Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage
Abstract
1. Introduction
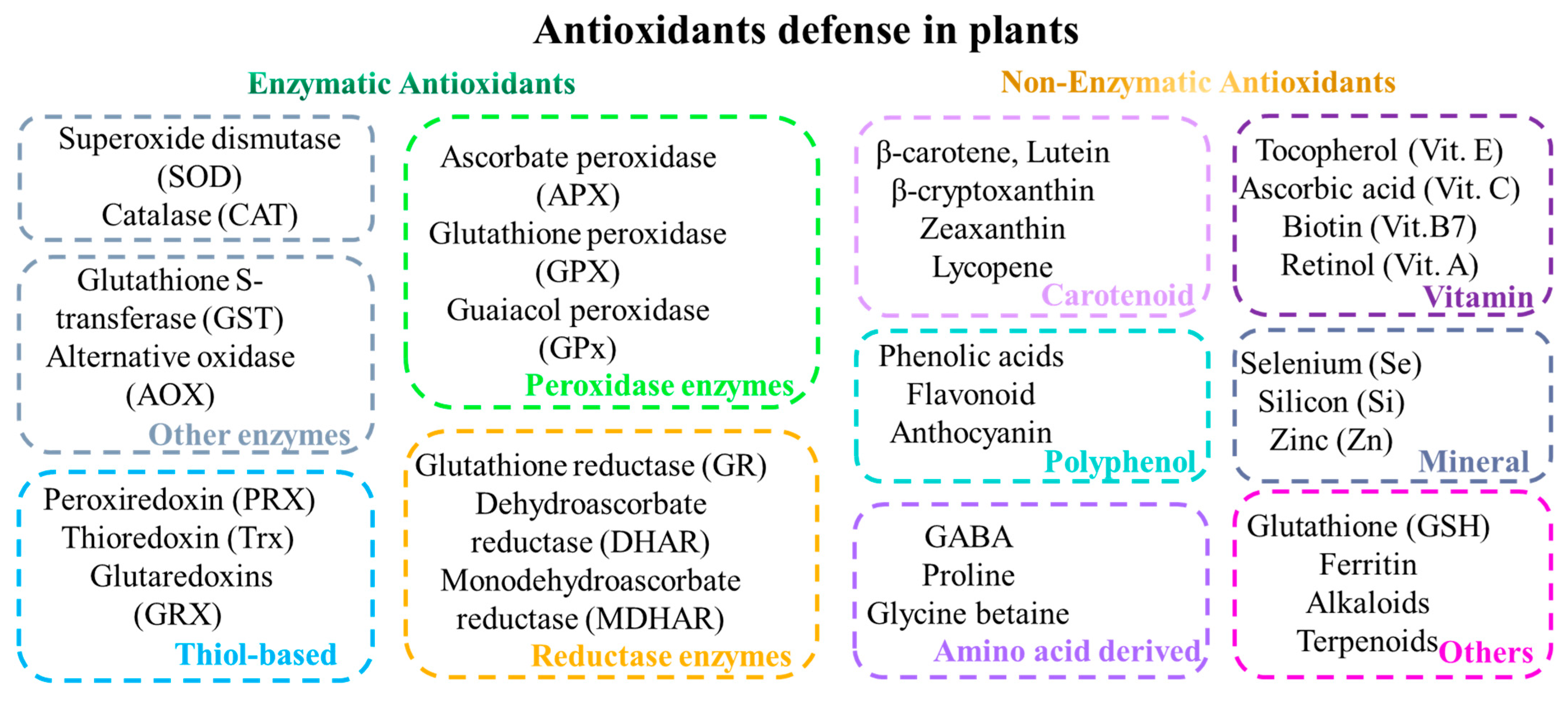
2. Antioxidative Defense Mechanisms in Plants
2.1. Enzymatic Antioxidants
| Serial No. | Enzymatic Antioxidant | Location | Function | Stress Response | Reference |
|---|---|---|---|---|---|
| 1 | Superoxide Dismutase (SOD) | Chloroplasts, mitochondria, cytosol | Catalyzes dismutation of O2•− to H2O2 and O2 | Increases during drought, salinity, and heat stress | [9,46] |
| 2 | Catalase (CAT) | Peroxisomes | Converts H2O2 to H2O and O2 | Upregulated during oxidative stress conditions | [47,48,49] |
| 3 | Guaiacol Peroxidase (GPx) | Cell wall, cytosol | Lignification Cell wall strengthening H2O2 scavenging | Response to mechanical stress and heavy metal stress | [9,50] |
| 4 | Ascorbate Peroxidase (APX) | Chloroplasts, cytosol | Reduces H2O2 to H2O using ascorbate as an electron donor | Enhanced activity during cold and drought stress | [9,40] |
| 5 | Glutathione Peroxidase (GPX) | Cytosol, chloroplasts | Reduces H2O2 and lipid hydroperoxides | Activated during heavy metal and UV stress | [9,40] |
| 6 | Glutathione Reductase (GR) | Chloroplasts, mitochondria | Maintains GSH/GSSG ratio by reducing GSSG to GSH | Elevated levels of salt and metal stress | [42] |
| 7 | Dehydroascorbate reductase (DHAR) | Chloroplasts, cytosol, mitochondria | Regenerates ascorbate from dehydroascorbate Maintains ascorbate pool | Enhances drought and salt tolerance; protects photosynthetic machinery | [42,51] |
| 8 | Monodehydro-ascorbate reductase (MDHAR) | Chloroplasts, cytosol, mitochondria, peroxisomes | Reduces mono-dehydroascorbate to ascorbate using NADPH Maintains cellular redox balance | Improves tolerance to oxidative stress Enhances aluminum stress tolerance | [9,52] |
| 9 | Peroxiredoxin (PRX) | Chloroplasts, mitochondria, nucleus | Reduces hydrogen peroxide and organic hydroperoxides | Scavenges peroxides and peroxynitrite (ONOO−); enhances heat drought, and cold tolerance | [34] |
| 10 | Thioredoxin (Trx) | Chloroplasts, mitochondria, cytosol | Maintains protein thiol–disulfide balance Maintains protein redox homeostasis and regulates enzyme activities | Protein disulfide reduction and redox regulation; improves drought and salt tolerance | [35] |
| 11 | Glutaredoxins (GRX) | Chloroplasts, mitochondria, nucleus, cytosol | Catalyzes thiol–disulfide exchange reaction Protein deglutathionylation | Glutathione-dependent protein repair, and iron–sulfur (Fe-S) assembly; enhances heavy metal tolerance | [53] |
| 12 | Glutathione S-transferase (GST) | Cytosol, chloroplasts | Conjugates glutathione to various substrates for detoxification | GSH conjugation and detoxification of lipid peroxidation products; improves drought and salt resistance | [54] |
| 13 | Alternative oxidase (AOX) | Mitochondrial inner membrane | Alternative respiratory pathway enzyme Reduces ROS production | Bypasses complexes III/IV in the mitochondrial electron transport chain; enhances drought and salinity stress tolerance | [55] |
| 14 | Polyphenol oxidase (PPO) | Chloroplasts | Oxidizes phenolic compounds into quinones | Oxidizes phenolics to quinones | [45]. |
2.2. Non-Enzymatic Antioxidants
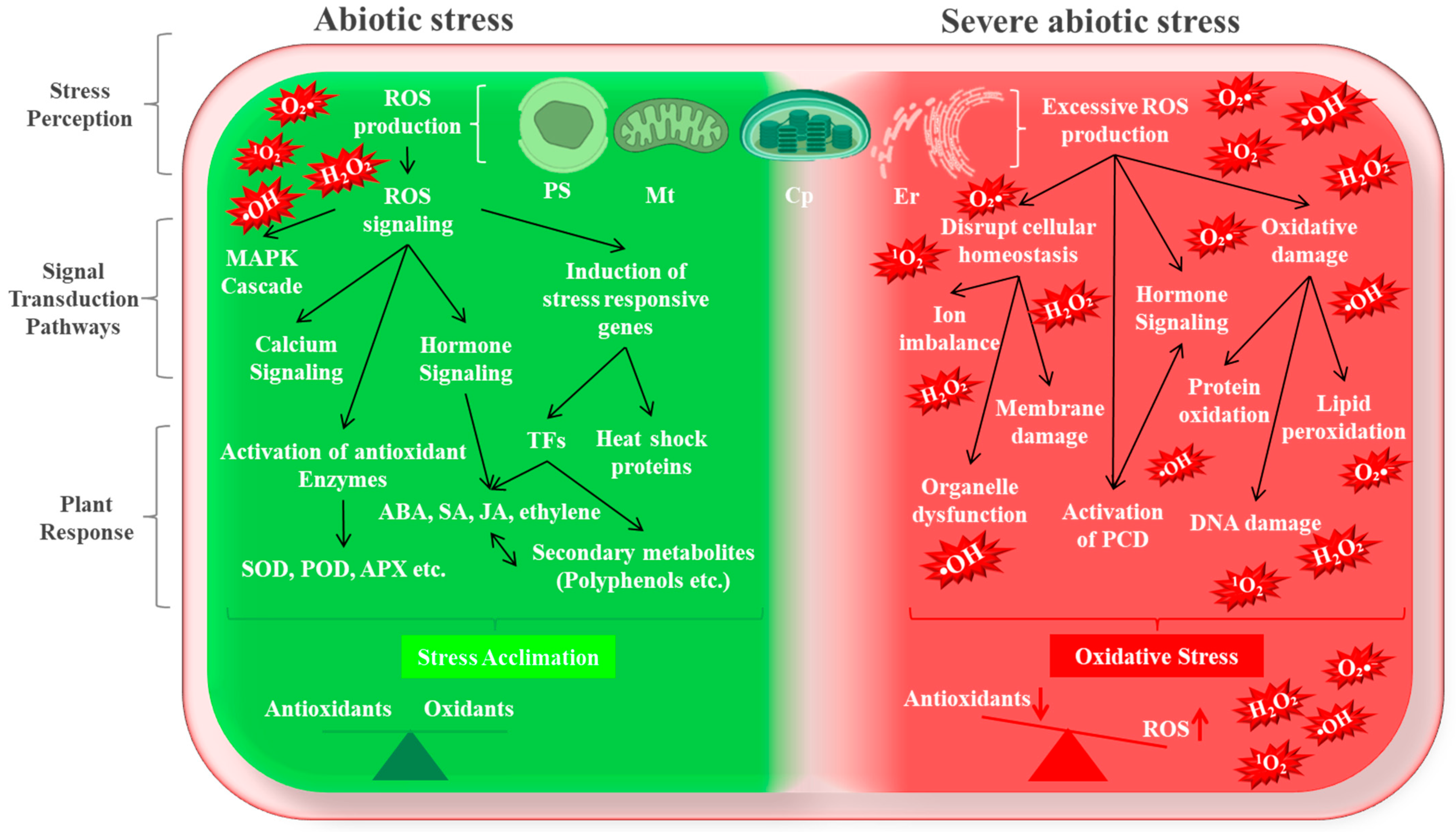
3. Types, Production Sites, and Signaling of Reactive Oxygen Species
3.1. Types of ROS
3.2. Production Sites of ROS
3.3. Signaling of Reactive Oxygen Species
4. Abiotic Stress-Induced Oxidative Stress
4.1. Heat (High Temperature) and Chilling (Low Temperature) Condition-Induced Oxidative Stress
4.2. Drought (Water Scarcity) and Flooding (Waterlogging)-Induced Oxidative Stress
4.3. Salt Stress-Induced Oxidative Stress
4.4. Heavy Metal Toxicity-Induced Oxidative Stress
5. Antioxidant Enzymatic and Non-Enzymatic Scavenging of ROS and Free Radicals
6. Antioxidative Defense Mechanisms Under Abiotic Stress
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Y.; Tilman, D.; Jin, Z.; Smith, P.; Barrett, C.B.; Zhu, Y.-G.; Burney, J.; D’Odorico, P.; Fantke, P.; Fargione, J. Climate Change Exacerbates the Environmental Impacts of Agriculture. Science 2024, 385, eadn3747. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, S.; Chen, J.; Yu, H.; Yang, T.; Wang, C.; Huang, S.; Chen, H.; Ao, X. Impacts of Global Climate Change on Agricultural Production: A Comprehensive Review. Agronomy 2024, 14, 1360. [Google Scholar] [CrossRef]
- Kumar, P.; Tokas, J.; Kumar, N.; Lal, M.; Singal, H.; Praveen Kumar, C. Climate Change Consequences and Its Impact on Agriculture and Food Security. Int. J. Chem. Stud. 2018, 6, 124–133. [Google Scholar]
- Liaqat, W.; Altaf, M.T.; Barutçular, C.; Nawaz, H.; Ullah, I.; Basit, A.; Mohamed, H.I. Ultraviolet-B Radiation in Relation to Agriculture in the Context of Climate Change: A Review. Cereal Res. Commun. 2024, 52, 1–24. [Google Scholar] [CrossRef]
- Rane, J.; Jangid, K.K. Impacts of Abiotic Stresses on Eco-Physiology of Crop in Changing Climate. In Climate Change Impacts on Soil-Plant-Atmosphere Continuum; Springer: Berlin/Heidelberg, Germany, 2024; pp. 427–445. [Google Scholar]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A Review of Plants Strategies to Resist Biotic and Abiotic Environmental Stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Wu, W.; Ma, B.-L. Assessment of Canola Crop Lodging under Elevated Temperatures for Adaptation to Climate Change. Agric. Meteorol. 2018, 248, 329–338. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; Al Harbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy Metal Induced Oxidative Stress Mitigation and ROS Scavenging in Plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of Organic Acids, Amino Acids and Phenolic Compounds on Antioxidant Characteristic of Zhenjiang Aromatic Vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Rai, G.K.; Kumar, P.; Choudhary, S.M.; Singh, H.; Adab, K.; Kosser, R.; Magotra, I.; Kumar, R.R.; Singh, M.; Sharma, R. Antioxidant Potential of Glutathione and Crosstalk with Phytohormones in Enhancing Abiotic Stress Tolerance in Crop Plants. Plants 2023, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Mukherjee, A.G.; Gopalakrishnan, A.V.; Wanjari, U.R.; Kannampuzha, S.; Murali, R.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; George, A. Protective Effects of Macromolecular Polyphenols, Metal (Zinc, Selenium, and Copper)-Polyphenol Complexes, and Pectin in Different Organs with an Emphasis on Arsenic Poisoning: A Review. Int. J. Biol. Macromol. 2023, 253, 126715. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Shad, M.A.; Aslam, M.Z.; Wang, J.; Wang, L. Widely Targeted LC-MS/MS Approach Provides Insights into Variations in Bioactive Flavonoid Compounds and Their Antioxidant Activities in Green, Red, and Purple Sugarcane. LWT Food Sci. Technol. 2024, 209, 116792. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.N.; Fujita, M. Hydrogen Peroxide Pretreatment Mitigates Cadmium-Induced Oxidative Stress in Brassica Napus, L.: An Intrinsic Study on Antioxidant Defense and Glyoxalase Systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef]
- Tanou, G.; Filippou, P.; Belghazi, M.; Job, D.; Diamantidis, G.; Fotopoulos, V.; Molassiotis, A. Oxidative and Nitrosative-based Signaling and Associated Post-translational Modifications Orchestrate the Acclimation of Citrus Plants to Salinity Stress. Plant J. 2012, 72, 585–599. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Liu, S.; Peng, X. Exogenous Application of Hydrogen Peroxide Alleviates Drought Stress in Cucumber Seedlings. South Afr. J. Bot. 2016, 106, 23–28. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N. Exogenous Low-Dose Hydrogen Peroxide Enhances Drought Tolerance of Soybean (Glycine max L.) through Inducing Antioxidant System. Acta Biol. Hung. 2016, 67, 169–183. [Google Scholar] [CrossRef]
- Basal, O.; Zargar, T.B.; Veres, S. Elevated Tolerance of Both Short-Term and Continuous Drought Stress during Reproductive Stages by Exogenous Application of Hydrogen Peroxide on Soybean. Sci. Rep. 2024, 14, 2200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shan, Y.; Li, Y.; He, J.; Jiang, Y. Hydrogen Peroxide Receptors Regulate Chilling Injury of Banana Fruit during Low-Temperature Storage. Postharvest Biol. Technol. 2024, 214, 112985. [Google Scholar] [CrossRef]
