chr4:3546270 3546572 (−) | Nucleus |
| Cucumis melo | |||||||
| CmDHN1 | MELO3C016401.2 | 303 | 100 | 11.28 | 5.39 | chr07:24180093 24180395 (+) | Nucleus |
| CmDHN2 | MELO3C016402.2 | 534 | 177 | 18.95 | 7.28 | chr07:24182063 24183504 (+) | Nucleus |
| CmDHN3 | MELO3C023323.2 | 477 | 158 | 16.9 | 8.89 | chr11:1435823 1437281 (+) | Nucleus |
| CmDHN4 | MELO3C026550.2 | 714 | 237 | 26.43 | 4.95 | chr03:24830447 24832398 (+) | Nucleus |
| Cucurbita maxima | |||||||
| CmaDHN1 | CmaCh00G004390 | 200 | 603 | 21.66 | 7.11 | Chr00:33264719 33266319 (+) | Nucleus |
| CmaDHN2 | CmaCh04G007640 | 172 | 519 | 18.5 | 7.28 | Chr04:3882339 3883409 (−) | Nucleus |
| CmaDHN3 | CmaCh05G009950 | 222 | 669 | 24.65 | 5.25 | Chr05:7991924 7993668 (+) | Nucleus |
| CmaDHN4 | CmaCh20G008280 | 204 | 615 | 21.51 | 6.84 | Chr20:3941796 3943080 (−) | Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Chen, Q.; Guo, X.; Pang, H.; Zhang, Y. Aluminum Stress of Oriental Melon (Cucumis melo L.) Is Linked to the Dehydrin CmDHN3. Horticulturae 2025, 11, 480. https://doi.org/10.3390/horticulturae11050480
Zhang C, Chen Q, Guo X, Pang H, Zhang Y. Aluminum Stress of Oriental Melon (Cucumis melo L.) Is Linked to the Dehydrin CmDHN3. Horticulturae. 2025; 11(5):480. https://doi.org/10.3390/horticulturae11050480
Chicago/Turabian StyleZhang, Chong, Qiang Chen, Xinqi Guo, Hongbo Pang, and Ying Zhang. 2025. "Aluminum Stress of Oriental Melon (Cucumis melo L.) Is Linked to the Dehydrin CmDHN3" Horticulturae 11, no. 5: 480. https://doi.org/10.3390/horticulturae11050480
APA StyleZhang, C., Chen, Q., Guo, X., Pang, H., & Zhang, Y. (2025). Aluminum Stress of Oriental Melon (Cucumis melo L.) Is Linked to the Dehydrin CmDHN3. Horticulturae, 11(5), 480. https://doi.org/10.3390/horticulturae11050480




