Brassica oleracea var. sabellica: A New Host of Agroathelia delphinii in Soilless Cultivation Systems in Central Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
2.2. Morphology Characteristics
2.3. DNA Isolations, Sequencing, and Phylogenetic Analyses
2.4. Phylogenetic Analyses
2.5. Pathogenicity Test
3. Results
3.1. Fungal Isolation
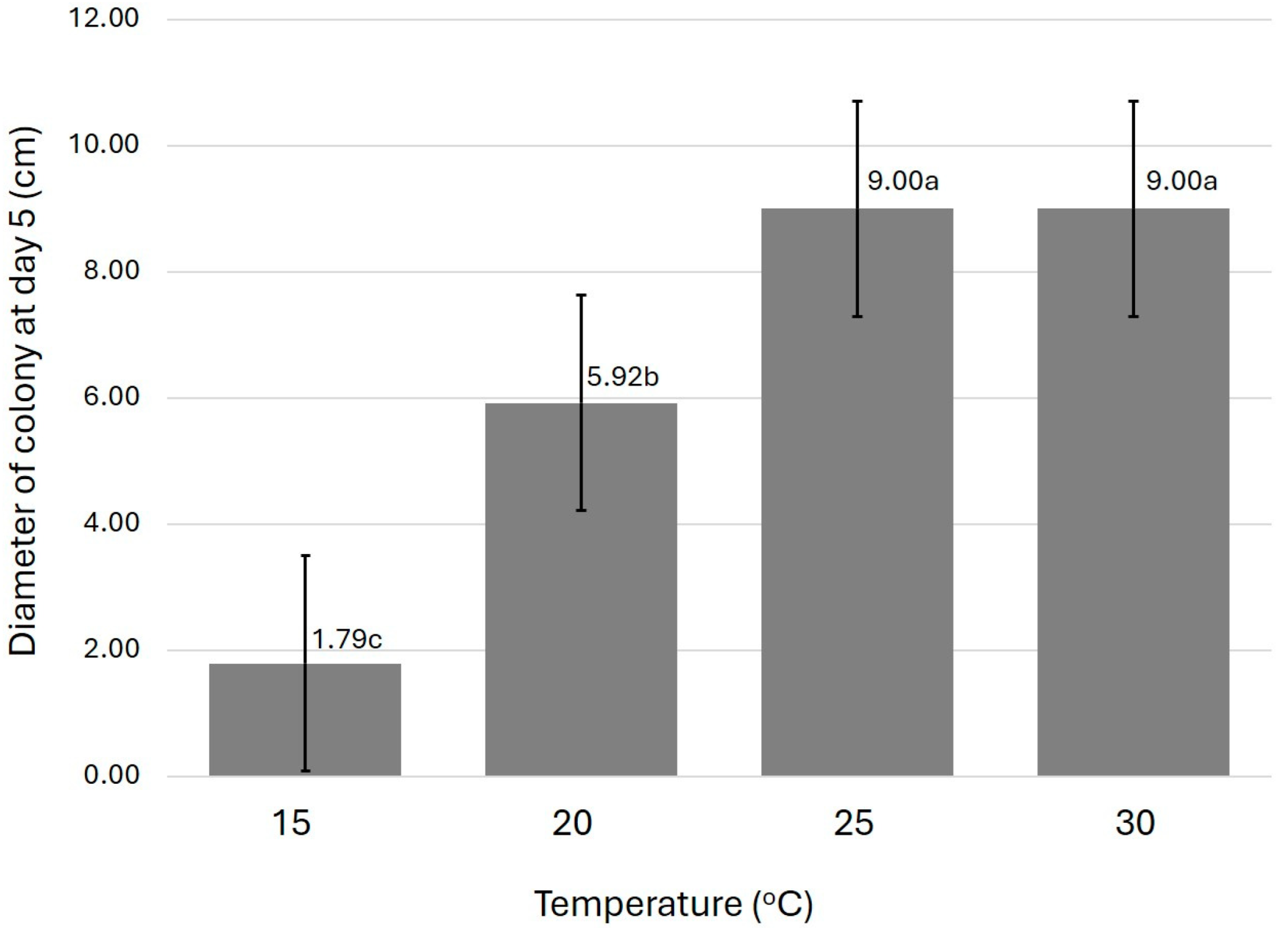
3.2. Morphology Characteristics
3.3. Molecular Identification and Phylogenetic Analyses
3.4. Pathogenicity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Zhou, R.; Qin, X.; Hou, J.; Liu, Y. Research progress on Brassicaceae plants: A bibliometrics analysis. Front. Plant Sci. 2024, 15, 1285050. [Google Scholar] [CrossRef]
- Mehmood, M.A.; Zhao, H.; Cheng, J.; Xie, J.; Jiang, D.; Fu, Y. Sclerotia of a phytopathogenic fungus restrict microbial diversity and improve soil health by suppressing other pathogens and enriching beneficial microorganisms. J. Environ. Manag. 2020, 259, 109857. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Cook, G.E. Survival of Whetzelinia sclerotiorum and initial infection of dry edible beans in Western Nebraska. Phytopathology 1975, 65, 250. [Google Scholar] [CrossRef]
- Hall, N.M.; Griffiths, H.; Corlett, J.A.; Jones, H.G.; Lynn, J.; King, G.J. Relationships between water-use traits and photosynthesis in Brassica oleracea resolved by quantitative genetic analysis. Plant Breed. 2005, 124, 557–564. [Google Scholar] [CrossRef]
- Dodd, I.C.; Puértolas, J.; Huber, K.; Pérez-Pérez, J.G.; Wright, H.R.; Blackwell, M.S.A. The importance of soil drying and re-wetting in crop phytohormonal and nutritional responses to deficit irrigation. J. Exp. Bot. 2015, 66, 2239–2252. [Google Scholar] [CrossRef]
- Barickman, T.C.; Ku, K.M.; Sams, C.E. Differing precision irrigation thresholds for Kale (Brassica oleracea L. var. Acephala) induces changes in physiological performance, metabolites, and yield. Environ. Exp. Bot. 2020, 180, 104253. [Google Scholar] [CrossRef]
- Dukes, M.D.; Zotarelli, L.; Morgan, K.T. Use of irrigation technologies for vegetable crops in Florida. Horttechnology 2010, 20, 133–142. [Google Scholar] [CrossRef]
- Frezza, D.; León, A.; Logegaray, V.; Chiesa, A.; Desimone, M.; Diaz, L. Soilless culture technology for high quality lettuce. Acta Hortic. 2005, 697, 43–48. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. An Overview of soil and soilless cultivation techniques—Chances, challenges and the neglected question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Gonnella, M.; Renna, M.; Fernández, J.A.; San Bautista, A. The evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Van Os, E.A. Disease management in soilless culture systems. Acta Hortic. 2010, 883, 385–394. [Google Scholar] [CrossRef]
