Abstract
Under nursery conditions, various organic and inorganic growing media can be used for plant propagation. However, a common fertigation program may have varying effects on plant performance. This study evaluated alternative growing media under the same fertigation scheme in three indigenous Cypriot grapevine cultivars (Xynisteri, Maratheftiko, Giannoudi). Rooted cuttings were grown in pots containing soil, perlite, river sand, peat, and cocosoil. The plants were fertigated with a hydroponic nutrient solution with an electrical conductivity of 2.4 dS/m and a pH of 5.8. Xynisteri grown in peat and cocosoil accumulated minerals such as N and P while showing reduced levels of Na, total phenols, antioxidant capacity, and total flavonoids in the leaves. Additionally, plants exhibited low hydrogen peroxide and malondialdehyde (MDA) content, indicating a non-stressful growing environment. Maratheftiko cultivar accumulated N in perlite, K in cocosoil, and P in peat and cocosoil media. When grown in soil, Maratheftiko showed higher phenol content and increased antioxidant capacity, which is correlated with elevated oxidative stress (higher MDA). Giannoudi appeared to be more adapted to soil and/or cocosoil media, as evidenced by its lower MDA content, total phenols, total flavonoids, and antioxidant activity, compared to plants grown in perlite, sand, and peat. Chlorophyll and total carotenoid levels were increased in Giannoudi grown in soil. In conclusion, both growing media and fertigation practices should be tailored to optimize plant performance under nursery conditions.
1. Introduction
According to the International Organization of Vine and Wine [1], the total global vineyard area is expected to reach 7.3 million hectares in the coming years, with approximately 3.3 million hectares (about 45%) located in the European Union. Mediterranean nations account for 40% of the world’s vineyard area, supporting millions of producers and wine industry workers [2]. Cyprus is recognized as having the longest winemaking heritage in the Mediterranean, with over 5500 years of history and a vineyard area exceeding 7000 hectares [3]. The island of Cyprus is home to more than ten indigenous grapevine cultivars, while in recent decades, several international cultivars have been introduced and their cultivation has expanded [4].
Grapevine cultivars vary widely across different regions of the world, with thousands of cultivars adapted to diverse climate conditions. This presents challenges for growers and nursery producers in standardizing cultivation management and the techniques applied [5]. While internationally recognized grapevine cultivars are widely used in different regions, indigenous cultivars have shown remarkable resilience to climate change challenges, often outperforming the international ones [6,7,8]. Previous reports have shown that the indigenous white Xynisteri cultivar adapts well to drought and heat waves, performing better under stress conditions than the international Chardonnay cultivar [9]. Additionally, Xynisteri had a lower carbon footprint production compared to the introduced Cabernet Sauvignon wine cultivar [4]. Similarly, the indigenous red Maratheftiko cultivar has demonstrated strong performance under drought conditions and various cultivation practices [3].
Grapevines are primarily produced by the vegetative propagation method [10]. Stem cutting offers several benefits, including cost-effectiveness, minimal space requirements, ease of execution, and, most importantly, the ability to produce true-to-type plants rapidly [11,12]. In nursery settings, grapevine cutting production and the initial stages of plant growth take place under controlled conditions, where a standardized growing environment and fertigation (providing mineral fertilizers through the irrigation system) scheme are desirable but not always achievable. However, grapevine cuttings are traditionally produced in the soil as growing media [13], though soil fertility and physicochemical properties can negatively impact plant development and crop yield [14]. Soilless cultivation, commonly known as hydroponics (which is the practice of growing plants in a nutrient solution with or without a soilless substrate to provide physical support), offers a promising alternative to the soil-based cultivation technique. It provides numerous benefits, including water and fertilizer savings, increased yields, improved quality of the harvested products [15,16], and reduced greenhouse gas emissions [17]. Previous studies have shown that the use of magnetized (after passed from a magnetic field generated by an apparatus) nutrient solution (NS), in hydroponically grown grapevines can act as an elicitor, simulating stress conditions and influencing various physiological and biochemical processes [14].
The effectiveness of different growing media used in soilless cultivation has attracted significant research interest in recent decades, with peat remaining the primary growing medium [18,19,20]. Although peat is the leading substrate used in greenhouse nurseries, it is an expensive and non-renewable resource with environmental constraints [21]. Consequently, researchers have focused on reducing peatland exploitation and exploring alternative materials as growing media with appropriate physicochemical properties [22,23]. Increased research efforts aim to either improve soil conditions where possible or identify suitable alternatives to peat [24,25,26,27]. Growing media play a crucial role in the viability and health of grapevine stem cuttings [13]. Several studies have investigated different growing media and their combinations for grapevine cutting production. For example, the development of Pusa Navrang variety cuttings was tested in soil alone or in mixtures of soil with sand, vermicompost, and cocopeat [13]. Similarly, grapevine cultivars Sundar Khani and Thompson were tested in soil, compost, sand, manure, and various combinations of these materials [10], highlighting the advantages of combining different substrates. Thompson Seedless grapevines have also been successfully cultivated in perlite under soilless conditions [14]. In addition, organic materials such as hazelnut husk compost and tea residue compost have been used in grapevine production and compared with soil, perlite, and manure [28].
Most studies on grapevine production under nursery conditions have focused on plant growth and rooting parameters [29,30,31], while relatively few have examined the mineral absorption and antioxidant capacity of the plants [14]. The present study evaluated the use of common growing media in nursery conditions, including mainly peat, followed by perlite, cocosoil, and sand. The above-mentioned media were compared with the commonly used soil for grapevine propagation, under the application of the same fertigation practice. This was achieved by employing a full-strength nutrient solution with the same mineral composition. This research is the first to assess alternative growing media in the three indigenous Cypriot grapevine cultivars during the cutting vegetative propagation stage, and their effects on plant growth, nutrient uptake, and biochemical responses, under nursery conditions.
