Abstract
Eruca sativa has been widely chosen among species to be cultivated in plant factories as microgreens, especially due to its nutraceutical and sensory qualities. Thus, the objective of this study was to evaluate the impact of blue light intensity (5 and 20 μmol m−2 s−1) and exposure time (1 and 2 h per day) on the yield and quality of arugula microgreens in plant factories. Blue light supplemental to white light for 1 h did not impair the hypocotyl lengths (HLs) or cotyledon area (CA) and yield of arugula microgreens compared with those grown only with white light. However, when the blue light time increased from 1 to 2 h, there were reductions in HL, CA and yield, with greater reductions under 20 μmol m−2 s−1. The concentrations of chlorophylls, carotenoids, vitamin C and antioxidant power responded similarly to the supply of blue light and were maximized with 20 μmol m−2 s−1. In view of these results, the supplementation of blue light with 20 μmol m−2 s−1 for 1 h is proposed, since it did not cause a reduction in growth and yield parameters and promoted the agronomic biofortification of arugula microgreens, bringing nutraceutical and, therefore, commercial benefits to the producer and consumer.
1. Introduction
Plant factories provide high production capacities thanks to the more efficient use of inputs, the absence of biotic and abiotic stresses and the verticalization of the production area [1], which help to improve food security. In addition, the global growth of this cultivation system can be attributed to the high degree of urbanization, which demands the generation of new jobs and the strengthening of local commerce and industry in addition to verification by the links of the production chain and society that this production system is directly or indirectly associated with eight (SDGs 1, 2, 3, 8, 9, 11, 12 and 13) of the 17 sustainable development goals proposed by the United Nations [2].
Microgreens have been one of the fastest-growing foods in this production system, gaining the attention of consumers, producers and researchers [3]. Currently, China is the country that produces the most microgreens, with an annual growth in the microgreen market of 12.3% between 2023 and 2030, jumping from 215.5 to 487.4 million dollars [4]. Arugula is among the most cultivated species of microgreens. It has a high yield in a very short production cycle [5] in addition to valuable and peculiar sensory and nutraceutical characteristics [6,7], which are phytochemical compounds associated with the combat of chronic diseases [3,8].
Although the market growth of plant factories has occurred rapidly, there is much to be improved in their management to maximize the production efficiency of this expensive environment, reducing the operating cost and price of the food produced. It is known that light is one of the main production factors of this cultivation system, and most studies have been conducted with a combination of red and blue light [9]. However, the use of white light in the production of microgreens in plant factories is growing because, in addition to providing greater visual comfort to workers, it has a lower cost than monochromatic LEDs [9], contributing to reduced costs and increasing companies’ competitiveness in the market. The results obtained with white light have proven the achievement of high yields and quality of vegetable products grown in vertical farms [5,10,11]. Thus, given the high control of irradiance enabled by advancement in artificial lighting, especially LEDs, light management in plant factories makes it possible to increase the concentration of phytochemicals beneficial to human health; therefore, it is necessary to generate irradiance protocols to obtain a product with the desired commercial quality [12].
In this context, the present study seeks to better understand the effect of blue light supplementation to white light on arugula in order to propose its biofortification. It is very well-known that blue light has a great influence on a range of physiological responses, translated into metabolic and genetic processes, that cause changes in plant development [13]. This light spectrum is important for chlorophyll formation, stomatal opening and the production of primary and secondary metabolites in plant tissues in addition to accumulation of macronutrients and micronutrients [13,14,15,16]. Therefore, in addition to its actions on crop biometry and productivity, blue light supplementing white light can be a means of achieving the agronomic biofortification of microgreen arugula, as reported by several studies attesting to an increase in nutrients and bioactive compounds in response to light management [17,18,19]. Agronomic biofortification is an excellent strategy to help combat hidden hunger, which affects 2 billion people worldwide and is characterized by a deficiency of one or more nutrients in the human body without showing symptoms, which can lead to effects from poor health to death [20,21].
However, there are few studies on the effect of blue light on microgreens, and the results reported are divergent according to species and blue light management. Kong et al. [22] observed that, compared to red light, blue light increased the hypocotyl lengths and petiole lengths of arugula microgreens regardless of the temperature of the growing environment. Divergent results were observed by Son and Oh [23] for lettuce, with lower shoot and root fresh and dry masses, leaf area and shoot/root ratios as the proportion of blue light to red light increased. Johnson et al. [24] also observed that increasing blue light intensity (20 to 650 μmol m−2 s−1) reduced hypocotyl length and cotyledon dry mass in arugula microgreens. Brazaityté et al. [19] found the same effect for mustard.
