Abstract
Passion fruit (Passiflora edulis), a commercially vital tropical crop, faces flowering instability due to photoperiod-sensitive flowering patterns, particularly under the cloudy, rainy climates of subtropical regions. To mitigate floral suppression during unfavorable light conditions, this study implemented a dual-modality strategy combining 16 h daily supplementary lighting (460 nm blue + 630 nm red spectrum) and foliar application of a high-phosphorus-containing nutrient, the Plant-Prod (nitrogen–phosphorus–potassium = 10:52:10) grown in field ‘Qinmi No. 9’. The treatment significantly stimulated lateral branch formation, internode elongation, flower retention, stage IV flower bud development, and enhanced photosynthetic efficiency. Physiological analyses revealed that the treatment increased the net photosynthetic rate (Pn), reduced the intercellular carbon dioxide concentration (Ci), and enhanced stomatal conductance (Gs), indicating the improvement of carbon assimilation. Controlled seedling trials further confirmed these effects, with treated groups exhibiting accelerated lateral branching and stress resilience. This integrated approach, combining optimized supplemental lighting and precision phosphorus fertilization, offers a practical and scalable strategy to stabilize passion fruit yields in climate-variable regions, with immediate potential for commercial orchards and greenhouse production.
1. Introduction
Passion fruit (Passiflora edulis), a globally significant tropical crop, is valued for its aromatic pulp, nutritional richness, and bioactive compounds, including polyphenols and carotenoids [1,2]. While the genus Passiflora encompasses over 500 species, fewer than 10 are commercially viable [3], with purple-fruited (Tainong No. 1, P. edulis Sims. f. edulis), yellow-fruited (Huangjin, P. edulis Sims. f. flavicarpa Deg., Qinmi No. 9, P. edulis f. flavicarpa), and large-fruited hybrids (Jinmi, P. quadrangularis L, Xiangmi, P. quadrangularis L) dominating global markets [4,5]. Among these, the yellow-fruited cultivar ‘Qinmi No. 9’ stands out for its exceptional sweetness, disease resistance, and subtropical adaptability [2,6]. However, chronic low-light stress suppresses flowering and reduces yields, particularly in monsoon-prone regions such as southern China, where over 60% of annual rainfall coincides with the March to April flowering [7,8]. For instance, in Guizhou, China, where the flowering period coincides with the rainy summer season, the implementation of rain-sheltering and supplemental lighting treatments has been shown to increase yields by 20–23% [9].
As a perennial vine, passion fruit displays unique reproductive strategies differing from woody perennials. Each axillary meristem bifurcates into a tendril and a floral primordium during reproductive phases, a developmental program conserved across Passiflora species [10,11]. This architectural plasticity enables concurrent vegetative and reproductive growth but requires strict regulation by environmental cues [12]. While passion fruit initiates flowers year-round, successful anthesis requires sustained long-day photoperiods to activate FLOWERING LOCUS T (FT) signaling in mature leaves [12,13,14]. In subtropical monsoon regions, chronic low-light stress triggered by prolonged cloud cover arrests floral primordia at early developmental stages, leading to yield losses exceeding 40% in commercial orchards [15].
Recent advances revealed that photoperiodic signaling and nutrient availability synergistically regulate floral induction [16]. Phosphorus (P), a core component of Adenosine Triphosphate (ATP) and nucleic acids, drives energy metabolism while modulating phloem-mobile flowering signals such as FT [17,18]. Field studies in barley (Hordeum vulgare L. var. nudum Hook.f.) and eggplant (Solanum melongena) demonstrate that P supplementation enhances photosynthetic carbon allocation to reproductive sinks, amplifying flowering efficiency when combined with extended photoperiods [19,20]. However, passion fruit responses to such interventions remain underexplored, particularly under low-light stress. Current management practices rely on reactive measures (pruning, hormone sprays), lacking proactive strategies to counteract environmental constraints [21,22].