2. Materials and Methods
2.1. Grapevine’s Cultivars and Growing Media Preparation
The present research was conducted at the greenhouse facilities, at Cyprus University of Technology, in Limassol, Cyprus, during the summer period of 2022. Three endemic Cypriot grapevine cultivars (Xynisteri, Maratheftiko, and Giannoudi) were selected due to the increased interest from both local and international markets. Xynisteri is a white cultivar, widely recognized by farmers for its tolerance to water deficit conditions [3]. Maratheftiko and Giannoudi are both red cultivars, with the former exhibiting moderate resistance to water shortages [3]. Limited information exists on Giannoudi, apart from its reputation as one of the most expensive Cypriot wines, recently gaining popularity in the local market [32]. However, Giannoudi cultivation practices were never studied before. Cuttings were collected in late January 2022 after pruning from mother plants (aged 18-year-old for Xynisteri, 12-year-old for Maratheftiko, 15-year-old for Giannoudi) in commercial vineyards located in Malia village, Limassol, Cyprus. Rooting was stimulated using Atonik SL (Asahi Chemical Europe s.r.o., Prague, Czech Republic), a synthetic biostimulant containing 0.3% sodium 5-nitroguaiacolate, 0.6% sodium o-nitrophenolate, and 0.9% sodium p-nitrophenolate (w/v). The biostimulant was exogenously applied by dipping the cuttings in a 7 mL/L H2O solution for 24 h, based on manufacturer’s recommendation and prior trials. Following treatment, cuttings (length of ~50 cm and width of 0.9–1.2 cm) were placed in a peat–perlite mixture (1:5 v/v) and periodically watered to promote rooting. After 50 days following initial bud outgrowth, cuttings were pruned to the three-bud stage to better support the rooting. Then, after 75 days from the Atonik SL application, rooted cuttings (with similar root volume) were transplanted into 7 L pots, containing different growing substrates. Two inorganic (perlite and sand) and two organic (peat and cocosoil) materials were used as a growing media and compared with vineyard soil collected from commercial vineyards in the Malia region and transferred to the greenhouse. The physicochemical properties of perlite [33], peat [23], and cocosoil [34] have been previously documented. Sand had a pH of 7.85, an electrical conductivity (EC) of 0.12 dS/m, and negligible mineral content. The soil had a clay loam texture, with an organic matter of 2.13%, a total CaCO3 content of 64.6%, a pH of 7.38, and an EC of 0.29 dS/m.
Each treatment (growing media) was tested with 12 replicate pots for each of the three examined grapevine cultivars. Each pot was placed in plastic trays to facilitate water absorption. Plants were fertigated with a full-strength NS, through plastic trays using capillary suction, and grown in the greenhouse under natural lighting. The composition of the NS was nitrogen (NO3−-N) = 14.96 mmol/L, potassium (K) = 8.75 mmol/L, phosphorus (PO43−-P) = 1.07 mmol/L, calcium (Ca) = 7.22 mmol/L, magnesium (Mg) = 3.04 mmol/L, sulfur (SO42−-S) = 3.37 mmol/L, and sodium (Na) = 0.34 mmol/L, respectively; while the micronutrient content was as follows: boron (B) = 25.00 μmol/L, iron (Fe) = 20.00 μmol/L, manganese (Mn) = 10.00 μmol/L, copper (Cu) = 0.77 μmol/L, zinc (Zn) = 4.00 μmol/L, and molybdenum (Mo) = 0.50 μmol/L, respectively [35]. The EC of the NS was adjusted to 2.4 dS/m and the pH was set to 5.8. No insecticides or protective agents were applied during plant growth. The average air temperature and relative humidity throughout the growing period were 27.2 °C and 61.8%, respectively, as it was recorded by the meteorological station in the greenhouse.
2.2. Plant Growth and Physiology Attributes
Leaf photochemistry was assessed by measuring the relative chlorophyll content using an optical chlorophyll meter (SPAD-502, Minolta, Osaka, Japan). Leaf chlorophyll fluorescence (Fv/Fm), which represents the maximum quantum efficiency of PSII, was recorded on two sun-exposed leaves for nine plants per cultivar and growing media using a fluorometer (Opti-sciences, OS-30p, Hertfordshire, UK) on a weekly basis.
After 39 days (end of the experiment), various plant growth and physiological attributes were evaluated in six cuttings per growing medium and per cultivar. The cuttings’ height (measured from the surface of the growing medium) and the leaf number were recorded. Plant tissue (leaves and stems) were sampled and used as raw material or dry material for further analysis.
At the end of the experiment, leaf chlorophyll content was assessed (six replications per treatment), following extraction with dimethyl sulfoxide (DMSO) [36]. The contents of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophylls (total Chl), and total carotenoids were calculated using the equations by Wellburn [37]. The results were expressed as mg of pigment per gram of fresh weight (FW). The ratio of Chl a:Chl b and Total Car/Total Chls were also computed.
2.3. Total Phenolics, Total Flavonoids, and Antioxidant Activity
Six leaf samples from each treatment and cultivar were used for polyphenol extraction. Samples (0.7 g) were extracted using methanol and the extracts were stored at −20 °C until analysis. Total phenols were determined from methanolic extracts using the Folin–Ciocalteu method (at 755 nm) with a microplate spectrophotometer (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA), as previously described [36]. Results were expressed in gallic acid equivalents (mg GAE/g of FW). The content of total flavonoids was determined based on the aluminum chloride colorimetric method [38], following modifications by Chrysargyris et al. [36]. The absorbance was measured at 510 nm and the content of total flavonoids was presented in rutin equivalents (mg rutin/g of FW).