- Ferreira Queiroz, M.; Araujo Gonçalves Lima, M.; Edinalva Pereira, J.; Alves Barroso, K.; Domingos Paz, C.D.; Maria Luchese, A.; Rosa Peixoto, A. Essential oils in the management of soft rot of kale in the Brazilian Semiarid Region. Biosci. J. 2020, 36, 143–155. [Google Scholar] [CrossRef]
- Kale|Diseases and Pests, Description, Uses, Propagation. Available online: https://plantvillage.psu.edu/topics/kale/infos (accessed on 2 September 2024).
- Kalayanamitra, P.; Kalayanamitra, K.; Nontajak, S.; Taylor, P.W.J.; Jonglaekha, N.; Bussaban, B. Identification, characterization, and control of black spot on chinese kale caused by Sphaerobolus Cuprophilus sp. nov. Plants 2023, 12, 480. [Google Scholar] [CrossRef]
- Nuñez, A.M.P.; Rodríguez, G.A.A.; Monteiro, F.P.; Faria, A.F.; Silva, J.C.P.; Monteiro, A.C.A.; Carvalho, C.V.; Gomes, L.A.A.; Souza, R.M.; De Souza, J.T.; et al. Bio-based products control black rot (Xanthomonas campestris pv. campestris) and increase the nutraceutical and antioxidant components in kale. Sci. Rep. 2018, 8, 10199. [Google Scholar] [CrossRef]
- Tode, H.J. Fungi Mecklenbvrgenses Selecti; Lemke: Lüneburg, Germany, 1790. [Google Scholar]
- Mahadevakumar, S.; Sarma, P.V.S.R.N.; Danteswari, C.; Joy, J.; Mahesh, M.; Mamathabhanu, L.S.; Santhosh, C.R.; Chandranayaka, S. First report of Athelia rolfsii (=Sclerotium rolfsii) associated with foot rot disease of Chrysanthemum morifolium in India. Plant Dis. 2023, 107, 2250. [Google Scholar] [CrossRef]
- Flores-Moctezuma, H.E.; Montes-Belmont, R.; Jiménez-Pérez, A.; Nava-Juárez, R. Pathogenic Diversity of Sclerotium rolfsii isolates from Mexico, and potential control of Southern blight through solarization and organic amendments. Crop Prot. 2006, 25, 195–201. [Google Scholar] [CrossRef]
- Fery, R.L.; Dukes, P.D. Southern blight (Sclerotium rolfsii Sacc.) of cowpea: Yield-loss estimates and sources of resistance. Crop Prot. 2002, 21, 403–408. [Google Scholar] [CrossRef]
- Blum, L.E.B.; Rodríguez-Kábana, R. Effect of organic amendments on sclerotial germination, mycelial growth, and Sclerotium rolfsii-induced diseases. Fitopatol. Bras. 2004, 29, 66–74. [Google Scholar] [CrossRef]
- Akem, C.N. First report of Southern blight caused by Sclerotium rolfsii on soybeans in Nigeria. Plant Dis. 1991, 75, 537D. [Google Scholar] [CrossRef]
- Paparu, P.; Acur, A.; Kato, F.; Acam, C.; Nakibuule, J.; Nkuboye, A.; Musoke, S.; Mukankusi, C. Morphological and pathogenic characterization of Sclerotium rolfsii, the causal agent of Southern blight disease on common bean in Uganda. Plant Dis. 2020, 104, 2130–2137. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E.; White, T.J. First report of Agroathelia rolfsii causing Southern blight on cowpea in China. Plant Dis. 2005, 97, 225. [Google Scholar] [CrossRef]
- Urbina, H.; Jones, C.; Moore, M.R.; Hansen, J. Southern blight of water lily: The first host record of Agroathelia rolfsii on Nelumbo nucifera discovered in Florida, U.S.A. Plant Dis. 2024, 108, 1404. [Google Scholar] [CrossRef] [PubMed]