This study tackles a critical gap in addressing low-light resilience in passion fruit cultivation by integrating supplemental lighting (460 nm blue + 630 nm red wavelengths) with targeted P fertilization. The synergistic combination of red–blue light supplementation and foliar P application mitigated photoperiodic constraints by simultaneously enhancing photosynthetic carbon assimilation and redirecting carbohydrate allocation to reproductive sinks, thereby overcoming floral suppression mechanisms. This dual-modality strategy bridges the gap between supplemental lighting and nutrient-mediated flowering regulation, proposing a scalable solution to improve yield resilience in climate-variable agricultural ecosystems.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Field experiments were conducted at Baiguo Baixiaong Garden Farm (23°19′05″ N, 113°35′25″ E) using two adjacent blocks of mature ‘Qinmi No. 9’ passion fruit (each experimental area was 100 m long and 2 m wide, and a standardized planting pattern of 1.6 m row spacing and 2.5 m plant spacing was adopted). To evaluate the effects of supplemental lighting and foliar phosphorus application on flower bud development, one block was exposed to natural daylight (control) while the other received supplemental lighting using LED lamps (Zhongke Ruifeng, Beijing, China). The supplemental lighting system delivered a defined red–blue spectrum (peak emissions at 460 nm and 630 nm) with a maintained photon flux density of 200 ± 20 μmol·m−2·s−1. The illumination protocol (18:00–22:00 daily) ensured a consistent 16 h/8 h light/dark cycle, with luminaires mounted on greenhouse purlins at 2.5 m above ground level, maintaining a 0.7 m clearance above the passionfruit canopy (average plant height: 1.8 m). Within each block, 30 uniformly sized plants were randomly assigned to three treatment groups (n = 10 per group), sprayed with 500 mg/L Plant-Prod (nitrogen–phosphorus–potassium elements =10 (7.6 ammonium and 2.4 urea):52:10, Plant Products Co., Ltd., Brampton, ON, Canada), 200 mg/L KH2PO4 (provided a standardized P source (200 mg/L, Shenzhen Dugao Biotechnology Co., Ltd., Shenzhen, China), and water as a control. Foliar applications were uniformly administered at 09:00 on 21 May 2023 using a handheld sprayer (volume: 50 mL per plant). Meteorological records indicated an average temperature of 25 ± 3 °C and a relative humidity of 85 ± 5% during the experimental period. All measurements (lateral branch counts, photosynthetic parameters) were collected 14 d post-treatment.
To further investigate the synergistic effects of supplemental lighting and high phosphorus application, seeds of ‘Qinmi No. 9’ passion fruit were germinated in growth pods containing a 3:1:1 (v/v/v) peat soil–vermiculite–perlite substrate. Seedlings at the two-leaf stage (V2) were randomly assigned to four treatment groups (n = 15 per group): natural light (NL); supplemental light (SL); Plant-Prod + NL (PNL); Plant-Prod + SL (PSL). Treatments were administered in climate-controlled greenhouses maintained at 24 ± 1 °C (relative humidity: 70 ± 5%). Supplemental lighting was provided by LED panels emitting a red–blue spectrum. The experiment spanned 40 d (1 July to 10 August 2023).
2.2. Changes in Growth Parameters and Determination of Horticultural Traits
For phenotypic trait quantification in the field lighting supplemental and foliar spray experiment, 10 secondary vine shoots per treatment were randomly selected and labeled for longitudinal monitoring. Baseline measurements (day 0) included lateral branch count, internode number per shoot, total flower buds, and developmental status of flower buds (Figure 1). Tagged plants were re-evaluated 14 d post-treatment to calculate treatment-induced changes: lateral branches (count at 14 d minus baseline), internodes (14 d count minus baseline), flower buds (14 d total minus baseline), and Stage IV flower bud increment (mature buds at 14 d minus baseline). All measurements adhered to standardized protocols for passion fruit phenotyping, with morphological classifications validated against reference specimens.

Figure 1.
Classification of flower bud developmental stages. Flower buds were categorized into four discrete stages (I, II, III, and IV) based on progressive morphological changes, with stage IV representing fully differentiated buds capable of anthesis (complete flower shown). Bar = 1 cm.
2.3. Measurement of Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters
TARGAS-1 portable photosynthesis system (brand: PP System) were used to measure the net photosynthetic rate (Pn), intercellular carbon dioxide concentration (Ci), transpiration rate (Tr), and stomatal conductance (Gs) of the leaf blades in four directions, namely east, west, south, and north, for the passion fruit.
Chlorophyll fluorescence analysis was conducted using an IMAGING-PAM system (Walz, Germany) to measure key photosynthetic parameters: maximum quantum yield of photosystem II (Fv/Fm, where Fv is variable fluorescence and Fm is maximum fluorescence), effective quantum yield of photosystem II [Y(II)], quantum yield of regulated energy dissipation [Y(NPQ)], and electron transport rate (ETR), with all parameters standardized against pre-dawn measurements following dark adaptation (30 min) [23].
2.4. Statistical Analysis
Statistical analyses were performed using the Student’s t-test and Duncan’s multiple range test in SPSS (v19.0, IBM Corporation, Armonk, NY, USA), with data visualized using GraphPad Prism (v8.0.2).