The activity of free radical scavenging was measured as previously described [36], by employing three assays: the 2,2-diphenyl-1-picrylhydrazyl (DPPH), the ferric reducing antioxidant power (FRAP), and the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS) methods. Specifically, the DPPH radical scavenging activity of the plant methanolic extracts was measured at 517 nm, and FRAP activity was measured at 593 nm, as described in Chrysargyris et al. [36]. The ABTS assay was implemented according to the methodology described by Woidjylo et al. [39]. Results were expressed as Trolox ((±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) equivalents (mg trolox/g of FW).
2.4. Hydrogen Peroxide Content and Lipid Peroxidation
Hydrogen peroxide (H2O2) content was evaluated following the method of Loreto and Velikova [40], using six leaf samples (two individual plants were pooled per sample) for each treatment. The H2O2 concentration was calculated using standards ranging from 5 to 1000 μM of H2O2 after absorbance at 390 nm and results were reported as μmol H2O2/g of FW.
Lipid peroxidation was quantified in terms of malondialdeyde content (MDA), as described by De Azevedo Neto et al. [41]. The absorbance was measured at 532 nm and corrected for non-specific absorbance at 600 nm. The quantity of MDA was calculated using an extinction coefficient of 155/mM/cm. The results were given in nmol of MDA/g of FW.
2.5. Plant Mineral Content
Leaf mineral content was determined using four replicates per treatment and cultivar. Plant samples were dried to a constant weight (at 65 °C for 3 d) and ground to 0.42 mm. The dried plant tissue (~0.3 g) was placed in an ash furnace (Carbolite, AAF 1100, GERO, Neuhausen, Germany) and ashed at 490 °C for 6 h. Afterward, the ash was then acid-digested with 2 N HCl and diluted to a final volume of 50 mL to facilitate the mineral measurements. Potassium (K) and sodium (Na) were determined employing a flame photometer (Lasany Model 1832, Lasany International, Panchkula, India), phosphorus (P) by spectrophotometry (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA), and nitrogen (N) by the Kjeldahl method (BUCHI, Digest automat K-439 and Distillation Kjelflex K-360, Flawil, Switzerland), following Chrysargyris et al. [23]. The data were reported in g/kg of dry weight (DW).
2.6. Statistical Analysis
The experimental design was a completely randomized design, examining the effect of the different growing media in grapevine cultivars. The IBM SPSS v22.0 (SPSS Inc., Chicago, IL, USA) program was used for statistical analysis. The significance of differences in average values was determined using Duncan’s Multiple Range Test (DMRT) at p ≤ 0.05 after a one-way analysis of variance (ANOVA).
3. Results and Discussion
Plant height and leaf number varied among the different tested growing media for each cultivar (Figure 1). In Xynisteri, plant height was significantly greater in plants grown in soil, peat, and cocosoil compared to those grown in inorganic materials such as perlite and sand (Figure 1A). A similar trend was observed for leaf number, with plants grown in sand producing significantly fewer leaves than those in the other treatments (Figure 1B). For Maratheftiko, plant height was the highest in perlite- and peat-grown plants, while the greatest number of leaves was recorded in plants grown in cocosoil (Figure 1A,B). In Giannoudi, plants grown in cocosoil exhibited the greatest height, whereas those grown in the sand had the lowest leaf number (Figure 1A,B). Previous studies have shown that the leaf number in grapevines was positively influenced by the combination of different components in the growing media, rather than by the use of a single material [42]. For instance, the highest leaf number was observed in the NARC black grapevine cultivar grown in a soil/sand mixture, while the lowest was recorded in the Perlette cultivar grown in sand [31]. In general, growing media influenced leaf number, leaf development, and flowering potential in various crops [34,43].
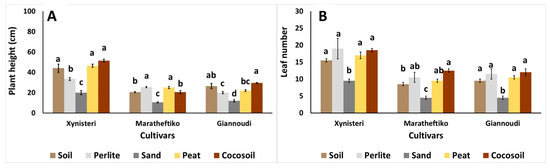
Figure 1.
Impact of growing media (soil, perlite, sand, peat, and cocosoil) on (A) plant height (cm) and (B) leaf number in Xynisteri, Maratheftiko, and Giannoudi plants. Values are mean ± SE (n = 6). Mean values followed by the same letter for each cultivar do not differ significantly at p ≥ 0.05 according to Duncan’s MRT.
Different growing media affected plant photosynthetic performance in various cultivars (Table 1), with Xynisteri being the least affected by the examined growing media, compared to Maratheftiko and Giannoudi. Xynisteri plants grown in perlite revealed the highest SPAD values, whereas those grown in peat had the lowest SPAD values and the highest total carotenoid content. Weekly monitoring of SPAD and chlorophyll fluorescence revealed that Xynisteri plants grown in organic media (cocosoil and peat) had lower SPAD and Fv/Fm values during the first 2–3 weeks, but these differences were diminished by the 5th week (Figure S1).

Table 1.
Impact of growing media (soil, perlite, sand, peat, and cocosoil) on chlorophyll fluorescence (Fv/Fm), SPAD values, chlorophylls (Chl a, Chl b, total Chls; mg/g FW) and total carotenoids (total Car; mg/g FW) content, Chl a:Chl b and Total Car/Total Chls ratios, in Xynisteri, Maratheftiko, and Giannoudi plants.
In Maratheftiko, plants grown in perlite had the highest chlorophyll content (Chl a, Chl b, total Chls) but lower total carotenoid content and Chl a:Chl b and total carotenoids/total chlorophylls ratios (Table 1). Since Chl b is converted to Chl a during chlorophyll degradation [44], this explains the lower chlorophyll content and the higher Chl a:Chl b ratio in plants grown in soil, sand, peat, and cocosoil. Leaf chlorophyll fluorescence was higher in plants grown in perlite, peat, and cocosoil, compared to those in soil and sand. Maratheftiko plants grown in sand exhibited the lowest SPAD and chlorophyll fluorescence values compared to plants grown in other media, which was evident from the first week of plant growth (Figure S1).
