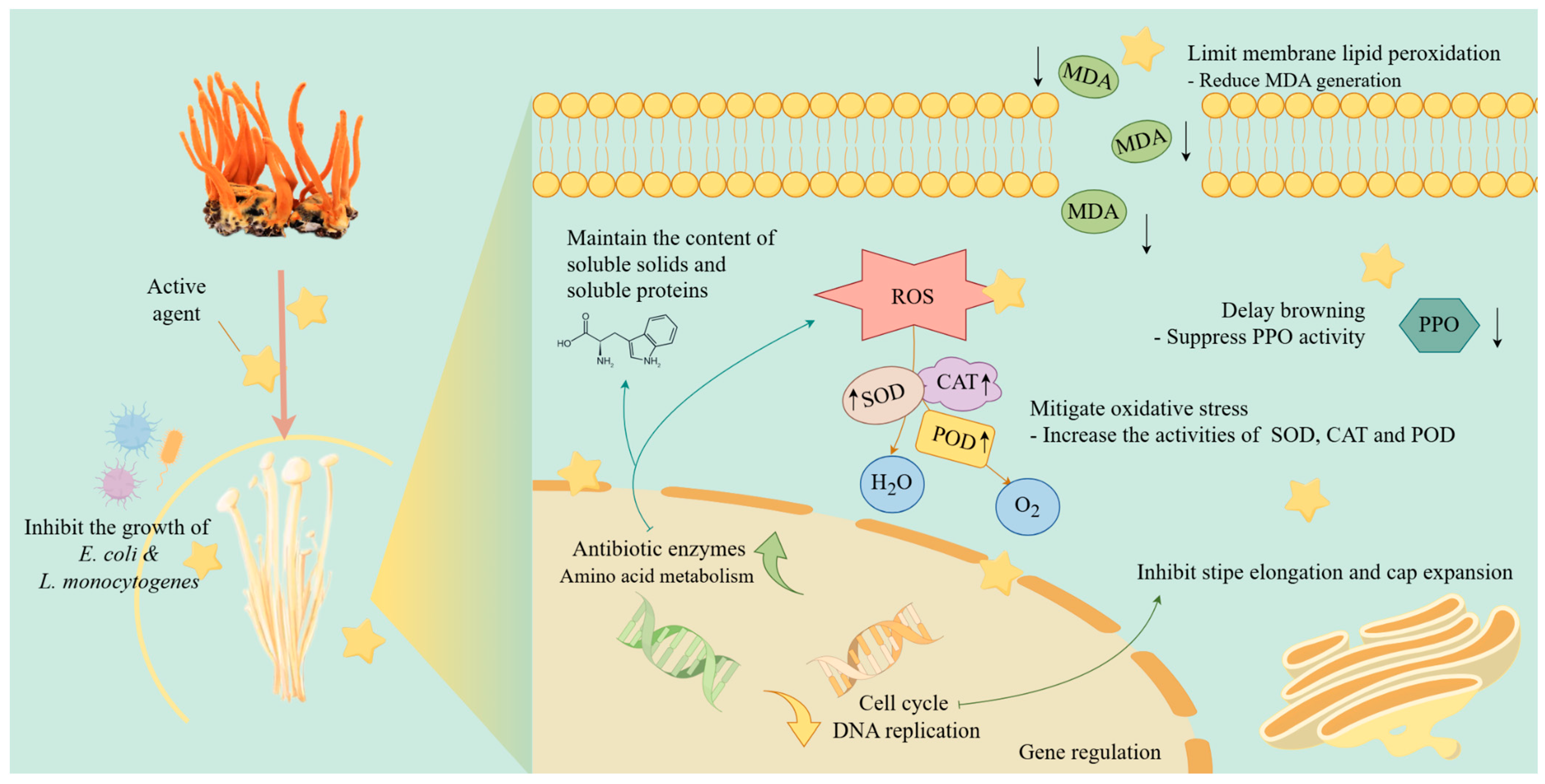
The Exploration of Cordyceps militaris Extract as a Postharvest Preservative for Flammulina filiformis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Cordyceps militaris Extract
2.3. Antibacterial Activity Test
2.3.1. Preparation of Bacterial Suspension
2.3.2. Determination of the Inhibition Zone
2.3.3. Determination of Minimum Inhibitory Concentration (MIC)
2.3.4. Evaluation of Environmental Microorganism Inhibition
2.3.5. Determination of Cordycepin Content
2.4. Preparation of Samples for Soaking
2.5. Weight Loss Rate, Cap Diameter, and Stipe Elongation Length
2.6. Evaluation of Quality Changes
2.6.1. Browning Index
2.6.2. The Content of Soluble Solids and Soluble Protein
2.6.3. Antioxidant Enzyme Activities and Malondialdehyde (MDA) Content
2.7. Transcriptome Sequencing and Analysis
2.7.1. Total RNA Extraction, Library Construction, and Illumina Sequencing
2.7.2. Transcriptome Sequencing Data Processing
2.7.3. Differential Gene Expression Analysis
2.7.4. GO Functional and KEGG Pathway Enrichment Analysis
2.7.5. Quantitative Real-Time PCR Validation
2.8. Data Statistics and Analysis
3. Results and Discussion
3.1. Antimicrobial Efficacy of CME
3.2. CME Alleviating Morphological Deterioration of F. filiformis During Storage
3.3. Benefit of CME Treatment to Quality Maintenance of F. filiformis During Storage
3.3.1. Effect on the Inhibition of Browning
3.3.2. Impact on the Retention of Soluble Solids, Protein Content, and Membrane Integrity
3.4. Effect of Exacts on Antioxidant Enzyme Activities in F. filiformis