3. Results
3.1. The Effects of Supplemental Lighting and Plant-Prod Spraying on the Horticultural Traits of Passion Fruit
We observed a significant amount of overcast weather in Guangzhou during the months of May and June, which severely impacted the flower bud development process of the passion fruit. We conducted an experiment where we applied six different treatments on the ‘Qinmi No. 9’ passion fruit, which is a highly light-sensitive variety and currently the highest selling on the market. As shown in Figure 2, supplemental lighting (SL) improved development in plants relative to natural light (NL) conditions, including enhanced lateral branch initiation, prolonged internode elongation, elevated total flower bud production, and increased stage IV flower buds. Under NL regimes, foliar treatments with Plant-Prod or KH2PO4 solely augmented floral bud proliferation (Figure 2C). Notably, the synergistic integration of Plant-Prod with SL elicited a marked synergistic interaction, yielding maximal performance across all evaluated morphological and reproductive parameters, surpassing outcomes achieved by SL alone (Figure 2). While KH2PO4 supplementation under illuminated conditions promoted lateral branch formation, no significant improvement in stage IV bud maturation was observed compared to the SL control group (Figure 2B). These findings suggest that SL regulates floral differentiation, while foliar application of Plant-Prod under SL not only enhanced plant growth but also significantly increased flower bud formation and the number of stage IV flower buds.
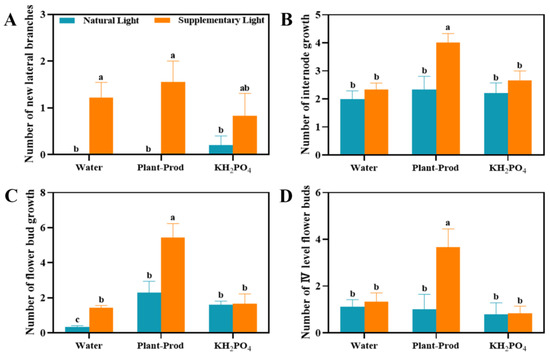
Figure 2.
Effect of spraying different chemicals on the number of new lateral branches (A), number of internode growth (B), number of flower bud growth (C), and number of IV level flower buds (D) in passion fruit. As detected by Duncan’s test, lowercase letters indicate significant differences at the p ≤ 0.05 (in ‘Qinmi No. 9’ passion fruit = 10) level.
3.2. The Effects of Supplemental Lighting and Plant-Prod Spraying on the Photosynthetic Characteristics of Passion Fruit
Physiological analyses revealed distinct photoregulatory patterns under SL regimes (Figure 3). Plants exposed to SL exhibited significantly elevated net photosynthetic rates (Pn) compared to NL controls, with foliar applications of Plant-Prod or KH2PO4 further enhancing Pn across both light conditions (Figure 3A). The SL + Plant-Prod combination (PSL) achieved maximal photosynthetic efficiency, surpassing all other treatments (Figure 3A). The intercellular carbon dioxide concentration (Ci) inversely correlated with light intensity, showing marked reductions under SL (Figure 3B). Both treatments reduced Ci in all illumination environments, with PSL yielding the lowest CO2 accumulation. Transpiration rates (Tr) displayed treatment-specific modulation. Plant-Prod increased Tr under NL, whereas KH2PO4 suppressed it, but both elevated Tr under SL (Figure 3C). Stomatal conductance (Gs) universally increased under SL, with Plant-Prod amplifying this effect across light regimes (Figure 3D). These findings suggest that SL + Plant-Prod enhances the photosynthetic capacity of passion fruit.
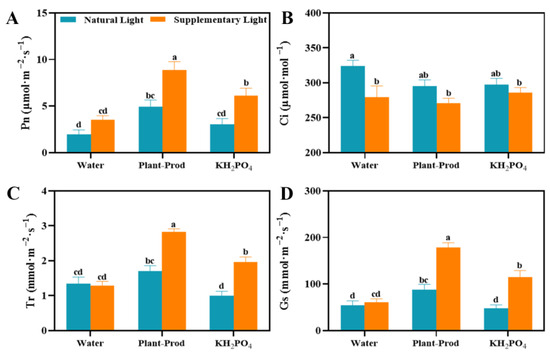
Figure 3.
Effect of spraying different chemicals on the changes in Pn values (A), changes in Ci values (B), changes in Tr values (C), and changes in Gs values (D) in passion fruit. As detected by Duncan’s test, lowercase letters indicate significant differences at the p ≤ 0.05 (in ‘Qinmi No. 9’ passion fruit = 10) level.
3.3. The Effects of Supplemental Lighting and Plant-Prod Spraying on the Chlorophyll Fluorescence Characteristics of Passion Fruit
Chlorophyll fluorescence analyses revealed light regime-specific photochemical adaptations (Figure 4). While plants under SL exhibited maximum quantum yield (Fv/Fm) values within the optimal range (0.75–0.8), SL consistently outperformed NL (Figure 4A). Notably, foliar treatments exhibited contrasting light-dependent responses. Plant-Prod reduced actual photochemical quantum yield of photosystem II (Y(II)) under NL enhanced it under SL, whereas KH2PO4 increased Y(II) in NL but suppressed it under SL (Figure 4B). Non-photochemical quenching (Y(NPQ)) inversely tracked Y(II) dynamics across treatments (Figure 4C). Electron transport rates (ETR) mirrored these patterns, with the PSL achieving maximal electron transfer efficiency (Figure 4D). The results showed that under SL, the Plant-Prod treatment exhibited minimal phytotoxicity and the highest electron-transfer efficiency.