Despite the contradictory results for plant growth and development, there seems to be a consensus on the positive effect of blue light on the quality of microgreens. When absorbed by chlorophyll, it promotes a very high energy gain by the plant photosystem. This excitation energy, in addition to being used in the photochemical phase, can be lost as heat and/or generate damage to the photosynthetic apparatus as it forms free radicals, which in turn stimulates the synthesis of antioxidants [25]. This knowledge supports our hypothesis that the supplying of blue light will promote arugula biofortification.
The objective of this study was to assess the effects of irradiance and time of exposure to blue light on the yield and quality of arugula microgreens in a plant factory.
2. Materials and Methods
2.1. Experimental Site
This experiment was conducted in July 2022 at the Plant Factory Laboratory, with temperatures ranging from 21.3 to 24.6 °C and relative humidities of 50.7 to 63.6%, at the Biology Department, São Paulo State University (UNESP), Jaboticabal campus, São Paulo, Brazil.
2.2. Treatments and Experimental Design
Five treatments were evaluated in a completely randomized design in a 2 × 2 factorial scheme, plus one additional treatment (control: 400 μmol m−2 s−1 of white light for 14 h a day and the absence of blue light) with four replicates. This experiment was repeated once. The blue light additional to the white light varied in irradiance intensity (5 and 20 μmol m−2 s−1) and time (1 and 2 h per day). The blue light was applied as soon as the 14 h period of white light ended. The characterizations of the white and blue lights are shown in Figure 1. Each experimental unit was composed of three transparent polypropylene trays (25 × 24 × 5 cm in length, width and height, respectively). Photosynthetic photon flux densities (PPFDs) were measured with a spectroradiometer (SpectraPen LM 500-PSI, Photon Systems Instruments, Drásov, Czech Republic).
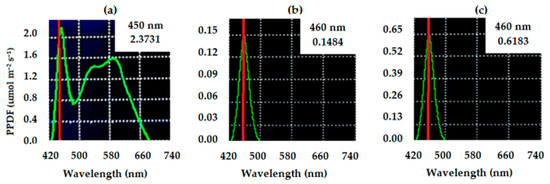
Figure 1.
Characterization of white light at 400 μmol m−2 s−1 (a) and blue light at 5 (b) and 20 μmol m−2 s−1 (c), used according to the treatment. The red vertical line in each panel marks the peak wavelength of the light source used in that treatment, and the green curve shows the spectral distribution of photon flux, basically how light energy is spread across different wavelengths.
2.3. Experimental Conditions
‘Folha Larga’ arugula was sown at a density of 102 g m−2 in trays filled with the organomineral substrate Bioplant® (pH of 6 ± 0.3 and electrical conductivity of 0.8 dS m−1). In the sequence, the trays were irrigated and placed in a dark environment for three days. Then, the trays containing seedlings with developed hypocotyls and cotyledons beginning to open were placed under the treatments. A nutrient solution [26] was supplied daily to the seedlings by sub-irrigation through small holes (2 mm) in the bottom of the trays. A total of 200 mL d−1 of nutrient solution was provided per tray for the first three days and 250 mL from then on until harvest. The arugula microgreens were harvested nine days after the light treatment, when the first leaf appeared, cutting the hypocotyl close to the substrate.
2.4. Evaluated Characteristics
- (a)
- Hypocotyl length (cm): A total of 50 seedlings were randomly and carefully cut at the level of the substrate, which, prior to sowing, had its surface flattened and the seeds distributed over it. After cutting, the seedlings were placed on the surface of the laboratory table and measured with a graduated ruler.
- (b)
- Cotyledon area (cm2 plant−1): The cotyledons from the 50 seedlings per experimental unit, used to measure the hypocotyl length, were separated from the seedlings and passed through an LI 3100 electronic bench area meter (LI-COR, Tucson, AZ, USA) to quantify the cotyledon area.
- (c)
- Yield (g m−2): The microgreens from one tray were harvested by cutting the hypocotyl close to the substrate and immediately weighed on a scale with 0.01 g precision.
- (d)
- Chlorophyll and carotenoid contents (μg g−1 fresh mass, FM): A hole punch was used to collect the discs of cotyledons with diameters of 0.5 cm, totaling 0.025 to 0.030 g of fresh mass. The leaf discs were placed in Eppendorf Tubes® with 1.5 mL of 80% acetone solution. The Eppendorf Tubes® were protected from light and placed in a refrigerator, where the samples remained for 48 h at 5–7 °C. Then, the samples were left at room temperature, and readings were performed at 663 nm for chlorophyll a, 647 nm for chlorophyll b and 470 nm for carotenoids with a Du Series 600 spectrophotometer (Beckman®, Brea, CA, USA). Subsequently, the pigment contents were calculated using the formulas proposed by Lichtenthaler [27]. The analyses were performed in triplicate.