In Giannoudi, plants grown in soil showed increased chlorophyll and carotenoid content, while those grown in perlite had the lowest values. Giannoudi plants grown in the sand had the lowest SPAD values and total carotenoids/total chlorophylls ratio. The SPAD values decreased after the 4th week, while chlorophyll fluorescence decreased only during the first 2 weeks of plant growth (Figure S1). This suggests that photosynthetic efficiency was influenced by the growing media and growth period for the different cultivars. Chlorophyll fluorescence (Fv/Fm) is a non-destructive indicator of recording the photosynthetic efficiency of the plants. Most plants exhibit strong photosynthetic performance when Fv/Fm averages around 0.8. For the tested grapevine cultivars, these values were averaged at 0.779 in Xynisteri, 0.741 in Maratheftiko, and 0.723 in Giannoudi. However, reduced values around 0.60 suggested plant stress [45]. Notably, Maratheftiko and Giannoudi grown in sand had significantly lower Fv/Fm values, suggesting plant stress conditions under sand substrate (Table 1). Previous reports indicated that grapevines grown in soil and perlite revealed lower SPAD values compared to those grown in organic media [28]. However, the present study differs, likely due to the standardized fertigation scheme using hydroponic nutrient solution and/or differences in cultivar selection and experimental setup.
Growing media significantly affected the phenolic and flavonoid content of plants, as well as the antioxidant capacity of the investigated grapevine cultivars, as illustrated in Figure 2. In Xynisteri, plants grown in organic-based materials (peat, cocosoil) exhibited reduced total phenolic content (up to 43.5%; Figure 2A), total flavonoids content (up to 41.1%; Figure 2B), and antioxidant capacity (DPPH, FRAP and ABTS; up to 59.7%, 52.2% and 55.7%, respectively; Figure 2C–E) compared to those grown in soil, perlite, or sand (Figure 2). These reductions could indicate that Xynisteri was either more tolerant to stress conditions in organic-based media or responded to stress earlier, thereby stabilizing stress responses by the end of the experiment. Future studies with periodic sampling (e.g., weekly) could confirm these hypotheses.
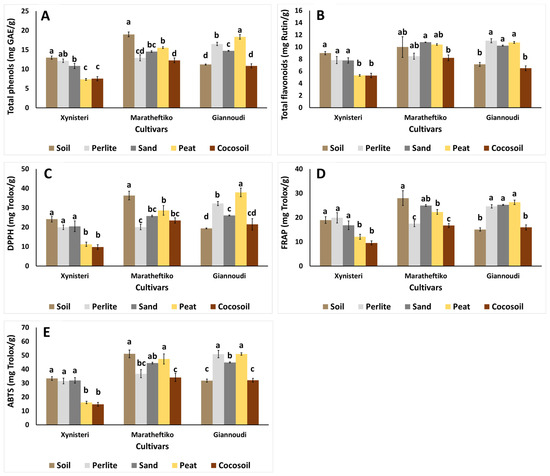
Figure 2.
Impact of growing media (soil, perlite, sand, peat, and cocosoil) on (A) total phenols (mg GAE/g FW), (B) total flavonoids (mg rutin/g FW), and antioxidant activity of (C) DPPH, (D) FRAP, and (E) ABTS (mg trolox/g FW) in Xynisteri, Maratheftiko, and Giannoudi plants. Values are mean ± SE (n = 6). Mean values followed by the same letter for each cultivar do not differ significantly at p ≥ 0.05, according to Duncan’s MRT.
In Maratheftiko, plants grown in soil had the highest total phenolic content (Figure 2A) and antioxidant capacity, as assayed by the DPPH, FRAP, and ABTS (Figure 2C–E). Maratheftiko plants grown in cocosoil had lower total flavonoid content (Figure 2B) compared to those grown in sand, whereas decreased antioxidant capacity was observed in plants grown in perlite, sand, and cocosoil. In Giannoudi, plants grown in peat and in perlite exhibited increased phenolics, with the former resulting in the highest content of total phenols (Figure 2A). Interestingly, plants grown in soil and cocosoil had the lowest content of total phenols (Figure 2A), total flavonoids (Figure 2B), and antioxidant capacity (Figure 2C–E). Previous studies indicated that Xynisteri was less affected by drought and heat stress than Maratheftiko, as it developed a coping mechanism within the first 4 days by closing stomata and stimulating antioxidant capacity [9,46]. The relevant resistance of the examined cultivars to stress events may be attributed to their adaptation to the local environment. Prior research had demonstrated that Xynisteri was more resilient to environmental stressors, such as heat and/or drought, compared to the international cultivar Chardonnay [9,47]. Plants cope with stress conditions in order to reduce reactive oxygen species, by activating several enzymatic (superoxide dismutase, catalase, peroxidase, glutathione peroxidase, glutathione reductase, glutathione S-transferases, ascorbate peroxidase, monodehydroascorbate reductase, and dehydroascorbate reductase) [48] and non-enzymatic (phenols, flavonoids, ascorbic acid, proline, etc.) responses [49]. For instance, Xynisteri produced more abscisic acid (ABA) than Chardonnay in response to drought stress. In the same study, it was concluded that ABA plays a more prominent role in disease resistance than jasmonic acid and salicylic acid [47]. In the Mediterranean region, leaves from several grapevine cultivars are harvested and used as edible leaves [50]. In such cases, methods that mimic oxidative stress to induce antioxidant capacity in edible plant parts may be highly desirable.