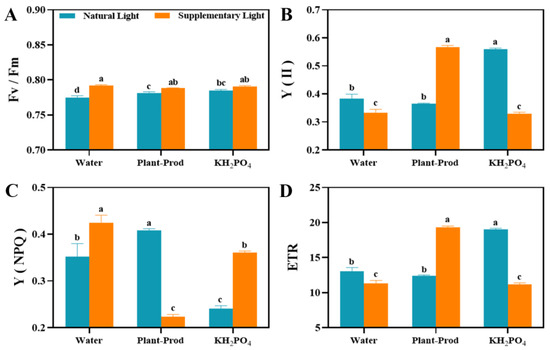
Figure 4.
Effects of spraying different chemicals in passion fruit on Fv/Fm values (A), Y(II) (B), Y(NPQ) (C), and ETR (D). As detected by Duncan’s test, lowercase letters indicate significant differences at the p ≤ 0.05 (in ‘Qinmi No. 9’ passion fruit = 10) level.
3.4. The Effects of Supplementary Light and Plant-Prod Spraying on the ‘Juvenile’ Period Development Stage of Passion Fruit
Comparative analyses of ‘Qinmi No. 9’ passion fruit seedling development demonstrated that foliar Plant-Prod application enhanced biomass accumulation across illumination regimes, with SL seedlings exhibiting superior morphological plasticity compared to NL (Figure 5). Crucially, SL synergized with Plant-Prod treatment (PSL) to drive apical dominance attenuation, increasing lateral branch primordia initiation relative to NL controls.
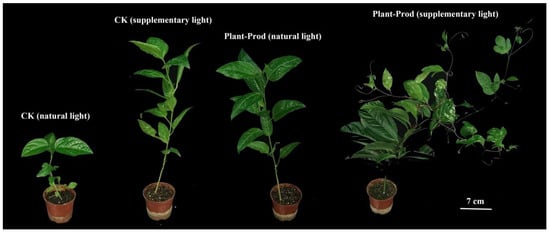
Figure 5.
The effects of supplementary light and Plant-Prod spraying on the growth of passion fruit seedlings. The 4 passion fruit plants in the figure respectively represent the comparison of plant morphology under natural light (12 h of light and 12 h of darkness, control, NL), supplementary light (16 h of light and 8 h of darkness, SL), Plant-Prod spraying with natural light (PNL), and Plant-Prod spraying with supplemental light (PSL) (from left to right). Bar = 7 cm.
3.5. The Impact of Supplementary Light and Plant-Prod Spraying on the Photosynthetic Characteristics of Passion Fruit
As shown in Figure 6, The PSL elicited the maximal enhancement of Pn, outperforming both isolated SL exposure and untreated controls (Figure 6A). This treatment concurrently minimized Ci (Figure 6B), with PSL reducing both Tr and Gs relative to SL conditions (Figure 6C,D). It is worth noting that only the Pn under SL was lower than the control group, which may be related to the light stress experienced by the passion fruit seedlings.
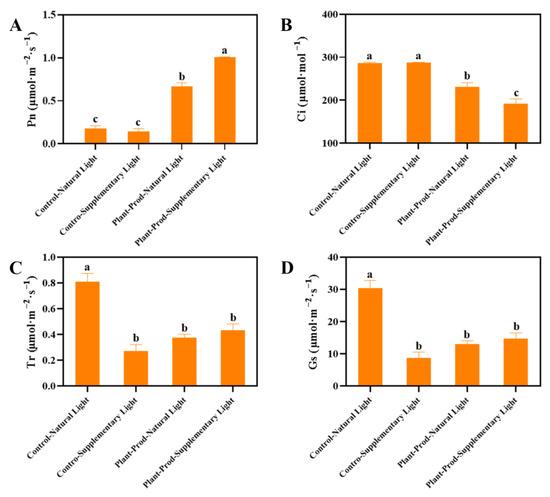
Figure 6.
Effect of spraying different chemicals on the changes in Pn values (A), changes in Ci values (B), changes in Tr values (C), and changes in Gs values (D) in passion fruit. As detected by Duncan’s test, lowercase letters indicate significant differences at the p ≤ 0.05 (in ‘Qinmi No. 9’ passion fruit = 3) level.