- Yamasaki, H.; Ogura, M.P.; Kingjoe, K.A.; Cohen, M.F. D-Cysteine-Induced Rapid Root Abscission in the Water Fern Azolla Pinnata: Implications for the Linkage between d-Amino Acid and Reactive Sulfur Species (RSS) in Plant Environmental Responses. Antioxidants 2019, 8, 411. [Google Scholar] [CrossRef]
- Sonmez, M.C.; Yirmibesoglu, S.S.S.; Ozgur, R.; Uzilday, B.; Turkan, I. Roles of Reactive Carbonyl Species (RCS) in Plant Response to Abiotic Stress. In ROS Signaling in Plants: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 101–130. [Google Scholar]
- Dutta, S.; Sengupta, P.; Samrot, A. V Physiological and Pathological Functions of Reactive Nitrogen Species (RNS) and Reactive Sulphur Species (RSS) on Male Reproductive Functions. J. Integr. Sci. Technol. 2024, 12, 755. [Google Scholar] [CrossRef]
- Rao, M.J.; Wu, S.; Duan, M.; Wang, L. Antioxidant Metabolites in Primitive, Wild, and Cultivated Citrus and Their Role in Stress Tolerance. Molecules 2021, 26, 5801. [Google Scholar] [CrossRef]
- Rao, M.J.; Ahmed, U.; Ahmed, M.H.; Duan, M.; Wang, J.; Wang, Y.; Wang, L. Comparison and Quantification of Metabolites and Their Antioxidant Activities in Young and Mature Leaves of Sugarcane. ACS Food Sci. Technol. 2021, 1, 362–373. [Google Scholar] [CrossRef]
- Rao, M.J.; Tahir Ul Qamar, M.; Wang, D.; Ali, Q.; Ma, L.; Han, S.; Duan, M.; Hu, L.; Wang, L. A High-Throughput Lipidomics and Transcriptomic Approach Reveals Novel Compounds from Sugarcane Linked with Promising Therapeutic Potential against COVID-19. Front. Nutr. 2022, 9, 988249. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Rai, K.K.; Kaushik, P. Free Radicals Mediated Redox Signaling in Plant Stress Tolerance. Life 2023, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Vidigal, P.; Carvalho, R.; Amâncio, S.; Carvalho, L. Peroxiredoxins Are Involved in Two Independent Signalling Pathways in the Abiotic Stress Protection in Vitis vinifera. Biol. Plant 2013, 57, 675–683. [Google Scholar] [CrossRef]
- Calderón, A.; Sevilla, F.; Jiménez, A. Redox Protein Thioredoxins: Function under Salinity, Drought and Extreme Temperature Conditions. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 123–162. [Google Scholar]
- Jiang, J.; Zhang, N.; Srivastava, A.K.; He, G.; Tai, Z.; Wang, Z.; Yang, S.; Xie, X.; Li, X. Superoxide Dismutase Positively Regulates Cu/Zn Toxicity Tolerance in Sorghum Bicolor by Interacting with Cu Chaperone for Superoxide Dismutase. J. Hazard. Mater. 2024, 480, 135828. [Google Scholar] [CrossRef]
- Carmo de Carvalho e Martins, M.d.; Martins; da Silva Santos Oliveira, A.S.; da Silva, L.A.A.; Primo, M.G.S.; de Carvalho Lira, V.B. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–25. [Google Scholar]
- Su, T.; Wang, P.; Li, H.; Zhao, Y.; Lu, Y.; Dai, P.; Ren, T.; Wang, X.; Li, X.; Shao, Q. The Arabidopsis Catalase Triple Mutant Reveals Important Roles of Catalases and Peroxisome-derived Signaling in Plant Development. J. Integr. Plant Biol. 2018, 60, 591–607. [Google Scholar] [CrossRef]
- Li, S. Novel Insight into Functions of Ascorbate Peroxidase in Higher Plants: More than a Simple Antioxidant Enzyme. Redox. Biol. 2023, 64, 102789. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Filiz, E.; Vatansever, R.; Kurtoglu, K.Y.; Koc, I.; Öztürk, M.X.; Anjum, N.A. Identification and Comparative Analysis of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Glutathione Peroxidase) in Selected Plants Employing Bioinformatics Approaches. Front. Plant Sci. 2016, 7, 301. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Rao, A.S.V.C.; Reddy, A.R. Glutathione Reductase: A Putative Redox Regulatory System in Plant Cells. In Sulfur Assimilation and Abiotic Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–147. [Google Scholar]
- Edwards, E.A.; Rawsthorne, S.; Mullineaux, P.M. Subcellular Distribution of Multiple Forms of Glutathione Reductase in Leaves of Pea (Pisum sativum L.). Planta 1990, 180, 278–284. [Google Scholar] [CrossRef]
- Madhu; Kaur, A.; Tyagi, S.; Shumayla; Singh, K.; Upadhyay, S.K. Exploration of Glutathione Reductase for Abiotic Stress Response in Bread Wheat (Triticum aestivum L.). Plant Cell Rep. 2022, 41, 639–654. [Google Scholar] [CrossRef]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Polidoros, A.N.; Scandalios, J.G. Role of Hydrogen Peroxide and Different Classes of Antioxidants in the Regulation of Catalase and Glutathione S-transferase Gene Expression in Maize (Zea mays L.). Physiol. Plant 1999, 106, 112–120. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–315. [Google Scholar]
- Uarrota, V.G.; Moresco, R.; Schmidt, E.C.; Bouzon, Z.L.; da Costa Nunes, E.; de Oliveira Neubert, E.; Peruch, L.A.M.; Rocha, M.; Maraschin, M. The Role of Ascorbate Peroxidase, Guaiacol Peroxidase, and Polysaccharides in Cassava (Manihot Esculenta Crantz) Roots under Postharvest Physiological Deterioration. Food Chem. 2016, 197, 737–746. [Google Scholar] [CrossRef]
- Romero-Puertas, M.C.; Corpas, F.J.; Sandalio, L.M.; Leterrier, M.; Rodríguez-Serrano, M.; Del Río, L.A.; Palma, J.M. Glutathione Reductase from Pea Leaves: Response to Abiotic Stress and Characterization of the Peroxisomal Isozyme. New Phytol. 2006, 170, 43–52. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Eltayeb, A.E.; Uddin, M.I.; Yamamoto, Y.; Tsuji, W.; Takeuchi, Y.; Tanaka, K. Overexpression of Dehydroascorbate Reductase, but Not Monodehydroascorbate Reductase, Confers Tolerance to Aluminum Stress in Transgenic Tobacco. Planta 2010, 231, 609–621. [Google Scholar] [CrossRef]
- Li, S. Redox Modulation Matters: Emerging Functions for Glutaredoxins in Plant Development and Stress Responses. Plants 2014, 3, 559–582. [Google Scholar] [CrossRef]
- Muslu, S.; Kasapoğlu, A.G.; Güneş, E.; Aygören, A.S.; Yiğider, E.; İlhan, E.; Aydın, M. Genome-Wide Analysis of Glutathione S-Transferase Gene Family in P. Vulgaris under Drought and Salinity Stress. Plant Mol. Biol. Rep. 2024, 42, 57–76. [Google Scholar] [CrossRef]
- Aziz, S.; Germano, T.A.; Thiers, K.L.L.; Batista, M.C.; de Souza Miranda, R.; Arnholdt-Schmitt, B.; Costa, J.H. Transcriptome Analyses in a Selected Gene Set Indicate Alternative Oxidase (AOX) and Early Enhanced Fermentation as Critical for Salinity Tolerance in Rice. Plants 2022, 11, 2145. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; De Oliveira, J.M.P.F.; Melo, P.; Santos, C. Tomato Plants Use Non-Enzymatic Antioxidant Pathways to Cope with Moderate UV-A/B Irradiation: A Contribution to the Use of UV-A/B in Horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Tang, X.; Huang, Y.; Liu, J.; Deng, X. CsCYT75B1, a Citrus CYTOCHROME P450 Gene, Is Involved in Accumulation of Antioxidant Flavonoids and Induces Drought Tolerance in Transgenic Arabidopsis. Antioxidants 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Xu, Y.; Huang, Y.; Tang, X.; Deng, X.; Xu, Q. Ectopic Expression of Citrus UDP-GLUCOSYL TRANSFERASE Gene Enhances Anthocyanin and Proanthocyanidins Contents and Confers High Light Tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 603. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Yang, M.; Li, M.; Wang, L. Sugarcane Rind Secondary Metabolites and Their Antioxidant Activities in Eleven Cultivated Sugarcane Varieties. Sugar Tech. 2022, 24, 1570–1582. [Google Scholar] [CrossRef]
- Szalai, G.; Kellős, T.; Galiba, G.; Kocsy, G. Glutathione as an Antioxidant and Regulatory Molecule in Plants under Abiotic Stress Conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Wang, C.; García-Caparros, P.; Li, Z.; Chen, F. A Comprehensive Review on Plant Ascorbic Acid. Trop. Plants 2024, 3, e042. [Google Scholar] [CrossRef]
- Wu, P.; Li, B.; Liu, Y.; Bian, Z.; Xiong, J.; Wang, Y.; Zhu, B. Multiple Physiological and Biochemical Functions of Ascorbic Acid in Plant Growth, Development, and Abiotic Stress Response. Int. J. Mol. Sci. 2024, 25, 1832. [Google Scholar] [CrossRef]
- Caferri, R.; Guardini, Z.; Bassi, R.; Dall’Osto, L. Assessing Photoprotective Functions of Carotenoids in Photosynthetic Systems of Plants and Green Algae. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 674, pp. 53–84. ISSN 00766879. [Google Scholar]