- Changtor, P.; Rodriguez-Mateos, P.; Buddhachat, K.; Wattanachaiyingcharoen, W.; Iles, A.; Kerdphon, S.; Yimtragool, N.; Pamme, N. Integration of IFAST-based nucleic acid extraction and LAMP for on-chip rapid detection of Agroathelia rolfsii in Soil. Biosens. Bioelectron. 2024, 250, 116051. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; De Wit, P.; Sánchez-García, M.; Ebersberger, I.; de Sousa, F.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Sulistyo, B.P.; Larsson, K.H.; Haelewaters, D.; Ryberg, M. Multigene phylogeny and taxonomic revision of Atheliales s.l.: Reinstatement of three Families and one new Family, Lobuliciaceae fam. nov. Fungal Biol. 2021, 125, 239–255. [Google Scholar] [CrossRef]
- Paul, S.K.; Gupta, D.R.; Mahapatra, C.K.; Rani, K.; Islam, T. Morpho-molecular, cultural, and pathological characterization of Athelia rolfsii causing Southern blight disease on common bean. Heliyon 2023, 9, e16136. [Google Scholar] [CrossRef]
- Wang, A.R.; Lin, W.W.; Chen, X.T.; Lu, G.D.; Zhou, J.; Wang, Z.H. Isolation, and identification of Sclerotinia stem rot causal pathogen in Arabidopsis thaliana. J. Zhejiang Univ. Sci. B 2008, 9, 818. [Google Scholar] [CrossRef]
- Lydeard, C.; Holznagel, W.E.; Schnare, M.N.; Gutell, R.R. Phylogenetic analysis of Molluscan Mitochondrial LSU rDNA sequences and secondary structures. Mol. Phylogenet. Evol. 2000, 15, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains archaea, bacteria, and eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Rattanakreetakul, C.; Keawmanee, P.; Bincader, S.; Mongkolporn, O.; Phuntumart, V.; Chiba, S.; Pongpisutta, R. Two newly identified Colletotrichum species associated with mango anthracnose in Central Thailand. Plants 2023, 12, 1130. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial atlas of soil and seed fungi. In Morphologies of Cultured Fungi and Key to Species, 2nd ed.; CRC Press: Washington, DC, USA, 2002; 500p. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification, and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More models, new heuristics, and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ho, S.Y.W.; Phillips, M.J.; Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006, 4, e88. [Google Scholar] [CrossRef]
- Abdelghany, M.M.A.; Kurikawa, M.; Watanabe, M.; Matsui, H.; Yamamoto, M.; Ichinose, Y.; Toyoda, K.; Kouzai, Y.; Noutoshi, Y. Surveillance of pathogenicity of Rhizoctonia solani Japanese isolates with varied anastomosis groups and subgroups on Arabidopsis thaliana. Life 2022, 12, 76. [Google Scholar] [CrossRef]
- Anderson, N.A. The genetics and pathology of Rhizoctonia solani. Annu. Rev. Phytopathol. 1982, 20, 329–347. [Google Scholar] [CrossRef]
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 11 September 2024).