3.6. The Effects of Supplementary Light and Plant-Prod Spraying on the Chlorophyll Fluorescence Parameters of Passion Fruit
Under SL conditions, the foliar application of Plant-Prod significantly enhanced photosynthetic electron transport rates in mature passion fruit plants without inducing phytotoxicity. To investigate the potential carryover effects of this treatment on early developmental stages, we conducted parallel experiments with passion fruit seedlings. As shown in Figure 7, the Fv/Fm values of the Plant-Prod treatment and the control group were between 0.75 and 0.8 under SL (Figure 7A). The Y(II) and ETR values were highest in the PSL (Figure 7B,D). The Y(NPQ) value was the lowest (Figure 7C). In summary, supplementary light during the seedling stage may be influenced by excessive light influence. In contrast, when the Plant-Prod agent is applied to passion fruit seedlings under SL conditions during the seedling stage, the seedlings are neither affected by the potential phytotoxicity of the agent nor by excessive light.
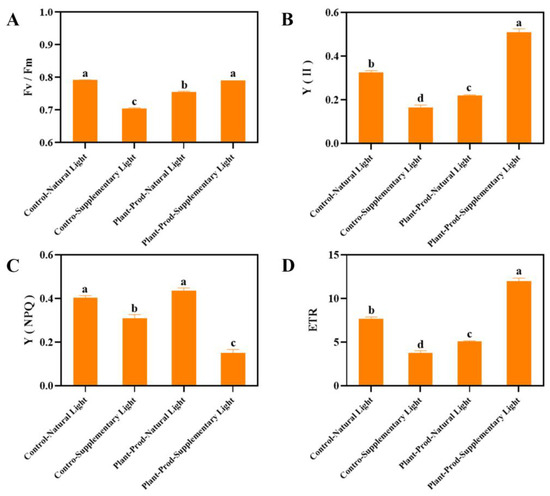
Figure 7.
Effects of spraying different chemicals in passion fruit on Fv/Fm values (A), Y(II) (B), Y(NPQ) (C), and ETR (D). As detected by Duncan’s test, lowercase letters indicate significant differences at the p ≤ 0.05 (in ‘Qinmi No. 9’ passion fruit = 3) level.
4. Discussion
Environmental cues trigger flowering at the right time [24]. Prolonged exposure to light is essential for normal flowering development [25]. It is shown that the yield and prices in the market are greatly influenced by seasonal factors, with unsuitable temperatures and rainfall significantly impacting production. Hence it is important to find solutions for stable production under unfavorable environmental conditions.
Passion fruit cultivation during the flowering season is frequently challenged by persistent overcast and rainy conditions in subtropical regions, where chronic low-light stress disrupts floral meristem activation and bud maturation [26]. To overcome these light limitations, we developed a dual-modality intervention combining spectral-tuned supplemental lighting (16 h/day; 460 nm blue + 630 nm red wavelengths) with precision foliar delivery of a phosphorus-enriched formulation (Plant-Prod; N:P:K = 10:52:10). The results demonstrate that the combined supplemental lighting and phosphorus supplementation (PSL) significantly enhanced lateral branch formation, internode elongation, total floral bud production, and stage IV bud differentiation in field vines (Figure 2). Controlled seedling trials confirmed these effects, with PSL plants exhibiting accelerated lateral meristem activation (Figure 5). This synergistic interaction between supplemental lighting and phosphorus-mediated metabolic remodeling effectively bypasses light-deficiency-induced floral suppression, establishing a climate-resilient cultivation framework adaptable to monsoon-affected agricultural systems.
Phosphorus (P) orchestrates metabolic coordination between nutrient signaling and developmental transitions, functioning as a central regulator of photofertilization efficacy [26,27]. Beyond its canonical roles in nucleic acid synthesis and energy transfer, phosphorus critically governs phase transitions from vegetative growth to reproductive commitment through the transcriptional regulation of FT homologs and redox-sensitive meristem activation [17,18]. Our data demonstrate that phosphorus supplementation enhances the thioredoxin-mediated redox regulation of RuBisCO activase, as evidenced by the elevated Fv/Fm ratios (Figure 4A) and reduced Y(NPQ) under supplemental lighting (Figure 4C). These processes synergistically optimize light energy conversion efficiency, mitigating RuBisCO carboxylation bottlenecks that typically limit Calvin cycle flux under suboptimal irradiance [5]. An increase in Pn (Figure 3A) following Plant-Prod application aligns with the phosphorus-mediated RuBisCO activase upregulation reported in eggplant (Solanum melongena) [21], confirming the conserved phosphorus-gated control of carbon fixation machinery across divergent angiosperm lineages.