- Zulfiqar, S.; Sharif, S.; Saeed, M.; Tahir, A. Role of Carotenoids in Photosynthesis. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 147–187. [Google Scholar]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H.M. Role of Glycine Betaine in the Thermotolerance of Plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, T.; Liu, Y.; Wang, J.; Wang, Q.; Zhu, W. Effect of Exogenous Glycine Betaine on the Germination of Tomato Seeds under Cold Stress. Int. J. Mol. Sci. 2022, 23, 10474. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.-D.; Abd El-Gawad, H.G.; El-Yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S. GABA: A Key Player in Drought Stress Resistance in Plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef]
- Ashraf, U.; Anjum, S.A.; Naseer, S.; Abbas, A.; Abrar, M.; Nawaz, M.; Luo, K. Gamma Amino Butyric Acid (GABA) Application Modulated the Morpho-Physiological and Yield Traits of Fragrant Rice under Well-Watered and Drought Conditions. BMC Plant Biol. 2024, 24, 569. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Lu, L.; Zihan, W.; Qianyue, B.; Chungang, Z.; Shuheng, Z.; Jiali, P.; Jiaxin, Y.; Shuang, Z.; Jian, W. Gamma-Aminobutyric Acid (GABA) Improves Salinity Stress Tolerance in Soybean Seedlings by Modulating Their Mineral Nutrition, Osmolyte Contents, and Ascorbate-Glutathione Cycle. BMC Plant Biol. 2024, 24, 365. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Feng, X.; Piechatzek, A.; Zhang, S.; Konrad, K.R.; Kromdijk, J.; Hedrich, R.; Gilliham, M. The GABA Shunt Contributes to ROS Homeostasis in Guard Cells of Arabidopsis. New Phytol. 2024, 241, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef]
- Faizan, M.; Alam, P.; Rajput, V.D.; Shareen; Kaur, K.; Faraz, A.; Minkina, T.; Maqbool Ahmed, S.; Rajpal, V.R.; Hayat, S. Potential Role of Tocopherol in Protecting Crop Plants against Abiotic Stresses. Physiol. Mol. Biol. Plants 2023, 29, 1563–1575. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Rao, M.J.; Duan, M.; Eman, M.; Yuan, H.; Sharma, A.; Zheng, B. Comparative Analysis of Citrus Species’ Flavonoid Metabolism, Gene Expression Profiling, and Their Antioxidant Capacity under Drought Stress. Antioxidants 2024, 13, 1149. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, X.; Wang, X.; Dai, Q.; Zhan, X.; Gong, H.; Guo, J. Effects of Combined Application of Selenium and Various Plant Hormones on the Cold Stress Tolerance of Tomato Plants. Plant Soil 2024, 509, 471–489. [Google Scholar] [CrossRef]
- Omar, A.A.; Heikal, Y.M.; Zayed, E.M.; Shamseldin, S.A.M.; Salama, Y.E.; Amer, K.E.; Basuoni, M.M.; Abd Ellatif, S.; Mohamed, A.H. Conferring of Drought and Heat Stress Tolerance in Wheat (Triticum aestivum L.) Genotypes and Their Response to Selenium Nanoparticles Application. Nanomaterials 2023, 13, 998. [Google Scholar] [CrossRef]
- Zeeshan, M.; Wang, X.; Salam, A.; Wu, H.; Li, S.; Zhu, S.; Chang, J.; Chen, X.; Zhang, Z.; Zhang, P. Selenium Nanoparticles Boost the Drought Stress Response of Soybean by Enhancing Pigment Accumulation, Oxidative Stress Management and Ultrastructural Integrity. Agronomy 2024, 14, 1372. [Google Scholar] [CrossRef]
- Jan, A.U.; Hadi, F.; Ditta, A.; Suleman, M.; Ullah, M. Zinc-Induced Anti-Oxidative Defense and Osmotic Adjustments to Enhance Drought Stress Tolerance in Sunflower (Helianthus annuus L.). Environ. Exp. Bot. 2022, 193, 104682. [Google Scholar] [CrossRef]
- Malik, M.A.; Wani, A.H.; Mir, S.H.; Rehman, I.U.; Tahir, I.; Ahmad, P.; Rashid, I. Elucidating the Role of Silicon in Drought Stress Tolerance in Plants. Plant Physiol. Biochem. 2021, 165, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, Y.; Zhang, Y.; Shi, Y.; Hou, L.; Khan, A.; Zhang, R.; Zhang, Y. Mechanism of Exogenous Silicon in Enhancing Cold Stress Tolerance in Solanum Lycopersicum L. Seedlings: Insights from Resistance and Quality Indicators. Horticulturae 2024, 11, 4. [Google Scholar] [CrossRef]
- Bouhadi, M.; El Kouali, M.; Samir, K.; Elbouhmadi, K.; Talbi, M.; Fougrach, H. Exogenous Application of Thiamine and Nicotinic Acid Improves Tolerance and Morpho-Physiological Parameters of Lens Culinaris Under Lead (Pb) Exposure. J. Plant Growth Regul. 2024, 43, 4185–4198. [Google Scholar] [CrossRef]
- Alvi, G.B.; Iqbal, M.S.; Ghaith, M.M.S.; Haseeb, A.; Ahmed, B.; Qadir, M.I. Biogenic Selenium Nanoparticles (SeNPs) from Citrus Fruit Have Anti-Bacterial Activities. Sci. Rep. 2021, 11, 4811. [Google Scholar] [CrossRef]
- Maurya, A.K. Oxidative Stress in Crop Plants. In Agronomic Crops: Volume 3: Stress Responses and Tolerance; Springer: Berlin/Heidelberg, Germany, 2020; pp. 349–380. [Google Scholar]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond 1–3. Am. J. Clin. Nutr. 2005, 81, 215–217. [Google Scholar] [CrossRef]
- Canan, İ.; Gündoğdu, M.; Seday, U.; Oluk, C.A.; Karaşahin, Z.; Eroğlu, E.Ç.; Yazici, E.; Ünlü, M. Determination of Antioxidant, Total Phenolic, Total Carotenoid, Lycopene, Ascorbic Acid, and Sugar Contents of Citrus Species and Mandarin Hybrids. Turk. J. Agric. For. 2016, 40, 894–899. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, R.S.; Mishra, S.; Jadaun, J.S.; Sabir, F.; Sangwan, N.S. Effect of Cadmium Stress on Inductive Enzymatic and Nonenzymatic Responses of ROS and Sugar Metabolism in Multiple Shoot Cultures of Ashwagandha (Withania Somnifera Dunal). Protoplasma 2014, 251, 1031–1045. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive Oxygen Species Signalling in Plant Stress Responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, R.; Handa, N.; Kaur, H.; Rattan, A.; Yadav, P.; Gautam, V.; Kaur, R.; Bhardwaj, R. Redox Homeostasis in Plants under Abiotic Stress: Role of Electron Carriers, Energy Metabolism Mediators and Proteinaceous Thiols. Front. Environ. Sci. 2015, 3, 13. [Google Scholar] [CrossRef]
- Wani, K.I.; Naeem, M.; Castroverde, C.D.M.; Kalaji, H.M.; Albaqami, M.; Aftab, T. Molecular Mechanisms of Nitric Oxide (NO) Signaling and Reactive Oxygen Species (ROS) Homeostasis during Abiotic Stresses in Plants. Int. J. Mol. Sci. 2021, 22, 9656. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Jatav, H.S.; Aftab, T.; Pandey, S.; Mishra, U.N.; Chauhan, J.; Chand, S.; Indu; Saha, D.; Dadarwal, B.K. Roles of Nitric Oxide in Conferring Multiple Abiotic Stress Tolerance in Plants and Crosstalk with Other Plant Growth Regulators. J. Plant Growth Regul. 2021, 40, 2303–2328. [Google Scholar] [CrossRef]
- Dogra, V.; Kim, C. Singlet Oxygen Metabolism: From Genesis to Signaling. Front. Plant Sci. 2020, 10, 1640. [Google Scholar] [CrossRef]
- Dietz, K.-J. Thiol-Based Peroxidases and Ascorbate Peroxidases: Why Plants Rely on Multiple Peroxidase Systems in the Photosynthesizing Chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [CrossRef]
- Tano, D.W.; Kozlowska, M.A.; Easter, R.A.; Woodson, J.D. Multiple Pathways Mediate Chloroplast Singlet Oxygen Stress Signaling. Plant Mol. Biol. 2023, 111, 167–187. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.-P.; Niyogi, K.K. Non-Photochemical Quenching. A Response to Excess Light Energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and Responding to Excess Light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet Oxygen Production in Photosystem II and Related Protection Mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Goggin, F.L.; Fischer, H.D. Singlet Oxygen Signalling and Its Potential Roles in Plant Biotic Interactions. Plant Cell Environ. 2024, 47, 1957–1970. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A. Singlet Oxygen Production in Photosynthesis. J. Exp. Bot. 2005, 56, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Flors, C.; Fryer, M.J.; Waring, J.; Reeder, B.; Bechtold, U.; Mullineaux, P.M.; Nonell, S.; Wilson, M.T.; Baker, N.R. Imaging the Production of Singlet Oxygen in Vivo Using a New Fluorescent Sensor, Singlet Oxygen Sensor Green®. J. Exp. Bot. 2006, 57, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kim, C. Singlet Oxygen in Plants: From Genesis to Signaling. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2023; Volume 105, pp. 1–42. ISSN 00652296. [Google Scholar]
- Huang, L.; Liu, Y.; Wang, X.; Jiang, C.; Zhao, Y.; Lu, M.; Zhang, J. Peroxisome-Mediated Reactive Oxygen Species Signals Modulate Programmed Cell Death in Plants. Int. J. Mol. Sci. 2022, 23, 10087. [Google Scholar] [CrossRef]
- He, A.; Dean, J.M.; Lodhi, I.J. Peroxisomes as Cellular Adaptors to Metabolic and Environmental Stress. Trends Cell. Biol. 2021, 31, 656–670. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive Oxygen Species and Cell Signaling. Review. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Kunert, K.J.; Foyer, C.H. The Ascorbate/Glutathione Cycle. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2023; Volume 105, pp. 77–112. ISSN 00652296. [Google Scholar]