Plants have developed several detoxification mechanisms to mitigate the accumulation of reactive oxygen species (ROS) in cells under stress [51]. One of the most widely used stress markers is the elevated concentration of malondialdehyde (MDA), which is linked to increased lipid peroxidation under stress. MDA levels rise when plant antioxidants lose their ability to scavenge ROS, serving as an initial step in detoxifying stressors [52,53]. Stress conditions may arise due to inappropriate material mixtures, inadequate physicochemical properties of the growth media, or mineral imbalances [54]. Plants exhibit varying responses to stressors, displaying either sensitivity or tolerance. In Xynisteri, MDA levels were elevated in plants grown in sand (Figure 3B). This corresponded with the increased levels of H2O2 (Figure 3A), indicating stress conditions associated with sand as a growing medium. In contrast, plants grown in organic media, such as peat and cocosoil, exhibited lower MDA and H2O2 content.
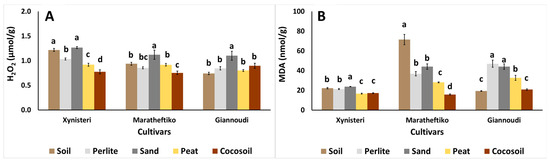
Figure 3.
Impact of growing media (soil, perlite, sand, peat, and cocosoil) on (A) hydrogen peroxide- H2O2 (μmol/g), and (B) lipid peroxidation-MDA (nmol/g) in Xynisteri, Maratheftiko, and Giannoudi plants. Values are mean ± SE (n = 6). Mean values followed by the same letter for each cultivar do not differ significantly at p ≥ 0.05, according to Duncan’s MRT.
Cocosoil proved to be the most suitable growing media for Maratheftiko, as evidenced by reduced MDA and H2O2 content (Figure 3A,B). Plants grown in soil revealed nearly double MDA levels compared to those grown in perlite or sand and four times higher MDA levels than those grown in peat or cocosoil. For Giannoudi, MDA levels were found elevated in plants grown in perlite and sand, indicating stress conditions, while plants grown in soil or cocosoil displayed lower MDA levels (Figure 3B). Furthermore, plants grown in sand exhibited higher H2O2 content compared to those grown in soil, perlite, peat, or cocosoil (Figure 3A).
The increased H2O2 levels observed in the plants appear to be linked to the stimulation of non-enzymatic antioxidants, including total phenols, total flavonoids, FRAP, DPPH, and ABTS. Less stressed plants can allocate more resources to chlorophyll production rather than stress responses, leading to increased chlorophyll content [55]. This was evident in Maratheftiko grown in perlite and Giannoudi grown in soil, where increased chlorophyll content was accompanied by reduced MDA levels.
Mineral accumulation in plants was influenced by the growing media used with variations observed among the tested cultivars (Figure 4). In Xynisteri, leaf N and K contents were increased in plants grown in cocosoil, while the lowest values were found in plants grown in the sand (Figure 4A,B). The phosphorus content was higher in plants grown in peat, whereas plants grown in sand had the lowest P levels compared to the examined treatments (Figure 4C). Sodium accumulation was lower in plants grown in organic-based media, peat, and cocosoil, compared to the soil, perlite, and sand-growing media (Figure 4D).
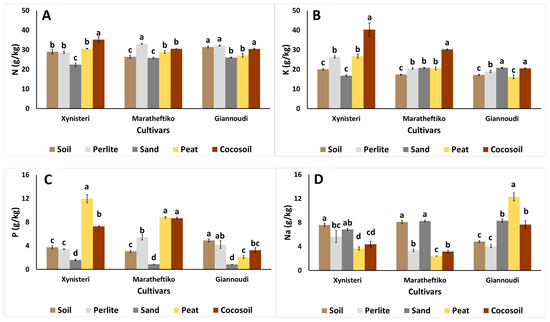
Figure 4.
Impact of growing media (soil, perlite, sand, peat, and cocosoil) on (A) nitrogen—N, (B) potassium—K, (C) phosphorus—P, and (D) sodium—Na content (g/kg) in Xynisteri, Maratheftiko, and Giannoudi plants. Values are mean ± SE (n = 4). Mean values followed by the same letter for each cultivar do not differ significantly at p ≥ 0.05, according to Duncan’s MRT.
For Maratheftiko, N accumulation was the highest in plants grown in perlite, K was the highest in those grown in cocosoil, and P was more abundant in plants grown in peat and cocosoil (Figure 4A–C). In contrast, Na was significantly accumulated in plants grown in soil and sand, while its levels were lower in plants grown in perlite, peat, and cocosoil (Figure 4D).
Giannoudi responded differently to growing media compared to Xynisteri and Maratheftiko. Nitrogen accumulation was higher in plants grown in soil, perlite, and cocosoil, and lower in plants grown in sand and peat (Figure 4A). Potassium levels were increased in plants grown in sand and cocosoil (Figure 4B), while P accumulation was lower in sand-grown plants (Figure 4C). Sodium accumulation was the highest in plants grown in peat, followed by those grown in sand and cocosoil (Figure 4D).
It has been previously reported that chlorophyll, flavonoid, and anthocyanin content in leaves serves as markers of photosynthetic capacity and plant vigor, which are closely linked to N uptake [56]. This correlation was evident in Maratheftiko, where increased N uptake in perlite-grown plants coincided with higher chlorophyll content, which may improve plant growth in the next developmental stages. Singh and Chauhan [57] reported that grapevines grown in soil had better rooting and plant growth compared to those grown in sand, due to the lower mineral content and buffering capacity of the latter. Indeed, early plant growth is closely associated with adequate P availability and uptake capacity [58], and the present findings indicate that sand as a growing medium restricted P uptake across all tested cultivars compared to soil. Phosphorus deficiency affects the first step of chlorophyll fluorescence [59], as indicated in plants grown in sand-growing media that had the lowest P levels and decreased chlorophyll fluorescence (Figure S1).