The integrative effects of spectral tuning and phosphorus signaling were further evidenced by coordinated photosynthetic responses. PSL induced a marked elevation in Pn accompanied by reduced Ci and enhanced Gs compared to NL (Figure 3), indicating optimized carbon assimilation efficiency. This integrative response mirrors the phosphorus-mediated photochemical optimization observed in Black Highland Barley (Hordeum vulgare L. var. nudum Hook.f.) and eggplant (Solanum melongena), where foliar P enrichment couples spectral tuning to amplify light-harvesting complex II (LHCII) phosphorylation efficiency, thereby minimizing CO2 diffusion limitations in the mesophyll [19,20]. Crucially, PSL-induced Ci reduction indicates RuBisCO carboxylation rate acceleration, a phenomenon previously linked to ATP synthase upregulation under Pi-enriched conditions [5,28]. These data collectively establish that Plant-Prod primes photosynthetic machinery for spectral inputs by synchronizing stomatal kinetics with chloroplast ATP synthase assembly, a dual coordination absent in isolated light or nutrient interventions.
The observed reduction in Ci aligns with enhanced RuBisCO carboxylation efficiency, where accelerated enzymatic activity exceeded the stromal CO2 diffusion capacity under optimized light–nutrient conditions. Elevated Gs and Tr were found in mature plants exposed to SL (Figure 3C,D), reflecting photochemical regulation through blue light-activated phototropin signaling, which primes guard cell turgor via K+ channel dynamics [29]. Concurrent phosphorus-mediated ATP synthesis synergistically amplifies proton pump activity, further expanding stomatal apertures [30]. While foliar phosphorus enrichment promoted hydraulic demand-driven increases in Gs and Tr in adult vines, seedlings exhibited contrasting responses, with suppressed stomatal opening and reduced transpiration. This developmental divergence likely arises from ontogenetic adaptation, where juvenile plants prioritize water conservation through ABA-mediated stomatal closure under resource allocation constraints, a strategy conserved in light-stressed angiosperms.
Chlorophyll fluorescence dynamics provide critical insights into the photochemical resilience of photosynthetic systems under environmental perturbations [31]. Our analyses revealed that passion fruit plants subjected to combined spectral modulation and phosphorus enrichment maintained fluorescence parameters within optimal ranges: Fv/Fm ratios approached theoretical maxima, indicative of intact photosystem II (PSII) integrity and minimal light stress (Figure 4). The concurrent enhancement of Y(II) and ETR demonstrated sustained light energy conversion efficiency, while suppressed Y(NPQ) reflected the precise regulation of excess excitation dissipation [32,33]. These coordinated responses contrast with photoinhibitory patterns typically observed under low-light stress, confirming that the intervention strategy preserved photostasis by synchronizing light harvesting with carbon assimilation capacity. Crucially, the conserved correlation between elevated Y(II) and ETR and reduced Y(NPQ) under treatment conditions underscores a systemic optimization of photosynthetic flux control, a hallmark of phosphorus-mediated metabolic priming in spectral-tuned environments.
This photoperiod–nutrient decoupling strategy establishes an adaptive framework for monsoon-affected agroecosystems, where spectral-tuned lighting systems synergize with phosphorus-mediated metabolic priming to stabilize reproductive output. Our schematic model illustrates an integrated intervention strategy to mitigate low-light stress in passion fruit cultivation (Figure 8). Under persistent cloudy and rainy conditions, the combined application of spectrum-optimized supplemental lighting (460 nm blue + 630 nm red wavelengths) and foliar Plant-Prod treatment synergistically enhances multiple physiological and developmental processes. This dual-modality approach promotes lateral branch formation, internode elongation, floral bud proliferation, and the maturation of stage IV flower buds capable of successful anthesis. Concurrently, the treatment significantly improves photosynthetic efficiency by optimizing light energy conversion and carbon assimilation. The model underscores how spectral tuning and phosphorus-mediated metabolic reprogramming collectively overcome environmental constraints, offering a scalable solution for yield stabilization in monsoon-prone agroecosystems.
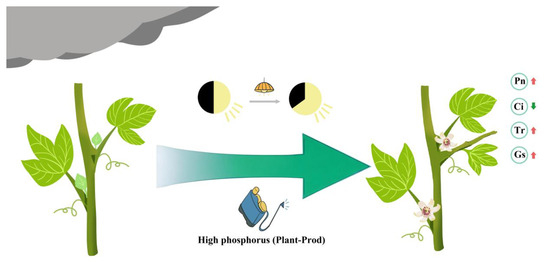
Figure 8.
Schematic model of the synergistic effects of supplemental lighting and foliar phosphorus application on passion fruit under low-light stress. Red arrows indicate increases in Pn, Tr, and Gs after treatment, while green arrows indicate a decrease in Ci after treatment.