- Petřivalský, M. Compartmentalization in the Production of ROS and RNS in Horticultural Crops. In Oxygen, Nitrogen and Sulfur Species in Post-Harvest Physiology of Horticultural Crops; Elsevier: Amsterdam, The Netherlands, 2024; pp. 141–162. [Google Scholar]
- Lennicke, C.; Cochemé, H.M. Redox Metabolism: ROS as Specific Molecular Regulators of Cell Signaling and Function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Gideon, D.A.; Nirusimhan, V.; E., J.C.; Sudarsha, K.; Manoj, K.M. Mechanism of Electron Transfers Mediated by Cytochromes c and b 5 in Mitochondria and Endoplasmic Reticulum: Classical and Murburn Perspectives. J. Biomol. Struct. Dyn. 2022, 40, 9235–9252. [Google Scholar] [CrossRef]
- Duan, M.; Bao, L.; Eman, M.; Han, D.; Zhang, Y.; Zheng, B.; Yang, S.; Rao, M.J. The Ectopic Expression of the MpDIR1(t) Gene Enhances the Response of Plants from Arabidopsis Thaliana to Biotic Stress by Regulating the Defense Genes and Antioxidant Flavonoids. Plants 2024, 13, 2692. [Google Scholar] [CrossRef]
- Rao, M.J.; Ding, F.; Wang, N.; Deng, X.; Xu, Q. Metabolic Mechanisms of Host Species Against Citrus Huanglongbing (Greening Disease). CRC Crit. Rev. Plant Sci. 2018, 37, 496–511. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Wang, J.; Han, S.; Ma, L.; Mo, X.; Li, M.; Hu, L.; Wang, L. Transcriptomic and Widely Targeted Metabolomic Approach Identified Diverse Group of Bioactive Compounds, Antiradical Activities, and Their Associated Genes in Six Sugarcane Varieties. Antioxidants 2022, 11, 1319. [Google Scholar] [CrossRef]
- Sewelam, N.; Kazan, K.; Schenk, P.M. Global Plant Stress Signaling: Reactive Oxygen Species at the Cross-Road. Front. Plant. Sci. 2016, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Schlauch, K.; Tam, R.; Cortes, D.; Torres, M.A.; Shulaev, V.; Dangl, J.L.; Mittler, R. The Plant NADPH Oxidase RBOHD Mediates Rapid Systemic Signaling in Response to Diverse Stimuli. Sci. Signal. 2009, 2, ra45. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zheng, S.; Jiang, D.; Lu, J.; Huang, Z.; Liu, Z.; Zhou, H.; Zhuang, C.; Li, J. Initiation and Execution of Programmed Cell Death and Regulation of Reactive Oxygen Species in Plants. Int. J. Mol. Sci. 2021, 22, 12942. [Google Scholar] [CrossRef]
- Woodson, J.D.; Chory, J. Coordination of Gene Expression between Organellar and Nuclear Genomes. Nat. Rev. Genet. 2008, 9, 383–395. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.H.; Bones, A.M.; Hall, R.D. Plant Molecular Stress Responses Face Climate Change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Heidarvand, L.; Maali Amiri, R. What Happens in Plant Molecular Responses to Cold Stress? Acta Physiol. Plant 2010, 32, 419–431. [Google Scholar] [CrossRef]
- Volkov, R.A.; Panchuk, I.I.; Mullineaux, P.M.; Schöffl, F. Heat Stress-Induced H2O2 Is Required for Effective Expression of Heat Shock Genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef]
- Pnueli, L.; Liang, H.; Rozenberg, M.; Mittler, R. Growth Suppression, Altered Stomatal Responses, and Augmented Induction of Heat Shock Proteins in Cytosolic Ascorbate Peroxidase (Apx1)-deficient Arabidopsis Plants. Plant J. 2003, 34, 187–203. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in Rice Roots during Lysigenous Aerenchyma Formation under Oxygen-Deficient Conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Hong, C.-P. The NADPH Oxidase Rboh D Is Involved in Primary Hypoxia Signalling and Modulates Expression of Hypoxia-Inducible Genes under Hypoxic Stress. Environ. Exp. Bot. 2015, 115, 63–72. [Google Scholar] [CrossRef]
- Banti, V.; Mafessoni, F.; Loreti, E.; Alpi, A.; Perata, P. The Heat-Inducible Transcription Factor HsfA2 Enhances Anoxia Tolerance in Arabidopsis. Plant Physiol. 2010, 152, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.; Sun, L.; Jiao, Y.; Zhang, G.; Miao, C.; Hao, F. NADPH Oxidase AtrbohD and AtrbohF Function in ROS-Dependent Regulation of Na+/K+ Homeostasis in Arabidopsis under Salt Stress. J. Exp. Bot. 2012, 63, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kim, B.-G.; Cheong, Y.H.; Pandey, G.K.; Luan, S. A Ca2+ Signaling Pathway Regulates a K+ Channel for Low-K Response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 12625–12630. [Google Scholar] [CrossRef]
- Shin, R.; Schachtman, D.P. Hydrogen Peroxide Mediates Plant Root Cell Response to Nutrient Deprivation. Proc. Natl. Acad. Sci. USA 2004, 101, 8827–8832. [Google Scholar] [CrossRef]
- Rao, M.J.; Wang, H.; Lei, H.; Zhang, H.; Duan, X.; Bao, L.; Yang, C.; Han, D.; Zhang, Y.; Yang, S.; et al. LC-MS/MS-Based Metabolomic Study Provides Insights into Altitude-Dependent Variations in Flavonoid Profiles of Strawberries. Front. Plant Sci. 2025, 15, 1527212. [Google Scholar] [CrossRef]
- Hendrix, S.; Dard, A.; Meyer, A.J.; Reichheld, J.-P. Redox-Mediated Responses to High Temperature in Plants. J. Exp. Bot. 2023, 74, 2489–2507. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Saud, S.; Khan, F.; Hassan, S.; Amanullah; Nasim, W.; Arif, M.; Wang, F.; Huang, J. Exogenously Applied Plant Growth Regulators Affect Heat-stressed Rice Pollens. J. Agron. Crop Sci. 2016, 202, 139–150. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; He, L.; Zhou, Q.; Yu, J.; Hui, D.; Huang, D. Exogenous Glutathione Improves High Root-Zone Temperature Tolerance by Modulating Photosynthesis, Antioxidant and Osmolytes Systems in Cucumber Seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef]
- Vacca, R.A.; de Pinto, M.C.; Valenti, D.; Passarella, S.; Marra, E.; De Gara, L. Production of Reactive Oxygen Species, Alteration of Cytosolic Ascorbate Peroxidase, and Impairment of Mitochondrial Metabolism Are Early Events in Heat Shock-Induced Programmed Cell Death in Tobacco Bright-Yellow 2 Cells. Plant Physiol. 2004, 134, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Perumal, R.; Jagadish, S.V.K.; Ciampitti, I.A.; Welti, R.; Prasad, P.V. V Sensitivity of Sorghum Pollen and Pistil to High-temperature Stress. Plant Cell Environ. 2018, 41, 1065–1082. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Rizwan, M.; Ali, S.; Shahid, M.R.; Alamri, S.A.; Alyemeni, M.N.; Ahmad, P. Exogenously Applied Growth Regulators Protect the Cotton Crop from Heat-Induced Injury by Modulating Plant Defense Mechanism. Sci. Rep. 2018, 8, 17086. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High Temperature and Drought Stress Cause Abscisic Acid and Reactive Oxygen Species Accumulation and Suppress Seed Germination Growth in Rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, K.; Wang, M.; Wang, X.; Li, H.; Wu, Y. Production, Functions and Scavenging Mechanisms of Reactive Oxygen Species in Plants under Low-Temperature Stress. Maejo Int. J. Sci. Technol. 2023, 17, 210. [Google Scholar]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S. Effects of Melatonin on Anti-Oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Liu, T.; Ye, X.; Li, M.; Li, J.; Qi, H.; Hu, X. H2O2 and NO Are Involved in Trehalose-Regulated Oxidative Stress Tolerance in Cold-Stressed Tomato Plants. Environ. Exp. Bot. 2020, 171, 103961. [Google Scholar] [CrossRef]
- Ghanbari, F.; Sayyari, M. Controlled Drought Stress Affects the Chilling-Hardening Capacity of Tomato Seedlings as Indicated by Changes in Phenol Metabolisms, Antioxidant Enzymes Activity, Osmolytes Concentration and Abscisic Acid Accumulation. Sci. Hortic. 2018, 229, 167–174. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of Polyamines, Abscisic Acid, Nitric Oxide, and Hydrogen Peroxide under Chilling Stress in Tomato (Lycopersicon Esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef]
- Xue, M.; Guo, T.; Ren, M.; Wang, Z.; Tang, K.; Zhang, W.; Wang, M. Constitutive Expression of Chloroplast Glycerol-3-Phosphate Acyltransferase from Ammopiptanthus Mongolicus Enhances Unsaturation of Chloroplast Lipids and Tolerance to Chilling, Freezing and Oxidative Stress in Transgenic Arabidopsis. Plant Physiol. Biochem. 2019, 143, 375–387. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; Wiley: Hoboken, NJ, USA, 2013; pp. 209–250. [Google Scholar]