3.5. Transcriptome Analysis Revealed Cell Activity Inhibition of F. filiformis by CME Treatment
3.6. The Advantages and Challenges of CME Preservatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukushima-Sakuno, E. Bioactive small secondary metabolites from the mushrooms Lentinula edodes and Flammulina velutipes. J. Antibiot. 2020, 73, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Research, Y. Flammulina filiformis Market Report: Industry Production Rebounds with Sustained Growth in Exports. Available online: https://www.yhresearch.cn/news/6895/flammulina-velutipes (accessed on 14 August 2024).
- Chen, M.; Wu, Q.; Zhang, J.; Guo, W.; Wu, S.; Yang, X. Prevalence and contamination patterns of Listeria monocytogenes in Flammulina velutipes plants. Foodborne Pathog. Dis. 2014, 11, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.E.; Reyes, J.E.; Rivera, C.S.; Oria, R.; Blanco, D. Microbiological quality and safety of fresh cultivated and wild mushrooms commercialized in Spain. Food Microbiol. 2011, 28, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cheng, J.; Wu, Q.; Zhang, J.; Chen, Y.; Zeng, H.; Ye, Q.; Wu, S.; Cai, S.; Wang, J. Prevalence, potential virulence, and genetic diversity of Listeria monocytogenes isolates from edible mushrooms in Chinese markets. Front. Microbiol. 2018, 9, 1711. [Google Scholar] [CrossRef]
- FDA. Outbreak Investigation of Listeria monocytogenes: Enoki Mushrooms. Available online: https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-listeria-monocytogenes-enoki-mushrooms-november-2022 (accessed on 7 April 2023).