5. Conclusions
This study demonstrates that spectrum-optimized supplemental lighting (460 nm blue + 630 nm red) combined with foliar phosphorus fertilization effectively mitigates low-light stress in subtropical passion fruit cultivation. The treatment significantly enhanced stage IV flower bud formation, lateral branching, and photosynthetic efficiency (increased Pn, decreased Ci), overcoming floral suppression under cloudy conditions.
In this scalable strategy, modular LED systems with precision phosphorus application decouple flowering from climatic variability, offering yield stability in monsoon-prone regions. Future research should validate field-scale energy efficiency and long-term soil phosphorus dynamics to ensure sustainability. Our findings advance climate-resilient horticulture for tropical crops.
Author Contributions
B.Z. and X.L. conceived and designed the experiments; D.S. and C.H. performed most of the experiments; Y.Y., H.W., T.Y., C.W. and Z.H. provided assistance; D.S. and B.Z. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovative Team Project of Modern Agricultural Industry Technology System of Guangdong Province (2024CXTD09), the Foundation Research Project of Kaili University (grant No. YTH-XM2024018), the Growth of Young Scientific and Technological Talents of Guizhou Educational Commission (grant No. QIAN JIAO HE KY [2022]078), and the Key Laboratory Program of Guizhou Provincial Department of Education (grant No. Qianjiaoji [2022]053).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Tongbo Yan was employed by the company Hainan Foreray Star Pro Biotecoch Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Mira, S.; Veiga-Barbosa, L.; González-Benito, M.E.; Pérez-García, F. Conductivity test in seeds of different passion flower species. Pesq. Agropec. Bras. 2015, 50, 510–513. [Google Scholar] [CrossRef]
- Pereira, Z.C.; dos Anjos Cruz, J.M.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; de Araújo Bezerra, J. Passion fruit (Passiflora spp.) pulp: A review on bioactive properties, health benefits and technological potential. Food Res. Int. 2023, 166, 112626. [Google Scholar] [CrossRef] [PubMed]
- De Melo, N.F.; Guerra, M. Variability of the 5S and 45S rDNA sites in Passiflora L. species with distinct base chromosome numbers. Ann. Bot. 2003, 92, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tan, F.; Deng, J. Research progress on Passiflora edulis. China J. Chin. Mat. Med. 2008, 1789–1793. (In Chinese) [Google Scholar]
- De Jesus, O.N.; Lima, L.K.S.; Soares, T.L.; da Silva, L.N.; dos Santos, I.S.; Sampaio, S.R.; de Oliveira, E.J. Phenotypic diversity and alternative methods for characterization and prediction of pulp yield in passion fruit (Passiflora spp.) germplasm. Sci. Hortic. 2022, 292, 110573. [Google Scholar] [CrossRef]
- Rudnicki, M.; Oliveira, M.R.D.; Pereira, T.D.V.; Reginatto, F.H.; Dal-Pizzol, F.; Moreira, J.C.F. Antioxidant and antiglycation properties of Passiflora alata and Passiflora edulis extracts. Food Chem. 2007, 100, 719–724. [Google Scholar] [CrossRef]
- Utsunomiya, N. Effect of temperature on shoot growth, flowering and fruit growth of purple passionfruit (Passiflora edulis Sims var. edulis). Sci. Hortic. 1992, 52, 63–68. [Google Scholar] [CrossRef]
- Li, L.; Luo, S.; Weng, S.; Liang, Q.; Feng, Y. Growth rhythm of three native lianas from Guangzhou and their application in landscape. Subtro. Plant Sci. 2023, 52, 534–539. [Google Scholar]
- Teng, Y.; Wang, Y.; Li, J.; Zhang, X.; Chen, C.; Zhang, S.; Long, X. Effect of rain shelter and light supplement on yield, fruit quality and photosynthetic characteristics in passion fruit. J. Fruit Sci. 2024, 41, 1170–1180. [Google Scholar]
- Cutri, L.; Nave, N.; Ami, M.B.; Chayut, N.; Samach, A.; Dornelas, M.C. Evolutionary, genetic, environmental and hormonal-induced plasticity in the fate of organs arising from axillary meristems in Passiflora spp. Mech. Dev. 2013, 130, 61–69. [Google Scholar] [CrossRef]
- Prenner, G. Floral ontogeny in Passiflora lobata (Malpighiales, Passifloraceae) reveals a rare pattern in petal formation and provides new evidence for interpretation of the tendril and corona. Plant Syst. Evol. 2014, 300, 1285–1297. [Google Scholar] [CrossRef]