- Mirza, H.; Kamrun, N.; Mahabub, A.M.; Masayuki, F. Regulation of Reactive Oxygen Species Metabolism and Glyoxalase Systems by Exogenous Osmolytes Confers Thermotolerance in Brassica Napus. Gesunde Pflanz. 2020, 72, 3–16. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Exogenous Nitric Oxide Donor and Arginine Provide Protection against Short-Term Drought Stress in Wheat Seedlings. Physiol. Mol. Biol. Plants 2018, 24, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Abideen, Z.; Koyro, H.; Huchzermeyer, B.; Ansari, R.; Zulfiqar, F.; Gul, B. Ameliorating Effects of Biochar on Photosynthetic Efficiency and Antioxidant Defence of Phragmites Karka under Drought Stress. Plant Biol. 2020, 22, 259–266. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin Reduces Oxidative Stress and Promotes Drought Tolerance in Young Coffea arabica L. Plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Saha, I.; De, A.K.; Sarkar, B.; Ghosh, A.; Dey, N.; Adak, M.K. Cellular Response of Oxidative Stress When Sub1A QTL of Rice Receives Water Deficit Stress. Plant Sci. Today 2018, 5, 84–94. [Google Scholar] [CrossRef]
- Satish, L.; Rency, A.S.; Ramesh, M. Spermidine Sprays Alleviate the Water Deficit-Induced Oxidative Stress in Finger Millet (Eleusine Coracana L. Gaertn.) Plants. 3 Biotech 2018, 8, 63. [Google Scholar] [CrossRef]
- Malhotra, C.; Kapoor, R.T.; Ganjewala, D.; Singh, N. Sodium Silicate Mediated Response of Antioxidative Defense System in Lycopersicon Esculentum Mill. under Water Stress. Int. J. Phytomed. 2017, 9, 364–378. [Google Scholar] [CrossRef]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Tahir Ul Qamar, M.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.B.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-Based Metabolomics Approach Identified Novel Antioxidant Flavonoids Associated with Drought Tolerance in Citrus Species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Khan, M.I.R.; Fujita, M. Silicon-Mediated Regulation of Antioxidant Defense and Glyoxalase Systems Confers Drought Stress Tolerance in Brassica napus L. S. Afr. J. Bot. 2018, 115, 50–57. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Anee, T.I.; Parvin, K.; Fujita, M. Nitric Oxide Pretreatment Enhances Antioxidant Defense and Glyoxalase Systems to Confer PEG-Induced Oxidative Stress in Rapeseed. J. Plant Interact. 2017, 12, 323–331. [Google Scholar] [CrossRef]
- Rezayian, M.; Ebrahimzadeh, H.; Niknam, V. Nitric Oxide Stimulates Antioxidant System and Osmotic Adjustment in Soybean under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 20, 1122–1132. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum Lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Filippou, P.; Antoniou, C.; Fotopoulos, V. Effect of Drought and Rewatering on the Cellular Status and Antioxidant Response of Medicago Truncatula Plants. Plant Signal Behav. 2011, 6, 270–277. [Google Scholar] [CrossRef]
- Kusvuran, S.; Dasgan, H.Y. Effects of Drought Stress on Physiological and Biochemical Changes in Phaseolus Vulgaris L. Legume Res.-Int. J. 2017, 40, 55–62. [Google Scholar]
- Nurrahma, A.H.I.; Putri, H.H.; Syahadat, R.M. Scientific Research Trends of Flooding Stress in Plant Science and Agriculture Subject Areas (1962–2021). ASEAN J. Sci. Eng. 2023, 3, 163–178. [Google Scholar] [CrossRef]
- Zhang, R.D.; Zhou, Y.F.; Yue, Z.X.; Chen, X.F.; Cao, X.; Xu, X.X.; Xing, Y.F.; Jiang, B.; Ai, X.Y.; Huang, R.D. Changes in Photosynthesis, Chloroplast Ultrastructure, and Antioxidant Metabolism in Leaves of Sorghum under Waterlogging Stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative Damage and Antioxidant Defense in Sesamum Indicum after Different Waterlogging Durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef]
- Rasheed, R.; Iqbal, M.; Ashraf, M.A.; Hussain, I.; Shafiq, F.; Yousaf, A.; Zaheer, A. Glycine Betaine Counteracts the Inhibitory Effects of Waterlogging on Growth, Photosynthetic Pigments, Oxidative Defence System, Nutrient Composition, and Fruit Quality in Tomato. J. Hortic. Sci. Biotechnol. 2018, 93, 385–391. [Google Scholar] [CrossRef]
- Luan, H.; Shen, H.; Pan, Y.; Guo, B.; Lv, C.; Xu, R. Elucidating the Hypoxic Stress Response in Barley (Hordeum vulgare L.) during Waterlogging: A Proteomics Approach. Sci. Rep. 2018, 8, 9655. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, E.J. Waterlogging Induced Oxidative Stress and the Mortality of the Antarctic Plant, Deschampsia antarctica. J. Ecol. Environ. 2019, 43, 29. [Google Scholar] [CrossRef]
- Wang, R.R.-C.; Xu, S.S.; Monaco, T.A.; Robbins, M.D. Dual Mechanisms of Salinity Tolerance in Wheat Germplasm Lines W4909 and W4910. Int. J. Mol. Sci. 2024, 25, 12892. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Farooq, A. Effect of Salinity on Cadmium Tolerance, Ionic Homeostasis and Oxidative Stress Responses in Conocarpus Exposed to Cadmium Stress: Implications for Phytoremediation. Ecotoxicol. Environ. Saf. 2019, 171, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-W.; Kong, X.-W.; Wang, N.; Wang, T.-T.; Chen, J.; Shi, Z.Q. Thymol Confers Tolerance to Salt Stress by Activating Anti-Oxidative Defense and Modulating Na+ Homeostasis in Rice Root. Ecotoxicol. Environ. Saf. 2020, 188, 109894. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined Effects of Brassinosteroid and Kinetin Mitigates Salinity Stress in Tomato through the Modulation of Antioxidant and Osmolyte Metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; El-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2019, 10, 26. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alam, P.; Alyemeni, M.N.; Wijaya, L.; Ali, S.; Ashraf, M. Silicon (Si) Supplementation Alleviates NaCl Toxicity in Mung Bean [Vigna radiata (L.) Wilczek] through the Modifications of Physio-Biochemical Attributes and Key Antioxidant Enzymes. J. Plant Growth Regul. 2019, 38, 70–82. [Google Scholar] [CrossRef]
- Hindala, M.R.; Joshi, R.; Patni, V. Reactive Oxygen Species and Oxidative Damage in Halophytes Under Salt Stress. In Physiology of Halophytes; Apple Academic Press: New Jersey, NJ, USA, 2025; pp. 337–372. [Google Scholar]
- Arora, M.; Saxena, P.; Abdin, M.Z.; Varma, A. Interaction between Piriformospora Indica and Azotobacter Chroococcum Diminish the Effect of Salt Stress in Artemisia Annua L. by Enhancing Enzymatic and Non-Enzymatic Antioxidants. Symbiosis 2020, 80, 61–73. [Google Scholar] [CrossRef]
- Lalarukh, I.; Shahbaz, M. Response of Antioxidants and Lipid Peroxidation to Exogenous Application of Alpha-Tocopherol in Sunflower (Helianthus annuus L.) under Salt Stress. Pak. J. Bot. 2020, 52, 75–83. [Google Scholar] [CrossRef]
- Tariq, A.; Shahbaz, M. Glycinebetaine Induced Modulation in Oxidative Defense System and Mineral Nutrients Sesame (Sesamum indicum L.) under Saline Regimes. Pak. J. Bot. 2020, 52, 775–782. [Google Scholar] [CrossRef]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Aouani, M.E.; Polidoros, A.N. Alternative Oxidase 1 (Aox1) Gene Expression in Roots of Medicago truncatula Is a Genotype-Specific Component of Salt Stress Tolerance. J. Plant Physiol. 2013, 170, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Alaraidh, I.A.; Migdadi, H.; Alghamdi, S.; Khan, M.A.; Ahmad, P. Physiological, Biochemical, and Antioxidant Properties of Two Genotypes of Vicia Faba Grown under Salinity Stress. Pak. J. Bot. 2019, 51, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Bouchagier, P.; Skotti, E.; Fotopoulos, V. Proline and Reactive Oxygen/Nitrogen Species Metabolism Is Involved in the Tolerant Response of the Invasive Plant Species Ailanthus altissima to Drought and Salinity. Environ. Exp. Bot. 2014, 97, 1–10. [Google Scholar] [CrossRef]
- Das, A.; Pal, S.; Hasanuzzaman, M.; Adak, M.K.; Sil, S.K. Mitigation of Aluminum Toxicity in Rice Seedlings Using Biofabricated Selenium Nanoparticles and Nitric Oxide: Synergistic Effects on Oxidative Stress Tolerance and Sulfur Metabolism. Chemosphere 2025, 370, 143940. [Google Scholar] [CrossRef]
- Mahajan, P.; Khanna, V. Nanoparticle-Mediated Regulation of Chromium Toxicity in Plants: Unveiling the Mechanism at Cellular Level. In Sustainable Development Goals Towards Environmental Toxicity and Green Chemistry; Springer: Berlin/Heidelberg, Germany, 2025; pp. 269–290. ISBN 3031773276. [Google Scholar]
- Kumar, U.; Kumar, I.; Singh, P.K.; Dwivedi, A.; Singh, P.; Mishra, S.; Seth, C.S.; Sharma, R.K. Nickel Contamination in Terrestrial Ecosystems: Insights into Impacts, Phytotoxicity Mechanisms, and Remediation Technologies. Rev. Environ. Contam. Toxicol. 2025, 263, 2. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.M.; Nahar, K.; Mohsin, S.M.; Bhuyan, M.H.M.B.; Parvin, K.; Hawrylak-Nowak, B.; Fujita, M. Silicon-Induced Antioxidant Defense and Methylglyoxal Detoxification Works Coordinately in Alleviating Nickel Toxicity in Oryza sativa L. Ecotoxicology 2019, 28, 261–276. [Google Scholar] [CrossRef]