- Agricolae: Statistical Procedures for Agricultural Research. Agricolae: Statistical Procedures for Agricultural Research. Available online: https://myaseen208.com/agricolae//index.html (accessed on 11 September 2024).
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi, 4th ed.; Macmillan Publishing Co.: New York, NY, USA, 1972; 241p. [Google Scholar]
- Asgharipour, M.R.; Amiri, Z.; Campbell, D.E. Evaluation of the sustainability of four greenhouse vegetable production ecosystems based on an analysis of emergy and social characteristics. Ecol. Modell. 2020, 424, 109021. [Google Scholar] [CrossRef]
- Fuentes-Peñailillo, F.; Gutter, K.; Vega, R.; Silva, G.C. New generation sustainable technologies for soilless vegetable production. Horticulturae 2024, 10, 49. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Editorial: Soilless cultivation through an intensive crop production scheme. Management strategies, challenges, and future directions. Front. Plant Sci. 2020, 11, 363. [Google Scholar] [CrossRef]
- Transporting Vegetables from Thailand: Maintaining Quality and Freshness. Available online: https://sse.co.th/transporting-vegetables/?srsltid=AfmBOooRC_f2fmQ0Q7Mbw_CaioigpPiMKMVJeR1R03TRMT1B0riIAo3F (accessed on 19 September 2024).
- Thailand—Agriculture. Available online: https://www.trade.gov/country-commercial-guides/thailand-agriculture (accessed on 19 September 2024).
- Nizamani, M.M.; Hughes, A.C.; Zhang, H.L.; Wang, Y. Revolutionizing agriculture with nanotechnology: Innovative approaches in fungal disease management and plant health monitoring. Sci. Total Environ. 2024, 928, 172473. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Epidemiology, and control of fungal diseases in crop plants. Agronomy 2023, 13, 2327. [Google Scholar] [CrossRef]
- Li, Z. Assessing potential soil pollution from plant waste disposal: A modeling analysis of pesticide contamination. Sci. Total Environ. 2024, 907, 167859. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K. Agricultural wastes: A practical and potential source for the isolation and preparation of cellulose and application in agriculture and different industries. Ind. Crops Prod. 2024, 208, 117904. [Google Scholar] [CrossRef]
- Garvey, M.; Meade, E.; Rowan, N.J. Effectiveness of front line and emerging fungal disease prevention and control interventions and opportunities to address appropriate eco-sustainable solutions. Sci. Total Environ. 2022, 851, 158284. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Mustafa, M.; Charles, K.; Wagara, I.W.; Kappel, N. Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. J. Agric. Food Res. 2023, 14, 100827. [Google Scholar] [CrossRef]
- Mahadevakumar, S.; Yadav, V.; Tejaswini, G.S.; Janardhana, G.R. Morphological and molecular characterization of Sclerotium rolfsii associated with fruit rot of Cucurbita maxima. Eur. J. Plant Pathol. 2016, 145, 215–219. [Google Scholar] [CrossRef]
- Hernández-Morales, J.; Ochoa-Martínez, D.L.; Ayala-Escobar, V.; Ortega-Acosta, S.Á. First report of Southern blight caused by Sclerotium rolfsii on sesame in Mexico. Plant Pathol. J. 2018, 100, 323. [Google Scholar] [CrossRef]
- Okabe, I.; Matsumoto, N. Phylogenetic relationship of Sclerotium rolfsii (Teleomorph Athelia rolfsii) and S. delphinii based on ITS sequences. Mycol. Res. 2003, 107, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Okabe, I. Variation in Sclerotium rolfsii isolates in Japan. Mycoscience 1998, 39, 399–407. [Google Scholar] [CrossRef]
- Garibaldi, A.; Gilardi, G.; Gullino, M.L. First report of Southern blight incited by Sclerotium rolfsii on potato (Solanum tuberosum) in Northern Italy. Plant Dis. 2006, 90, 1114. [Google Scholar] [CrossRef]
- Sun, S.; Sun, F.; Deng, D.; Zhu, X.; Duan, C.; Zhu, Z. First report of Southern blight of mung bean caused by Sclerotium rolfsii in China. Crop Prot. 2020, 130, 105055. [Google Scholar] [CrossRef]
- Song, J.; Liu, X.Y.; Wang, M.; Cui, B.K. Phylogeny, and taxonomy of the Genus Anomoloma (Amylocorticiales, Basidiomycota). Mycol. Prog. 2016, 15, 11. [Google Scholar] [CrossRef]
- Index Fungorum—Names Record. Available online: https://www.indexfungorum.org/Names/namesrecord.asp?RecordID=251780 (accessed on 19 September 2024).