- Guo, Y.; Chen, X.; Gong, P.; Deng, Z.; Qi, Z.; Wang, R.; Long, H.; Wang, J.; Yao, W.; Yang, W.; et al. Recent advances in quality preservation of postharvest golden needle mushroom (Flammulina velutiper). J. Sci. Food Agric. 2023, 103, 5647–5658. [Google Scholar] [CrossRef]
- Barido, F.H.; Kang, S.M.; Lee, S.K. The quality and functional improvement of retorted Korean ginseng chicken soup (Samgyetang) by enzymolysis pre-treatment with Cordyceps militaris mushroom extract. Foods 2022, 11, 422. [Google Scholar] [CrossRef]
- Xiong, S.; Jiang, J.; Wan, F.; Tan, D.; Zheng, H.; Xue, H.; Hang, Y.; Lu, Y.; Su, Y. Cordyceps militaris extract and cordycepin alleviate oxidative stress, modulate gut microbiota and ameliorate intestinal damage in LPS-induced piglets. Antioxidants 2024, 13, 441. [Google Scholar] [CrossRef]
- Bhattacharjee, M.K. Better visualization and photodocumentation of zone of inhibition by staining cells and background agar differently. J. Antibiot. 2015, 68, 657–659. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.; Dupont, B.; Fegeler, W.; Moore, C. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2003, 9, 1–8. [Google Scholar] [CrossRef]
- Shan, N.; Yang, Q.; Yang, W.; Liu, Y.; Zhao, L.; Xin, Z.; Fang, Y.; Hu, Q.; An, K. Effect of nano-packing on storage quality of Flammulina velutipes. Food Sci. 2012, 33, 262–266. [Google Scholar]
- Grintzalis, K.; Georgiou, C.D.; Schneider, Y.-J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal. Biochem. 2015, 480, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Naphtali, J.; Schellhorn, H.E. High-throughput DNA sequencing technologies for water and wastewater analysis. Sci. Prog. 2019, 102, 351–376. [Google Scholar] [CrossRef] [PubMed]
- Alexa, A.; Rahnenführer, J. Gene set enrichment analysis with topGO. Bioconductor Improv. 2009, 27, 776. [Google Scholar]
- Miao, M.; Yu, W.-Q.; Li, Y.; Sun, Y.-L.; Guo, S.-D. Structural elucidation and activities of Cordyceps militaris-derived polysaccharides: A review. Front. Nutr. 2022, 9, 898674. [Google Scholar] [CrossRef]
- Afzal, M.; Abusalah, M.A.H.A.; Shehzadi, N.; Absar, M.; Ahmed, N.; Khan, S.; Naseem, Y.; Mehmood, N.; Singh, K.K.B. Investigation of biometabolites and novel antimicrobial peptides derived from promising source Cordyceps militaris and effect of non-small cell lung cancer genes computationally. PLoS ONE 2025, 20, e0310103. [Google Scholar] [CrossRef]
- Yoon, J.; Jeong, D.; Lee, S.; Kim, S. Control of Listeria monocytogenes and Escherichia coli O157: H7 in enoki mushrooms (Flammulina velutipes) by combined treatments with organic acids, nisin, and ultrasound. Food Control 2021, 129, 108204. [Google Scholar] [CrossRef]
- Wei, Q.; Pan, X.; Jia, Z.; Li, C.; Chen, B.; Fang, T.; Jiang, Y. Comparative study of ε-polylysine or nisin inhibition kinetics of Lactococcus lactis and spoilage microorganisms in fresh Flammulina velutipes fruiting bodies. J. Food Qual. 2022, 1, 9135887. [Google Scholar] [CrossRef]
- Yang, W.; Wu, Y.; Hu, Q.; Pei, F.; Mariga, A.M. Preharvest treatment of Agaricus bisporus with methyl jasmonate inhibits postharvest deterioration. LWT 2019, 106, 158–163. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Gong, P.; Wang, R.; Han, A.; Deng, Z.; Qi, Z.; Long, H.; Wang, J.; Yao, W. Advances in the role and mechanisms of essential oils and plant extracts as natural preservatives to extend the postharvest shelf life of edible mushrooms. Foods 2023, 12, 801. [Google Scholar] [CrossRef]