- El-Amier, Y.; Elhindi, K.; El-Hendawy, S.; Al-Rashed, S.; Abd-ElGawad, A. Antioxidant System and Biomolecules Alteration in Pisum Sativum under Heavy Metal Stress and Possible Alleviation by 5-Aminolevulinic Acid. Molecules 2019, 24, 4194. [Google Scholar] [CrossRef]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and Biochemical Mechanisms of Spermine-Induced Cadmium Stress Tolerance in Mung Bean (Vigna radiata L.) Seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218. [Google Scholar] [CrossRef]
- Gupta, D.K.; Pena, L.B.; Romero-Puertas, M.C.; Hernández, A.; Inouhe, M.; Sandalio, L.M. NADPH Oxidases Differentially Regulate ROS Metabolism and Nutrient Uptake under Cadmium Toxicity. Plant Cell Environ. 2017, 40, 509–526. [Google Scholar] [CrossRef]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the Signaling Molecules Hydrogen Sulfide and Hydrogen Peroxide and Their Role in Vacuolar H+-ATPase Regulation in Cadmium-stressed Cucumber Roots. Physiol. Plant 2019, 166, 688–704. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Combined Kinetin and Spermidine Treatments Ameliorate Growth and Photosynthetic Inhibition in Vigna Angularis by Up-Regulating Antioxidant and Nitrogen Metabolism under Cadmium Stress. Biomolecules 2020, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant Growth Regulators Improve Growth, Photosynthesis, Mineral Nutrient and Antioxidant System under Cadmium Stress in Menthol Mint (Mentha Arvensis L.). Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hasanuzzaman, M.; Suzuki, T.; Fujita, M. Polyamines-Induced Aluminum Tolerance in Mung Bean: A Study on Antioxidant Defense and Methylglyoxal Detoxification Systems. Ecotoxicology 2017, 26, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous Glutathione Attenuates Lead-Induced Oxidative Stress in Wheat by Improving Antioxidant Defense and Physiological Mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Yadu, B.; Chandrakar, V.; Tamboli, R.; Keshavkant, S. Dimethylthiourea Antagonizes Oxidative Responses by Up-Regulating Expressions of Pyrroline-5-Carboxylate Synthetase and Antioxidant Genes under Arsenic Stress. Int. J. Environ. Sci. Technol. 2019, 16, 8401–8410. [Google Scholar] [CrossRef]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of Salinity on Physiological, Biochemical and Photostabilizing Attributes of Two Genotypes of Quinoa (Chenopodium Quinoa Willd.) Exposed to Arsenic Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109814. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V. Influence of Heavy Metals (Ni, Cu, and Zn) on Nitro-Oxidative Stress Responses, Proteome Regulation and Allergen Production in Basil (Ocimum Basilicum L.) Plants. Front. Plant Sci. 2018, 9, 862. [Google Scholar] [CrossRef]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-Induced Oxidative Stress and Plant Mitochondria. Int. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef]
- Smejkal, G.B.; Kakumanu, S. Enzymes and Their Turnover Numbers. Expert Rev. Proteom. 2019, 16, 543–544. [Google Scholar] [CrossRef]
- Veljović Jovanović, S.; Kukavica, B.; Vidović, M.; Morina, F.; Menckhoff, L. Class III Peroxidases: Functions, Localization and Redox Regulation of Isoenzymes. In Antioxidants and Antioxidant Enzymes in Higher Plants; Springer: Berlin/Heidelberg, Germany, 2018; pp. 269–300. [Google Scholar]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An Introduction to Antioxidants and Their Roles in Plant Stress Tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–23. [Google Scholar]
- Singh, A.; Satheeshkumar, P.K. Reactive Oxygen Species (ROS) and ROS Scavengers in Plant Abiotic Stress Response. In Stress Biology in Photosynthetic Organisms: Molecular Insights and Cellular Responses; Springer: Berlin/Heidelberg, Germany, 2024; pp. 41–63. [Google Scholar]
- Dixon, D.P.; Skipsey, M.; Edwards, R. Roles for Glutathione Transferases in Plant Secondary Metabolism. Phytochemistry 2010, 71, 338–350. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbate, Tocopherol and Carotenoids: Metabolism, Pathway Engineering and Functions. In Antioxidants and Reactive Oxygen Species in Plants; Wiley: Honoken, NJ, USA, 2005; pp. 53–86. [Google Scholar]
- Sita, K.; Kumar, V. Role of Gamma Amino Butyric Acid (GABA) against Abiotic Stress Tolerance in Legumes: A Review. Plant Physiol. Rep. 2020, 25, 654–663. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; López-Martínez, L.X.; Contreras-Angulo, L.A.; Elizalde-Romero, C.A.; Heredia, J.B. Plant Alkaloids: Structures and Bioactive Properties. In Plant-Derived Bioactives; Springer: Berlin/Heidelberg, Germany, 2020; pp. 85–117. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Cizmarova, B.; Hubkova, B.; Birkova, A. Quercetin as an Effective Antioxidant against Superoxide Radical. Funct. Food Sci.-Online 2023, 3, 15–25. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Saviranta, N.M.M.; Veeroos, L.; Granlund, L.J.; Hassinen, V.H.; Kaarniranta, K.; Karjalainen, R.O. Plant Flavonol Quercetin and Isoflavone Biochanin a Differentially Induce Protection against Oxidative Stress and Inflammation in ARPE-19 Cells. Food Res. Int. 2011, 44, 109–113. [Google Scholar] [CrossRef]
- Wang, F.; Ren, X.; Zhang, F.; Qi, M.; Zhao, H.; Chen, X.; Ye, Y.; Yang, J.; Li, S.; Zhang, Y. A R2R3-Type MYB Transcription Factor Gene from Soybean, GmMYB12, Is Involved in Flavonoids Accumulation and Abiotic Stress Tolerance in Transgenic Arabidopsis. Plant Biotechnol. Rep. 2019, 13, 219–233. [Google Scholar] [CrossRef]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunková, M.; Barbierikova, Z.; Malcek, M.; Alwasel, S.H.; Alhazza, I.M.; Rhodes, C.J.; Valko, M. The Effect of Luteolin on DNA Damage Mediated by a Copper Catalyzed Fenton Reaction. J. Inorg. Biochem. 2022, 226, 111635. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and Calcium Modulate the Production of Rosmarinic Acid, Luteolin, and Apigenin in Dracocephalum Kotschyi under Salinity Stress. Phytochemistry 2020, 177, 112422. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Geng, Z.; Wang, Y.; Wang, Y.; Liu, S.; Chen, C.; Song, A.; Jiang, J.; Chen, S.; Chen, F. A Novel Transcription Factor CmMYB012 Inhibits Flavone and Anthocyanin Biosynthesis in Response to High Temperatures in Chrysanthemum. Hortic. Res. 2021, 8, 248. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The Antioxidant Activity of Coumarins and Flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Dędek, K.; Rosicka-Kaczmarek, J.; Nebesny, E.; Kowalska, G. Characteristics and Biological Properties of Ferulic Acid. Biotechnol. Food Sci. 2019, 83, 71–85. [Google Scholar]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants; IntechOpen: London, UK, 2019; ISBN 1789239206. [Google Scholar]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Mughal, A.; Jabeen, N.; Ashraf, K.; Sultan, K.; Farhan, M.; Hussain, M.I.; Deng, G.; Alsudays, I.M.; Saleh, M.A.; Tariq, S. Exploring the Role of Caffeic Acid in Mitigating Abiotic Stresses in Plants: A Review. Plant Stress 2024, 12, 100487. [Google Scholar] [CrossRef]
- Wilson, R.A.; Sangha, M.K.; Banga, S.S.; Atwal, A.K.; Gupta, S. Heat Stress Tolerance in Relation to Oxidative Stress and Antioxidants in Brassica juncea. J. Environ. Biol. 2014, 35, 383. [Google Scholar]
- Chugh, P.; Kaur, J.; Grewal, S.K.; Singh, S.; Agrawal, S.K. Upregulation of Superoxide Dismutase and Catalase along with Proline Accumulation Mediates Heat Tolerance in Lentil (Lens culinaris Medik.) Genotypes during Reproductive Stage. Indian J. Agric. Biochem. 2017, 30, 195–199. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective Role of Antioxidant Enzymes under High Temperature Stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Mazumdar, P.; Kumar, D.; Sharma, G.D.; Sahoo, L.; Sanjib, P. Excess Copper Induced Oxidative Stress and Response of Antioxidants in Rice. Plant Physiol. Biochem. 2012, 53, 33–39. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and Metabolic Changes of Purslane (Portulaca Oleracea L.) in Response to Drought, Heat, and Combined Stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate Peroxidase 1 Plays a Key Role in the Response of Arabidopsis Thaliana to Stress Combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of Antioxidant Defense System Is Associated with Combined Drought and Heat Stress Tolerance in Citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef]