The variation in mineral accumulation observed in this study was likely influenced by the physicochemical properties of the examined growing media [33,60,61] and the cultivar-specific responses, as all plants received the same volume of nutrient solution with a consistent composition. Previous research has shown that both growing media (e.g., cocopeat, basaltic tuff, peat/perlite) and mineral concentrations (N and K) significantly impacted grapevine growth and mineral uptake, with higher nutrient levels leading to improved plant performance [62]. Notably, in this study, the N (NO3−-N: 14.96 mmol/L) and K (K: 8.75 mmol/L) concentrations used in the NS were in line with the higher mineral levels used in the study of Tangolar et al. [62].
In addition to effectiveness, the cost of purchase and availability of growing media in the market are key factors to consider. The price of peat varies, typically costing around EUR 25.0/m3, while coconut fiber (cocosoil) is priced at approximately EUR 20.0/m3 and green compost at EUR 12.5/m3. Other materials, such as biochar, can be up to five times more expensive than peat [63]. Furthermore, Mediterranean countries, including Cyprus, often rely on imports for both peat and cocosoil. Environmental concerns also play a crucial role in substance selection. Peat extraction has a significant carbon footprint compared to alternative materials such as wood fiber, compost, and hydrochar-based components [64]. To mitigate the environmental impact of peat use, researchers have suggested mixing peat with other materials to achieve optimal physicochemical properties, including total porosity, air capacity, and water-holding capacity [23,55,65]. Additionally, natural substances such as Aloe vera gel, cinnamon powder, and undiluted honey have been reported to enhance grapevine cutting growth when applied as rooting treatments [66].
4. Conclusions
Plant propagation primarily takes place in nurseries, an intensive agricultural sector that requires a high amount of fertilizers, water, resources, labor, and space. In the present study, under a uniform fertigation scheme using a hydroponic nutrient solution, Xynisteri grown in organic materials (peat and cocosoil) effectively accumulated minerals as N and P but exhibited decreased total phenols, flavonoids, and antioxidant status due to the reduced oxidative stress. Maratheftiko accumulated different minerals depending on the growing medium; however, when cultivated in soil, lipid peroxidation was observed to induce oxidative stress, activating various plants’ antioxidant mechanisms. Giannoudi appeared to be more adapted to soil and/or cocosoil media, exhibiting lower MDA content, total phenols, total flavonoids, and antioxidant status compared to plants grown in perlite, sand, and peat media. These findings suggest that both substrate selection and fertigation practices must be tailored to each cultivar to optimize plant performance in a nursery environment. However, in cases where grapevine cutting propagation takes place outside of nurseries, directly by the farmers, various environmental conditions might affect the influence of growing media on oxidative stress and antioxidant responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11050479/s1, Figure S1: Impact of growing media (soil, perlite, sand, peat, and cocosoil) on Xynisteri, Maratheftiko, and Giannoudi cuttings chlorophyll fluorescence (Fv/Fm; as the maximum quantum efficiency of PSII), and SPAD on a weekly basis.
Author Contributions
Conceptualization, N.T.; methodology, A.C. and N.T.; software, A.C.; validation, A.C. and N.T.; formal analysis, A.C. and N.T.; investigation A.C. and N.T.; resources, N.T.; data curation; A.C. and N.T.; writing—original draft preparation, A.C. and N.T.; writing—review and editing, A.C. and N.T.; visualization, A.C.; supervision, N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was financed by PRIMA (MiDiVine project), a program supported by the European Union with co-funding by the Funding Agencies RIF—Cyprus.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Organization of Vine and Wine State of the World Vine and Wine Sector 2021. Int. Organ. Vine Wine Intergov. Organ. 2022, 1–19. Available online: https://www.oiv.int/sites/default/files/documents/OIV_State_of_the_world_Vine_and_Wine_sector_in_2022_2.pdf (accessed on 4 March 2025).
- Santillán, D.; Sotés, V.; Iglesias, A.; Garrote, L. Adapting viticulture to climate change in the Mediterranean region: Evaluations accounting for spatial differences in the producers-climate interactions. BIO Web Conf. 2019, 12, 01001. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Litskas, V.; Mandoulaki, A.; Antoniou, D.; Boyias, T.; Stavrinides, M.; Tzortzakis, N. Drought stress and soil management practices in grapevines in Cyprus under the threat of climate change. J. Water Clim. Chang. 2018, 9, 703–712. [Google Scholar] [CrossRef]
- Litskas, V.D.; Irakleous, T.; Tzortzakis, N.; Stavrinides, M.C. Determining the carbon footprint of indigenous and introduced grape varieties through Life Cycle Assessment using the island of Cyprus as a case study. J. Clean. Prod. 2017, 156, 418–425. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Copper, A.W.; Johnson, T.E.; Danner, L.; Bastian, S.E.P.; Collins, C. Preliminary Sensory and chemical profiling of Cypriot wines made from indigenous grape varieties Xynisteri, Maratheftiko and Giannoudhi and acceptability to Australian consumers. OENO One 2019, 53, 229–248. [Google Scholar] [CrossRef]