- Wang, B.; Yun, J.; Ye, C.; Xu, S.; Guo, W.; Zhao, F.; Qu, Y.; Bi, Y. A novel polyethylene nanopackaging combined with ozone fumigation delayed the browning and softening of Agaricus bisporus during postharvest storage. Postharvest Biol. Technol. 2024, 210, 112771. [Google Scholar] [CrossRef]
- Xue, L.; Shi, K.; Zhang, Y.; Song, H.; Liao, Y.; Shi, H.; Shi, W. Evaluation of the umami in edible fungi and study on umami extraction of Agaricus bisporus. J. Food Composit. Anal. 2024, 128, 106069. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, X.; Huang, W.; Zhan, J.; He, Y. Drought stress influences the growth and physiological characteristics of Solanum rostratum dunal seedlings from different geographical populations in China. Front. Plant Sci. 2021, 12, 733268. [Google Scholar] [CrossRef]
- Srinontong, P.; Wandee, J.; Aengwanich, W. The effect of gallic acid on malondialdehyde, hydrogen peroxide and nitric oxide that influence viability of broiler blood cells at high ambient temperatures. Br. Poult. Sci. 2023, 64, 512–517. [Google Scholar] [CrossRef]
- Fang, D.; Yang, W.; Benard Muinde, K.; An, X.; Hu, Q.; Zhao, L. Effect of nanocomposite packaging on postharvest quality and reactive oxygen species metabolism of mushrooms (Flammulina velutipes). Postharvest Biol. Technol. 2016, 119, 49–57. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, P.; Teng, Q.; Chen, T.; Tian, G.; Yao, C.; Yalimaimaiti, N.; Liu, Q. Postharvest preservation of Flammulina velutipes with isoamyl isothiocyanate. Agronomy 2023, 13, 1771. [Google Scholar] [CrossRef]
- Qian, X.; Hou, Q.; Liu, J.; Huang, Q.; Jin, Z.; Zhou, Q.; Jiang, T.; Zheng, X. Inhibition of browning and shelf life extension of button mushroom (Agaricus bisporus) by ergothioneine treatment. Sci. Hortic. 2021, 288, 110385. [Google Scholar] [CrossRef]
- Prabsangob, N.; Sittiketgorn, S. Profile of taste-related compounds and bioactivity of split gill mushroom (Schizophyllum commune) as affected by blanching and drying. Int. J. Food Prop. 2023, 26, 2078–2090. [Google Scholar] [CrossRef]
- Hu, Y.; Xue, F.; Chen, Y.; Qi, Y.; Zhu, W.; Wang, F.; Wen, Q.; Shen, J. Effects and mechanism of the mycelial culture temperature on the growth and development of Pleurotus ostreatus (Jacq.) P. Kumm. Horticulturae 2023, 9, 95. [Google Scholar] [CrossRef]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Saxena, P.; Selvaraj, K.; Khare, S.K.; Chaudhary, N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: Role in human diseases. Biotechnol. Lett. 2022, 44, 1–22. [Google Scholar] [CrossRef]
- Fu, Y.; Tan, H.; Wang, B.; Peng, W.; Sun, Q.; Yu, Y. Integrated multi-omic analyses on yellow Flammulina filiformis cultivar reveal postharvest oxidative damage responses. Postharvest Biol. Technol. 2023, 195, 112111. [Google Scholar] [CrossRef]
- Shao, X.; Niu, B.; Fang, X.; Wu, W.; Liu, R.; Mu, H.; Gao, H.; Chen, H. Pullulan-stabilized soybean phospholipids/cinnamaldehyde emulsion for Flammulina velutipes preservation. Int. J. Biol. Macromol. 2023, 246, 125425. [Google Scholar] [CrossRef] [PubMed]
- Greenacre, M.; Groenen, P.J.; Hastie, T.; d’Enza, A.I.; Markos, A.; Tuzhilina, E. Principal component analysis. NRMP Natl. Resid. Matching Program 2022, 2, 100. [Google Scholar] [CrossRef]
- Mikhaylina, A.; Nikonova, E.; Kostareva, O.; Tishchenko, S. Regulation of ribosomal protein synthesis in prokaryotes. Mol. Biol. 2021, 55, 16–36. [Google Scholar] [CrossRef]
- Schwabe, D.; Formichetti, S.; Junker, J.P.; Falcke, M.; Rajewsky, N. The transcriptome dynamics of single cells during the cell cycle. Mol. Syst. Biol. 2020, 16, e9946. [Google Scholar] [CrossRef] [PubMed]