- Selote, D.S.; Khanna-Chopra, R. Drought Acclimation Confers Oxidative Stress Tolerance by Inducing Co-ordinated Antioxidant Defense at Cellular and Subcellular Level in Leaves of Wheat Seedlings. Physiol. Plant 2006, 127, 494–506. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High Temperature Stress Tolerance in Wheat Genotypes: Role of Antioxidant Defence Enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Esim, N.; Atici, O. Nitric Oxide Improves Chilling Tolerance of Maize by Affecting Apoplastic Antioxidative Enzymes in Leaves. Plant Growth Regul. 2014, 72, 29–38. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, M.S.; Mahmud, J.-A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-Induced Salt Stress Tolerance in Rice Seedlings: Regulation of Ion Homeostasis, Antioxidant Defense and Glyoxalase Systems. Physiol. Mol. Biol. Plants 2016, 22, 291–306. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Xie, J.; Li, T.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Herbicide Isoproturon Aggravates the Damage of Low Temperature Stress and Exogenous Ascorbic Acid Alleviates the Combined Stress in Wheat Seedlings. Plant Growth Regul. 2018, 84, 293–301. [Google Scholar] [CrossRef]
- Islam, F.; Ali, B.; Wang, J.; Farooq, M.A.; Gill, R.A.; Ali, S.; Wang, D.; Zhou, W. Combined Herbicide and Saline Stress Differentially Modulates Hormonal Regulation and Antioxidant Defense System in Oryza Sativa Cultivars. Plant Physiol. Biochem. 2016, 107, 82–95. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, C.B. Chilling Stress-Induced Changes of Antioxidant Enzymes in the Leaves of Cucumber: In Gel Enzyme Activity Assays. Plant Sci. 2000, 159, 75–85. [Google Scholar] [CrossRef]
- Costa, H.; Gallego, S.M.; Tomaro, M.L. Effect of UV-B Radiation on Antioxidant Defense System in Sunflower Cotyledons. Plant Sci. 2002, 162, 939–945. [Google Scholar] [CrossRef]
- Qi, C.; Lin, X.; Li, S.; Liu, L.; Wang, Z.; Li, Y.; Bai, R.; Xie, Q.; Zhang, N.; Ren, S. SoHSC70 Positively Regulates Thermotolerance by Alleviating Cell Membrane Damage, Reducing ROS Accumulation, and Improving Activities of Antioxidant Enzymes. Plant Sci. 2019, 283, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Aftab, T.; Khan, M.M.A.; Idrees, M.; Naeem, M.; Ram, M. Boron Induced Oxidative Stress, Antioxidant Defence Response and Changes in Artemisinin Content in Artemisia annua L. J. Agron. Crop Sci. 2010, 196, 423–430. [Google Scholar] [CrossRef]
| Serial No. | Non-Enzymatic Antioxidant | Location | Function | Stress Tolerance | Reference |
|---|---|---|---|---|---|
| 1 | Ascorbic Acid (Vitamin C) | Chloroplasts, cytosol, vacuoles, mitochondria | Neutralizes ROS Regenerates vitamin E Acts as an enzyme cofactor in various physiological processes Key role in photoprotection | Acts as the first line of defense against ROS Protects photosynthetic machinery during heat/drought Maintains cell membrane integrity under salt stress Enhances cold tolerance Crucial for stomatal regulation during water stress | [61] |
| 2 | Glutathione (GSH) | Chloroplasts, mitochondria, cytosol, nucleus | Maintains cellular redox balance Detoxifies heavy metals Protects proteins from oxidation Regenerates ascorbate (AsA-GSH cycle) | Primary defense against heavy metal toxicity Maintains cellular redox state during oxidative stress Key role in xenobiotic detoxification Protects protein thiols during heat stress Essential for drought-tolerance mechanisms | [13] |
| 3 | Tocopherols (Vitamin E) | Cell membranes, chloroplast membranes, thylakoids | Protects membrane lipids Scavenges lipid peroxyl radicals (prevents lipid peroxidation) Maintains membrane stability Protects photosystem II | Prevents lipid peroxidation under stress Stabilizes membranes during temperature extremes Protects chloroplast function during high light stress Enhances drought resistance Critical for cold stress tolerance | [73] |
| 4 | Carotenoids | Chloroplasts, chromoplasts | Quenches singlet oxygen Protects chlorophyll Dissipates excess light energy | Protects photosynthetic apparatus from photo-oxidation Dissipates excess light energy as heat Stabilizes thylakoids under high temperatures Light stress adaptation by dissipating energy as heat | [63,64,74] |
| 5 | Phenolic Compounds | Vacuoles, cell walls, cytosol | ROS scavenging Metal chelation UV screening Cell wall lignification | Abiotic stress protection Enhanced pathogen resistance UV radiation screening Antioxidant activity | [75,76] |
| 6 | Flavonoids | Vacuoles, cell wall | UV-B radiation protection ROS scavenging Osmolyte function during drought Metal chelation | Strong UV-B radiation protection Drought stress tolerance Salt-stress amelioration Metal toxicity chelation | [58,77] |
| 7 | GABA (Gamma-aminobutyric acid) | Cytosol, mitochondria | Regulates stomatal closure Protects cellular membranes Maintains ion homeostasis Stabilizes membrane integrity Scavenges ROS Enhances antioxidant enzyme activities Maintains redox homeostasis | Chelates heavy metals Enhances frost tolerance Improves salt and drought tolerance Acts as a heat shock protectant Maintains photosynthetic efficiency under stress | [68,69,70] |
| 8 | Proline | Cytosol, chloroplasts | Osmolyte function Membrane stabilizer ROS scavenger Metal chelator Protein structure stabilizer | Osmolyte function during drought/salt stress Membrane stabilization under extreme temperatures ROS scavenging during oxidative stress Protein stabilization during dehydration Metal stress tolerance | [65] |
| 9 | Glycine Betaine | Chloroplasts, cytosol | Osmotic adjustment Protein structure protection Membrane integrity maintenance Photosynthetic apparatus protection ROS detoxification | Temperature stress tolerance Minimizes ROS during water deficit conditions. Cold stress protection | [66,67] |
| 10 | Selenium (Se) | Throughout plant tissues | Activates antioxidant defense mechanisms Enhances photosynthetic efficiency Enhances selenoproteins which combat ROS | Increases drought tolerance by regulating water status Protects against oxidative damage during salt stress Improves heavy metal stress tolerance | [78,79,80] |
| 11 | Zinc (Zn) | Cell wall, membranes, cytoplasm | Cofactor for antioxidant enzymes Maintains membrane stability Regulates stomatal function under drought Enhances root growth and water uptake | Regulates stomatal function during drought Involved in auxin metabolism and stress signaling Protects chloroplast structures during heat stress | [81] |
| 12 | Silicon (Si) | Cell walls, epidermis | Strengthens cell walls Improves water retention Forms a protective layer in cell walls Improves nutrients and water uptake | Improves water use efficiency during drought Reduces metal toxicity through complexation Enhances structural stability against lodging Regulates osmolyte accumulation under salt stress | [82,83] |
| 13 | Thiamine and nicotinic acid | Chloroplasts, mitochondria, and cytoplasm | Modulates MAPK and ABA pathways Upregulates phytochelatins Enhances GR, APX, and phenolic compounds Chelates lead (Pb) via phenolics/NADPH systems | Reduced Pb-induced oxidative damage (lower H2O2 and MDA) Enhanced activity of SOD, CAT, and POD, improving redox balance Higher photosynthetic efficiency | [84] |
| Serial No. | ROS Type | ROS Production Sites | Primary Function | Cellular Reactions and Damage | Effects on Plants | Key Signaling Roles | Scavenging Mechanisms |
|---|---|---|---|---|---|---|---|
| 1 | Superoxide (O2•−) | Chloroplasts, mitochondria, cell membrane | Electron transport chain byproduct, signaling molecule | Damages Fe-S centers, membrane lipid peroxidation | Membrane damage, protein oxidation | Pathogen defense, cell death signaling, growth regulation | Superoxide dismutase |
| 2 | Hydrogen Peroxide (H2O2) | Peroxisomes, chloroplasts, cytosol | Cell signaling, pathogen defense | Oxidizes protein thiols, inactivates enzymes, DNA damage | Cell wall modification, root growth, stress signaling | Stress-response signaling, root growth regulation, stomatal closure | Catalase, ascorbate peroxidase |