- Koufos, G.; Mavromatis, T.; Koundouras, S.; Fyllas, N.M.; Jones, G.V. Viticulture-climate relationships in Greece: The impacts of recent climate trends on harvest date variation. Int. J. Climatol. 2014, 34, 1445–1459. [Google Scholar] [CrossRef]
- Fraga, H.; García de Cortázar Atauri, I.; Malheiro, A.C.; Santos, J.A. Modelling climate change impacts on viticultural yield, phenology and stress conditions in Europe. Glob. Chang. Biol. 2016, 22, 3774–3788. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef]
- Shah, S.U.; Ayub, Q.; Hussain, I.; Khan, S.M.; Ali, S.; Khan, M.A.; Haq, N.; Mehmood, A.; Khan, T.; Brahmi, N.C. Effect of different growing media on survival and growth of Grape (Vitus vinifera) cuttings. J. Adv. Nutr. Sci. Technol. 2021, 1, 117. [Google Scholar] [CrossRef]
- Alikhani, L.; Ansari, K.; Jamnezhad, M.; Tabatabaie, Z. The effect of different mediums and cuttings on growth and rooting of pomegranate cuttings. Iran. J. Plant Physiol. 2011, 1, 199–203. [Google Scholar]
- Waite, H.; Whitelaw-Weckert, M.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Zeal. J. Crop Hortic. Sci. 2015, 43, 144–161. [Google Scholar] [CrossRef]
- Ausari, P.K.; Soni, N.; Kanpure, R.N.; Ninama, N.; Bhandari, J. Effect of Different Growing Media on Hardwood Cutting of Grapes (Vitis vinifera L.) cv. Pusa Navrang. Int. J. Plant Soil Sci. 2023, 35, 978–988. [Google Scholar] [CrossRef]
- Zareei, E.; Zaare-Nahandi, F.; Hajilou, J.; Oustan, S. Eliciting effects of magnetized solution on physiological and biochemical characteristics and elemental uptake in hydroponically grown grape (Vitis vinifera L. cv. Thompson Seedless). Plant Physiol. Biochem. 2021, 167, 586–595. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Ferrón-Carrillo, F.; Cunha-Chiamolera, T.P.L.d.; Urrestarazu, M. Effect of ammonium nitrogen on pepper grown under soilless culture. J. Plant Nutr. 2021, 45, 113–122. [Google Scholar] [CrossRef]
- Specht, K.; Siebert, R.; Hartmann, I.; Freisinger, U.B.; Sawicka, M.; Werner, A.; Thomaier, S.; Henckel, D.; Walk, H.; Dierich, A. Urban agriculture of the future: An overview of sustainability aspects of food production in and on buildings. Agric. Hum. Values 2014, 31, 33–51. [Google Scholar] [CrossRef]
- Huang, L.; Gu, M.; Yu, P.; Zhou, C.; Liu, X. Biochar and vermicompost amendments affect substrate properties and plant growth of basil and tomato. Agronomy 2020, 10, 224. [Google Scholar] [CrossRef]
- Wystalska, K.; Malińska, K.; Sobik-Szołtysek, J.; Dróżdż, D.; Meers, E. Properties of Poultry-Manure-Derived Biochar for Peat Substitution in Growing Media. Materials 2023, 16, 6392. [Google Scholar] [CrossRef] [PubMed]
- Hirschler, O.; Thrän, D. Peat Substitution in Horticulture: Interviews with German Growing Media Producers on the Transformation of the Resource Base. Horticulturae 2023, 9, 919. [Google Scholar] [CrossRef]
- Herrera, F.; Castillo, J.E.; Chica, A.F.; López Bellido, L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Niu, G.; Starman, T.; Volder, A.; Gu, M. Poinsettia Growth and Development Response to Container Root Substrate with Biochar. Horticulturae 2018, 4, 1. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Tzortzakis, N. Wood-Based Biochar Ratio Used for Partial Peat Replacement in Growing Media for Antirrhinum majus Pot Production. Agriculture 2024, 14, 1860. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, W.; Shafique Ahmad, K.; Shafi, J.; Asif Shehzad, M.; Aqeel Sarwar, M. Comparative Effect of Different Potting Media on Vegetative and Reproductive Growth of Floral Shower (Antirrhinum majus L.). Univers. J. Plant Sci. 2013, 1, 104–111. [Google Scholar] [CrossRef]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant nutrient availability and pH of biochars and their fractions, with the possible use as a component in a growing media. Agronomy 2020, 10, 10. [Google Scholar] [CrossRef]
- Gruda, N. Current and future perspective of growing media in Europe. Acta Hortic. 2012, 960, 37–43. [Google Scholar] [CrossRef]
- Messiga, A.J.; Hao, X.; Ziadi, N.; Dorais, M. Reducing peat in growing media: Impact on nitrogen content, microbial activity, and CO2 and N2O emissions. Can. J. Soil Sci. 2021, 102, 77–87. [Google Scholar] [CrossRef]
- Ekbic, H.B.; Yaman, E.; Özenç, D.B.; Ekbic, E. Effects of Hazelnut Husk Compost and Tea Residue Compost on Quality and Performance of 5 Bb American Grapevine Rootstock Saplings. Acta Sci. Pol. Hortorum Cultus 2022, 21, 15–23. [Google Scholar] [CrossRef]
- Saqib, M.; Razzaq, K.; Ullah, S.; Hussain, A.; Rajwana, I.A.; Naz, A.; Akhtar, G.; Amin, M.; Faried, H.N.; Zafar, M.S.; et al. Standardization of Growing Media for Grapes Nursery Production. J. Plant Environ. 2021, 3, 115–124. [Google Scholar] [CrossRef]
- Ronga, D.; Francia, E.; Allesina, G.; Pedrazzi, S.; Zaccardelli, M.; Pane, C.; Tava, A.; Bignami, C. Valorization of Vineyard By-Products to Obtain Composted Digestate and Biochar Suitable for Nursery Grapevine (Vitis vinifera L.) Production. Agronomy 2019, 9, 420. [Google Scholar] [CrossRef]
- Amir, M.; Hussain, S.; Martin, S.; Asad Latif, M.; Basit, A.; Ahmad, N.; Khan, F.; Ullah, W.; Ullah, R.; Abbas, M. Different media effect on various cultivars of grapes (Vitis vinifera) cuttings. J. Xi’an Shiyou Univ. 2023, 19, 468–484. [Google Scholar]