- Berti, M.; Cortez, D.; Lopes, M. The plasticity of DNA replication forks in response to clinically relevant genotoxic stress. Nat. Rev. Mol. Cell Biol. 2020, 21, 633–651. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, T.; Tang, C.; He, M.; Li, Y.; Li, X. Effects of different dryingmethods on amino acid metabolite content and quality of Ophiocordyceps sinensis by LC-MS/MS combined with multivariate statistical methods. Metabolites 2024, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Wassie, T.; Duan, X.; Xie, C.; Wang, R.; Wu, X. Dietary Enteromorpha polysaccharide-Zn supplementation regulates amino acid and fatty acid metabolism by improving the antioxidant activity in chicken. J. Anim. Sci. Biotechnol. 2022, 13, 18. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Wohlschlager, L.; Kracher, D.; Scheiblbrandner, S.; Csarman, F.; Ludwig, R. Spectroelectrochemical investigation of the glyoxal oxidase activation mechanism. Bioelectrochemistry 2021, 141, 107845. [Google Scholar] [CrossRef]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef] [PubMed]






| Test Strain | Concentration of CME (mg/mL) | MIC (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 240 | 120 | 60 | 30 | 15 | 7.5 | 3.75 | 1.875 | ||
| Escherchiacoli | - | - | - | - | + | ++ | ++++ | ++++ | 30 |
| Listeria monocytogenes | - | - | - | - | ++ | +++ | ++++ | ++++ | 30 |
| Test Strain | Concentration of Cordycepin (mg/mL) | MIC (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4.0 | 2.0 | 1.0 | 0.5 | 0.25 | 0.125 | 0.0625 | 0.03125 | ||
| E. coli | - | - | - | + | ++ | +++ | ++++ | ++++ | 1.0 |
| L. monocytogenes | - | - | ++ | +++ | +++ | ++++ | ++++ | ++++ | 2.0 |
| Test Strain | Concentration of Cordyceps Polysaccharide (mg/mL) | MIC (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 80 | 40 | 20 | 10 | 5.0 | 2.5 | 1.25 | 0.625 | ||
| E.coli | - | - | + | ++ | ++ | +++ | ++++ | ++++ | 40 |
| L. monocytogenes | - | - | ++ | +++ | +++ | ++++ | ++++ | ++++ | 40 |
| Treatment | Water Extract of Dried C. militaris | Sterile Water |
|---|---|---|
| Detected Bacteria | Micrococcus luteus uncultured Staphylococcus sp. uncultured bacterium | Alkalicoccobacillus gibsonii Staphylococcus hominis Staphylococcus epidermidis Staphylococcus sp. ZWS13 Roseomonas mucosa Bacillus sp. (in: firmicutes) uncultured soil bacterium uncultured bacterium uncultured Propionibacterium sp. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Li, Y.; Shao, Y.; Chen, H.; Gong, M.; Wang, L.; Li, N.; Wang, Y.; Zou, G. The Exploration of Cordyceps militaris Extract as a Postharvest Preservative for Flammulina filiformis. Horticulturae 2025, 11, 472. https://doi.org/10.3390/horticulturae11050472
Chen W, Li Y, Shao Y, Chen H, Gong M, Wang L, Li N, Wang Y, Zou G. The Exploration of Cordyceps militaris Extract as a Postharvest Preservative for Flammulina filiformis. Horticulturae. 2025; 11(5):472. https://doi.org/10.3390/horticulturae11050472
Chicago/Turabian StyleChen, Wenjing, Yan Li, Youran Shao, Hongyu Chen, Ming Gong, Li Wang, Nanyi Li, Ying Wang, and Gen Zou. 2025. "The Exploration of Cordyceps militaris Extract as a Postharvest Preservative for Flammulina filiformis" Horticulturae 11, no. 5: 472. https://doi.org/10.3390/horticulturae11050472
APA StyleChen, W., Li, Y., Shao, Y., Chen, H., Gong, M., Wang, L., Li, N., Wang, Y., & Zou, G. (2025). The Exploration of Cordyceps militaris Extract as a Postharvest Preservative for Flammulina filiformis. Horticulturae, 11(5), 472. https://doi.org/10.3390/horticulturae11050472