| 3 | Hydroxyl Radical (•OH) | Cell wall, all cellular compartments | Highly reactive oxidant | Severe DNA/RNA damage, protein oxidation, cell death | DNA damage, lipid peroxidation | Programmed cell death, stress response, defense activation | Non-enzymatic antioxidants |
| 4 | Singlet Oxygen (1O2) | Photosystem II, chloroplasts | Light harvesting byproduct | Chlorophyll bleaching, lipid peroxidation, PSII damage | Chloroplast damage, PSII inhibition | Photosynthetic signaling, chloroplast-nucleus communication, stress acclimation | Carotenoids, tocopherols |
| Serial No: | Compound | Primary Functions During Stress | Specific Roles | Response Mechanisms | Antioxidant Activity | Modulation of Stress-Responsive Pathways | Improvement of Physiological Parameters | References |
|---|---|---|---|---|---|---|---|---|
| 1- | Quercetin | -ROS scavenging -Membrane stabilization -Osmolyte accumulation -Metal ion chelation | -Enhances drought tolerance -Improves salt resistance -Mediates temperature stress response -Protects UV radiation | -Regulates stress-responsive genes -Enhanced enzyme activities -Aids cellular homeostasis -Membrane integrity maintenance | -Direct ROS scavenging -SOD, CAT, and POD enzyme activation -Reduced lipid peroxidation -Protection of cellular components | -ABA signaling pathway regulation -MAPK cascade activation -Calcium signaling enhancement -Transcription factor regulation | -Enhanced photosynthetic efficiency -Better water relations -Improved nutrient uptake -Increased chlorophyll content | [204,205,206,207] |
| 2- | Kaempferol | -ROS scavenging -Membrane stability enhancement -Osmolyte regulation -Signal molecule activation -Stress-tolerance improvement | -Direct scavenging of free radicals -Prevention of lipid peroxidation -Protection of cellular membranes -Enhancement of antioxidant enzyme activities | -Upregulation of stress-responsive genes -Activation of defense pathways -Modulation of hormone signaling -Enhancement of stress-tolerance genes | -Direct free radical neutralization -Support of enzymatic antioxidants -Protection of cellular components -Reduction in oxidative damage | -MAPK signaling cascade activation -ABA-dependent pathway regulation -Stress-responsive transcription factors -Heat shock protein expression | -Enhanced photosynthetic efficiency -Enhanced growth -Better water relations -Improved nutrient uptake | [11,77,153,205,208] |
| 3- | Luteolin | -Acts as ROS scavenger -Maintains cell membrane integrity -Enhances stress tolerance -Regulates osmolytes | -Protects photosynthetic machinery -Stabilizes cellular proteins -Reduces lipid peroxidation -Enhances membrane stability | -Activates stress-responsive genes -Induces enzymatic antioxidants -Modulates hormone signaling -Enhances proline accumulation | -Directly scavenges free radicals -Increases SOD, CAT, and POD activities -Reduces H2O2 accumulation -Prevents oxidative damage | -Activates MAPK signaling -Regulates ABA-dependent pathways -Enhances calcium signaling -Influences transcription factors | -Maintains water relations -Enhances photosynthetic efficiency -Improves nutrient uptake -Stabilizes chlorophyll content | [209,210] |
| 4- | Apigenin | -ROS scavenging -Membrane stability enhancement -Osmolyte regulation -Signal molecule activation | -Increases antioxidant defense -Reduces reactivity of free radicals -Increases antioxidant enzyme activities -Protection of cellular membranes | -Activation of stress-responsive genes -Upregulation of heat shock proteins -Enhancement of proline accumulation -Modulation of ion channels | -Quench free radicals -Photosystem II protection -Membrane thermostability -Minimizes oxidative damage -Osmolyte accumulation | -MAPK cascade activation -ABA signaling pathway modulation -Calcium signaling enhancement -Hormone crosstalk regulation | -Improved water retention -Enhanced photosynthetic efficiency -Better nutrient uptake -Increased chlorophyll content | [210,211] |
| 5- | Naringenin | -Acts as a potent ROS scavenger -Functions as stress-signaling molecules -Enhances membrane stability -Regulates osmolyte production -Maintains cellular homeostasis | -Drought-tolerance enhancement -Temperature stress mitigation -Salinity stress protection -Heavy metal stress resistance -UV radiation protection | -Regulates stress-responsive genes -Activation of antioxidant enzymes -Membrane lipid preservation -Ion homeostasis regulation -Photosynthetic efficiency maintenance | -Direct ROS neutralization -Antioxidant enzyme activation -Lipid peroxidation reduction -Free radical chain-breaking -Metal ion chelation | -ABA signaling modulation -MAPK cascade regulation -Heat shock protein induction -Transcription factor activation -Hormone signaling crosstalk | -Enhanced water retention -Better nutrient uptake -Improved growth parameters -Higher photosynthetic rate -Increased stress tolerance | [212,213] |
| 6- | Ferulic acid | -Acts as a potent antioxidant -Membrane stabilizer -Osmolyte accumulation -Cell wall strengthening -Signal molecule | -Direct neutralization of ROS -Scavenges peroxyl radicals -Prevention of lipid peroxidation -Protection of cellular membranes | -Enhancement of SOD, CAT, and POD -Upregulation of stress-responsive genes -Improvement in osmolyte synthesis -Strengthening of cell wall structure | -Electron donation capacity -Free radical neutralization -Metal ion chelation -Prevention of oxidative damage | -Activation of MAPK cascades -Regulation of transcription factors -Hormonal signaling modulation | -Enhanced photosynthetic efficiency -Better water relations -Improved nutrient uptake -Increased biomass accumulation | [12,214,215,216] |
| 7- | Chlorogenic acid | -Acts as a powerful antioxidant -Protection from oxidative damage -Maintains cellular homeostasis -Contributes to stress-tolerance mechanisms | -Scavenges reactive oxygen species -Stabilizes cell membranes -Protects photosynthetic machinery -Enhance stress-signaling pathways | -Accumulates rapidly during stress exposure -Activates defense-related genes -Triggers enzymatic antioxidants -Modulates hormone signaling | -Directly neutralizes free radicals -Reduces lipid peroxidation -Prevents oxidative damage to proteins -Maintains redox balance | -Activates MAPK signaling cascades -Induces stress-responsive transcription factors -Enhances expression of defense genes -Regulates stress hormone synthesis | -Maintains membrane integrity -Enhance water retention -Improves photosynthetic efficiency -Promotes osmolyte accumulation | [215,217] |
| 8- | Caffeic acid | -Acts as a natural antioxidant -Precursor for lignin biosynthesis -Strengthens cell wall and its stability -Functions as a stress-signaling molecule | -UV radiation protection by absorption -Membrane integrity maintenance -Osmolyte accumulation support -Free radical scavenging -Cell wall reinforcement | -Activates phenylpropanoid pathway -Synthesized protective compounds -Cross-linking of cell wall components -Activation of stress-responsive genes | -Direct ROS scavenging -Reduction in oxidative damage -Protection of cellular components -Regulates antioxidant enzymes -Prevention of lipid peroxidation | -Regulate phenylpropanoid pathway -Activation of defense genes -Interacts with hormone signaling -Stress-responsive protein regulation -Enhanced secondary metabolites | -Better water retention efficiency -Increased membrane stability -Enhanced photosynthetic efficiency -Improved nutrient uptake -Better growth maintenance under stress | [218] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. https://doi.org/10.3390/horticulturae11050477
Rao MJ, Duan M, Zhou C, Jiao J, Cheng P, Yang L, Wei W, Shen Q, Ji P, Yang Y, et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae. 2025; 11(5):477. https://doi.org/10.3390/horticulturae11050477
Chicago/Turabian StyleRao, Muhammad Junaid, Mingzheng Duan, Caixia Zhou, Jiejie Jiao, Peiwen Cheng, Lingwei Yang, Wei Wei, Qinyuan Shen, Piyu Ji, Ying Yang, and et al. 2025. "Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage" Horticulturae 11, no. 5: 477. https://doi.org/10.3390/horticulturae11050477
APA StyleRao, M. J., Duan, M., Zhou, C., Jiao, J., Cheng, P., Yang, L., Wei, W., Shen, Q., Ji, P., Yang, Y., Conteh, O., Yan, D., Yuan, H., Rauf, A., Ai, J., & Zheng, B. (2025). Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae, 11(5), 477. https://doi.org/10.3390/horticulturae11050477