- Sclerotium delphinii. Available online: https://www.mycobank.org/page/Name%20details%20page/field/Mycobank%20%23/251780 (accessed on 19 September 2024).
- Scott, A. Redhead & Sahra-Taylor Mullineux 2023: Nomenclatural novelties. Index Fungorum 550: 1.—Biota of NZ. Available online: https://biotanz.landcareresearch.co.nz/references/6d322bd2-05d3-4ed5-b0d5-f0b73c8d3e32 (accessed on 19 September 2024).
- Chen, J.; Cong, L.; Zhou, R.; Li, Z.; Piao, J.; Hao, N. Identification, and characterization of Sclerotium delphinii causing Southern blight on Aconitum kusnezoffii in Northeast China. Plant Dis. 2022, 106, 2031–2038. [Google Scholar] [CrossRef]
- Severo, R.; Shibutani, L.J.S.; Sousa, E.S.; Matos, K.S.; Beserra, J.E.A.; de Melo, M.P. Sclerotium delphinii causing concentric leaf spots in Piper nigrum in Brazil. Austral. Plant Pathol. 2021, 50, 661–670. [Google Scholar] [CrossRef]
- Javed, Z.U.R.; Coley-Smith, J.R. Studies on germination of Sclerotia of Sclerotium delphinii. Trans. Br. Mycol. Soc. 1973, 60, 441-IN6. [Google Scholar] [CrossRef]
- Obee, D.J. Studies on the host range of Sclerotium delphinii Welch. Trans. Kans. Acad. Sci. 1937, 40, 89. [Google Scholar] [CrossRef]





| Species Name | Culture Collection No. 1 | Host/Substrate 2 | Origin | Accession Number | |
|---|---|---|---|---|---|
| ITS-Region | LSU | ||||
| Agroathelia coffeicola | CBS 115.19 | N/A | Suriname | MH854677 | MH866193 |
| Agroathelia delphinii | BOS-AYA001 | B. oleracea var. sabellica | Thailand | LC835020 | LC835030 |
| BOS-AYA002 | B. oleracea var. sabellica | Thailand | LC835021 | LC835031 | |
| BOS-AYA003 | B. oleracea var. sabellica | Thailand | LC835022 | LC835032 | |
| BOS-AYA004 | B. oleracea var. sabellica | Thailand | LC835019 | LC835029 | |
| BOS-NPT001 | B. oleracea var. sabellica | Thailand | LC835015 | LC835025 | |
| BOS-NPT002 | B. oleracea var. sabellica | Thailand | LC835016 | LC835026 | |
| BOS-NPT003 | B. oleracea var. sabellica | Thailand | LC835017 | LC835027 | |
| BOS-NPT004 | B. oleracea var. sabellica | Thailand | LC835018 | LC835028 | |
| BOS-SPB001 | B. oleracea var. sabellica | Thailand | LC835013 | LC835023 | |
| BOS-SPB002 | B. oleracea var. sabellica | Thailand | LC835014 | LC835024 | |
| CBS 221.46 | Ranunculus sp. | The Netherlands | MH856168 | - | |
| CBS 272.30 | Iris germanica | Canada | MH855140 | MH866588 | |
| CBS 305.32 | Delphinium sp. | USA | NR_189755 | MH866785 | |
| Athelia rolfsii | CBS 115.22 | Oryza sativa L. | USA | MH854711 | - |
| CBS 132553 | Vigna unguiculata | Laos | JX566993 | - | |
| CBS 191.62 | Ficus repens | Italy | MH858139 | MH869724 | |
| CBS 305.32 | Delphinium sp. | USA | NR_189755 | MH866785 | |
| Athelia arachnoidea | CBS 418.72 | Populus sp. | The Netherlands | MH860510 | - |
| CBS 105.18 | N/A | Germany | MH854664 | MH866181 | |
| Athelia decipiens | CBS 103869 | Picea sp. | Finland | KY025593 | - |
| Athelia neuhoffii | CBS 463.72 | Angiosperm wood | The Netherlands | MH860532 | - |
| Rhizoctonia solani | CBS 124594 | Coprosma repens | Italy | MH863394 | - |
| Rhizoctonia fragariae | CBS 335.62 | Fragaria x ananassa | Canada | MH858171 | MH869763 |