- Vrontis, D.; Paliwoda, S.J. Branding and the Cyprus wine industry. J. Brand Manag. 2008, 16, 145–159. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Shredded maize stems as an alternative substrate medium: Effect on growth, flowering and yield of tomato in soilless culture. J. Veg. Sci. 2005, 11, 57–70. [Google Scholar] [CrossRef]
- Marinou, E.; Chrysargyris, A.; Tzortzakis, N. Use of sawdust, coco soil and pumice in hydroponically grown strawberry. Plant Soil Environ. 2013, 59, 452–459. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Pitsikoulaki, G.; Stamatakis, A.; Chrysargyris, A. Ammonium to Total Nitrogen Ratio Interactive Effects with Salinity Application on Solanum lycopersicum Growth, Physiology, and Fruit Storage in a Closed Hydroponic System. Agronomy 2022, 12, 386. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; Abreu, C.E.B.D.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Farooq, M.; Kakar, K.; Golly, M.K.; Ilyas, N.; Zib, B.; Khan, I.; Khan, S.; Khan, I.; Saboor, A.; Bakhtiar, M. Comparative Effect of Potting Media on Sprouting and Seedling Growth of Grape Cuttings. Int. J. Environ. Agric. Res. 2018, 4, 82–89. [Google Scholar]
- Popescu, G.C.; Popescu, M. Effects of different potting growing media for Petunia grandiflora and Nicotiana alata Link & Otto on photosynthetic capacity, leaf area, and flowering potential. Chil. J. Agric. Res. 2015, 75, 21–26. [Google Scholar]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Bresson, J.; Vasseur, F.; Dauzat, M.; Koch, G.; Granier, C.; Vile, D. Quantifying spatial heterogeneity of chlorophyll fluorescence during plant growth and in response to water stress. Plant Methods 2015, 11, 23. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Antoniou, O.; Tzortzakis, N. Climate change due to heat and drought stress can alter the physiology of Maratheftiko local cyprian grapevine variety. J. Water Clim. Chang. 2018, 9, 715–727. [Google Scholar] [CrossRef]
- Heyman, L.; Chrysargyris, A.; Demeestere, K.; Tzortzakis, N.; Höfte, M. Responses to drought stress modulate the susceptibility to Plasmopara viticola in Vitis vinifera self-rooted cuttings. Plants 2021, 10, 273. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Pintać, D.; Četojević-Simin, D.; Berežni, S.; Orčić, D.; Mimica-Dukić, N.; Lesjak, M. Investigation of the chemical composition and biological activity of edible grapevine (Vitis vinifera L.) leaf varieties. Food Chem. 2019, 286, 686–695. [Google Scholar] [CrossRef]
- Dorion, S.; Ouellet, J.C.; Rivoal, J. Glutathione metabolism in plants under stress: Beyond reactive oxygen species detoxification. Metabolites 2021, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.; Chrysargyris, A. Olive-mill and grape-mill residue impact the growth, physiology and nutrient status of grapevines young cuttings. Sustain. Chem. Pharm. 2024, 37, 101362. [Google Scholar] [CrossRef]
- Singh, A.; Satheeshkumar, P.K. Reactive Oxygen Species (ROS) and ROS Scavengers in Plant Abiotic Stress Response. In Stress Biology in Photosynthetic Organisms; Mishra, A.K., Ed.; Springer: Singapore, 2024; pp. 44–64. ISBN 9789819718825. [Google Scholar]
- Agarwal, P.; Saha, S.; Hariprasad, P. Agro-industrial-residues as potting media: Physicochemical and biological characters and their influence on plant growth. Biomass Convers. Biorefin. 2021, 13, 9601–9624. [Google Scholar] [CrossRef]
- Dubey, T.; Mishra, D.S. Effect of rooting media and different polythene wrappers on air layering of pomegranate (Punica granatum) cv. Bhagwa. Int. J. Adv. Biochem. Res. 2024, 8, 401–406. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Singh, K.K.; Chauhan, J.S. A review on vegetative propagation of grape (Vitis vinifera L.) through cutting. Glob. J. Biosci. Biotechnol. 2020, 9, 50–55. [Google Scholar]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Cetner, M.D.; Kalaji, H.M.; Borucki, W.; Kowalczyk, K. Phosphorus deficiency affects the i-step of chlorophyll a fluorescence induction curve of radish. Photosynthetica 2020, 58, 671–681. [Google Scholar] [CrossRef]
- Méndez, A.; Paz-Ferreiro, J.; Gil, E.; Gascó, G. The effect of paper sludge and biochar addition on brown peat and coir based growing media properties. Sci. Hortic. 2015, 193, 225–230. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef]
- Tangolar, S.; Tangolar, S.; Tarım, G.; Ada, M.; Torun, A.A.; Ertargın, E. The effects of different nitrogen and potassium levels on yield and quality of two early grape cultivars grown in different soilless media. Acta Hortic. 2019, 1242, 349–356. [Google Scholar] [CrossRef]
- Baronti, S.; Montagnoli, A.; Beatrice, P.; Danieli, A.; Maienza, A.; Vaccari, F.P.; Casini, D.; Di Gennaro, S.F. Above- and below-ground morpho-physiological traits indicate that biochar is a potential peat substitute for grapevine cuttings nursery production. Sci. Rep. 2024, 14, 17185. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.; Mogensen, L.; Smith, A.M.; Larsen, S.U.; Knudsen, M.T. Greenhouse gas emissions from bio-based growing media: A life-cycle assessment. Sci. Total Environ. 2024, 907, 167977. [Google Scholar] [CrossRef] [PubMed]
- Papafotiou, M.; Kargas, G.; Lytra, I. Olive-mill waste compost as a growth medium component for foliage potted plants. HortScience 2005, 40, 1746–1750. [Google Scholar] [CrossRef]
- Jamal Uddin, A.F.M.; Rakibuzzaman, M.; Raisa, I.; Maliha, M.; Husna, M.A. Impact of natural substances and synthetic hormone on grapevine cutting. J. Biosci. Agric. Res. 2020, 25, 2069–2074. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).