- Nave, N.; Katz, E.; Chayut, N.; Gazit, S.; Samach, A. Flower development in the passion fruit Passiflora edulis requires a photoperiod-induced systemic graft-transmissible signalpce. Plant Cell Environ. 2010, 33, 2065–2083. [Google Scholar] [CrossRef] [PubMed]
- Kiyak, A.; Mutlu, A.G. Molecular cloning, characterization and expression profile of FLOWERING LOCUS T (FT) gene from Prunus armeniaca L. S. Afr. J. Bot. 2023, 155, 330–339. [Google Scholar] [CrossRef]
- Nascimento, R.P.; Reguengo, L.M.; Machado, A.P.F.; Junior, M.R.M. The preventive and therapeutic potential of native Brazilian fruits on colorectal cancer. Food Biosci. 2022, 46, 101539. [Google Scholar] [CrossRef]
- Menzel, C.M.; Simpson, R.D. Effect of intermittent shading on growth, flowering and nutrient uptake of passionfruit. Sci. Hortic. 1989, 41, 83–96. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, W.; Chen, Y.; Tian, H.; Yang, Z.; Liu, S.; Li, X.; Song, C.; Ye, Z.; Guo, W.; et al. BRASSINAZOLE RESISTANT 1 delays photoperiodic flowering by repressing CONSTANS transcription. Plant Physiol. 2025, 197, kiaf032. [Google Scholar] [CrossRef]
- Cheng, G.; Jiao, J.; Jiang, J.; Xue, Y.; Zheng, B.; Yan, D. Research progress on growth, physiology, and molecular response of plants to nitrogen. Hubei Fore. Sci. Tech. 2024, 53, 1–14. [Google Scholar]
- Navaneethakrishnan, K.S.; Dhaliwal, H.S.; Gill, M.I.S.; Brar, J.S. Influence of nitrogen and phosphorus fertilization on fruiting and yield characteristics in ratoon crop of banana (Musa spp. AAA) cv. Grande Naine. J. Hortl. Sci. 2016, 11, 80–82. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, X.; Chen, G.; Shi, K.; Wang, J. Effects of different phosphorus fertilizer treatments on photosynthetic characteristics of flag leaves of Black Highland Barley. J. Plant Agri. 2024, 8, 404–412. [Google Scholar]
- Meng, Q.; Fan, W.; Liu, F.; Wang, G.; Yu, M.; Tian, L. The effects of different phosphate fertilizers on photosynthesis and antioxidant enzyme system of eggplant seedlings under cadmium stress. China Soils Fert. 2024, 3, 112–120. [Google Scholar]
- Zheng, X.; Wei, F. The impact of unfavorable weather conditions on the production of Golden passionfruit. Fujian Sci. Tech. Trop. Crop. 2018, 43, 34–36. [Google Scholar]
- Atsushi, S.; Tatsuya, K.; Shigeto, T.; Masashi, Y. Effect of temperature on photosynthesis characteristics in the passion fruits ‘Summer Queen’ and ‘Ruby Star’. Horticul. J. 2017, 86, 194–199. [Google Scholar]
- Ren, H.; Luo, F.; Yu, Y.; Xu, R. Comparison on methods of chlorophyll extraction in flowering Chinese cabbage. J. Anhui Agric. Sci. 2012, 40, 1455–1456. [Google Scholar]
- Bajpai, A.; Muthukumar, M.; Bajpai, Y.; Khan, K.; Rajan, S.; Singh, N.K.; Singh, V.K. Isoforms diversity and transcriptional regulation of floral meristem identity and pathway integrators, driving floral transition in mango under subtropics. Sci. Hortic. 2024, 334, 113301. [Google Scholar] [CrossRef]
- Scorza, L.C.T.; Hernandes-Lopes, J.; Melo-de-Pinna, G.F.A.; Dornelas, M.C. Expression patterns of Passiflora edulis APETALA1/FRUITFULL homologues shed light onto tendril and corona identities. EvoDevo 2017, 8, 3. [Google Scholar] [CrossRef]
- Meng, X.; Chen, W.; Wang, Y.; Huang, Z.; Ye, X.; Chen, L.; Yang, L. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus. grandis. PLoS ONE 2021, 16, e0246944. [Google Scholar] [CrossRef]
- Poudyal, S.; Owen, J.S.; Sharkey, T.D.; Fernandez, R.T.; Cregg, B. Phosphorus requirement for biomass accumulation is higher compared to photosynthetic biochemistry for three ornamental shrubs. Sci. Hortic. 2021, 275, 109719. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Chen, Y.; Liu, C.; Yang, Y. Light regulation of potassium in plants. Plant Physiol. Bioch. 2022, 170, 316–324. [Google Scholar] [CrossRef]
- Rascher, U.; Liebig, M.; Luttge, U. Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ. 2010, 23, 1397–1405. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.; Niyogi, K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jin, Z.; He, W.; Wang, X.; Che, X. Effects of different day/night warming on the photosynthetic characteristics and chlorophyll fluorescence parameters of Sinocalycanthus chinensis seedlings. Acta Ecol. Sin. 2012, 32, 6343–6353. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).