| Rhizoctonia carotae | CBS 464.48 | Daucus carota | USA | MH856434 | MH867980 |
| Rhizoctonia globuris | CBS 262.60 | N/A | Canada | MH857978 | MH869533 |
| Rhizoctonia callae | CBS 310.35 | Calla aethiopica | Italy | MH855686 | MH867201 |
| Sclerotium cacticola | CBS 304.32 | Opuntia sp. | The Netherlands | MH855330 | - |
| Sclerotium costantinii | CBS 288.38 | N/A | France | MH855965 | MH867461 |
| Sclerotium glucanicum | CBS 520.71 | N/A | USA | MH860245 | - |
| Sclerotium hydrophilum | CBS 385.63 | Submerged leaf in garden pond | Italy | FJ212350 | - |
| Sclerotium perniciosum | CBS 275.93 | Tulipa sp. | The Netherlands | MH862400 | FJ212355 |
| Sclerotium tuliparum | CBS 206.25 | N/A | USA | MH854847 | MH866346 |
| Sclerotium wakkeri | CBS 386.63 | Tulipa cv. Carrara | The Netherlands | MH858312 | - |
| Stromatinia cepivora | CBS 276.93 | Allium sp. | The Netherlands | MH862401 | MH874059 |
| CBS 402.85 | N/A | The Netherlands | MH861892 | - | |
| CBS 342.47 | N/A | The Netherlands | MH856279 | FJ212344 | |
| Phytophthora citrophthora | CBS 581.69 | Hevea brasiliensis | Malaysia | MH401211 | - |
| Isolate | Disease Incident (%) | Disease Severity (cm2) 1 |
|---|---|---|
| BOS-AYA001 | 0.00 | 0.00 ± 0.00 e |
| BOS-AYA002 | 100.00 | 4.47 ± 0.03 d |
| BOS-AYA003 | 100.00 | 5.79 ± 0.78 b |
| BOS-AYA004 | 100.00 | 5.55 ± 0.50 bc |
| BOS-NPT001 | 100.00 | 7.38 ± 0.41 a |
| BOS-NPT002 | 100.00 | 8.02 ± 0.41 a |
| BOS-NPT003 | 100.00 | 4.67 ± 0.55 c |
| BOS-NPT004 | 100.00 | 4.62 ± 0.53 d |
| BOS-SPB001 | 100.00 | 5.62 ± 0.57 b |
| BOS-SPB002 | 100.00 | 5.00 ± 0.66 bc |
| C.V. (%) | 4.9705 | |
| F-test | *** | |
| MSE | 3.9975 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bincader, S.; Pongpisutta, R.; Tiansawang, T.; Khienman, S.; Boonyaritthongchai, P.; Phuntumart, V.; Rattanakreetakul, C. Brassica oleracea var. sabellica: A New Host of Agroathelia delphinii in Soilless Cultivation Systems in Central Thailand. Horticulturae 2025, 11, 411. https://doi.org/10.3390/horticulturae11040411
Bincader S, Pongpisutta R, Tiansawang T, Khienman S, Boonyaritthongchai P, Phuntumart V, Rattanakreetakul C. Brassica oleracea var. sabellica: A New Host of Agroathelia delphinii in Soilless Cultivation Systems in Central Thailand. Horticulturae. 2025; 11(4):411. https://doi.org/10.3390/horticulturae11040411
Chicago/Turabian StyleBincader, Santiti, Ratiya Pongpisutta, Thipwara Tiansawang, Sirorat Khienman, Panida Boonyaritthongchai, Vipaporn Phuntumart, and Chainarong Rattanakreetakul. 2025. "Brassica oleracea var. sabellica: A New Host of Agroathelia delphinii in Soilless Cultivation Systems in Central Thailand" Horticulturae 11, no. 4: 411. https://doi.org/10.3390/horticulturae11040411
APA StyleBincader, S., Pongpisutta, R., Tiansawang, T., Khienman, S., Boonyaritthongchai, P., Phuntumart, V., & Rattanakreetakul, C. (2025). Brassica oleracea var. sabellica: A New Host of Agroathelia delphinii in Soilless Cultivation Systems in Central Thailand. Horticulturae, 11(4), 411. https://doi.org/10.3390/horticulturae11040411