- (e)
- Vitamin C (mg 100 g−1): The ascorbic acid content was determined by weighing 2 g of fresh microgreens and adding 10 mL of 0.5% cold oxalic acid solution. Then, the mixture was filtered. Amounts of 1 mL of the filtered extract and 4 mL of 0.5% oxalic acid were transferred to an Erlenmeyer® flask, mixed and titrated with 2,6-dichloroindophenol sodium salt up to the turning point, as described by Strohecker and Henning [28]. The analyses were performed in triplicate.
- (f)
- Phenolic compounds (mg gallic acid equivalent (GAE) 100 g−1 FM): This was determined by the spectrophotometry of an extract obtained according to Larrauri et al. [29] with the use of 70% acetone and 50% methanol solutions and subsequent addition of the Folin–Ciocalteu reagent, being based on an alkaline oxidation–reduction reaction where the phenolate ion was oxidized and the Folin reagent was reduced. A total of 300 mg of cotyledon FM was used. After the reaction, a blue color was produced, and the reading was performed at an absorbance of 700 nm, being expressed as the GAE. The analyses were performed in triplicate.
- (g)
- Antioxidant power (%): A total of 1 g of the sample was weighed in a dark environment and placed in a beaker, where 5 mL of 50% methanol was added and the mixture was homogenized and left to rest for 60 min. Subsequently, it was centrifuged at 15,000× g for 15 min, and supernatant 1 was placed in a 10 mL volumetric flask. In the second step, 4 mL of 70% acetone was added to the residue of the first extraction and homogenized, left at rest for 60 min; then, this residue was centrifuged at 15,000× g for 15 min, and supernatant 2 was collected in the same 10 mL volumetric flask. Subsequently, the flask was completed with distilled water, resulting in the extract. In the third step, 0.1 mL of the extract and 3.9 mL of the 0.06 mM DPPH radical were added to a test tube and homogenized in a tube shaker. Methyl alcohol was used as a blank to calibrate the spectrophotometer. Readings were taken at 515 nm, as described by Larrauri et al. [29]. The analyses were performed in triplicate.
- (h)
- Macronutrient (g kg−1), iron and zinc (mg kg−1) contents: The aerial parts of the microgreens were washed in deionized water and dried in an oven at 65 °C. After drying using the method described by Miyasawa et al. [30], the samples were prepared for reading of the N content using the Kjeldahl method; P and S by spectrophotometry; and K, Ca, Mg, Fe and Zn by atomic absorption.
2.5. Statistical Analysis
An analysis of variance (F test) was performed on the data of each characteristic of each experiment and then the joint analysis of the experiments. Since there was no interaction between the treatments and experiments, the effects of isolated or interacting factors (blue light irradiation time and PPFD) whose means were compared by the Tukey test (p < 0.05) were presented. The mean of each level of the factors was compared with the mean of the control treatment by the t-test (p < 0.05). The analyses were performed in the AgroEstat program (v.1.1.0.712 rev 77) [31].
3. Results
According to the joint analysis (Supplemental Tables S1–S6), for all the characteristics evaluated, there was no interaction between the treatments in this experiment. There was an interaction between the factors of PPFD and blue light irradiance time (Supplemental Table S7) for hypocotyl length (HL) and cotyledon area (CA) (Table 1) and yield (Table 2). Higher HLs and CAs were observed in the control treatment, in which the microgreens received only white light. However, the HLs and CAs of the microgreens under 1 h of blue light did not differ from those of the microgreens that received only white light, regardless of the intensity of the blue light. The supplying of 2 h of blue light was harmful to the growth of the arugula microgreens, and the damage was more intense when greater fluency and time of blue light were combined (Table 1). The yield responded to the application of the blue light similarly to the HL and CA. Only when the blue light was applied for 2 h was there a reduction in the yield (22.6%) of the arugula microgreens compared to the plants grown only with the white light, and a greater reduction (26.9%) was observed when the period of blue light was associated with higher intensity (Table 2).

Table 1.
Hypocotyl lengths (HLs) and cotyledon areas (CAs) of microgreen arugula as functions of photosynthetic photon flux density (PPFD), blue light irradiation time and absence of blue light (control: white light).

Table 2.
Yield and phenol compounds of microgreen arugula as functions of photosynthetic photon flux density (PPFD), blue light irradiation time and absence of blue light (control: white light).
The phenolic compounds were also influenced by the interaction between the factors (Supplemental Table S8). The concentrations observed in the microgreens grown with a lower PPFD (5 μmol m−2 s−1) and time (1 h) of blue light did not differ from those found in the microgreens grown under the white light but were lower than those observed in the microgreens irradiated with 20 μmol m−2 s−1 under 2 h of light. In these cultivation conditions, the concentration of the phenolic compounds was 49.3% higher than the concentration in the microgreens grown only under the white light (Table 2).
Regarding the concentrations of chlorophylls and carotenoids, there was no interaction between the factors and no single effect of the blue light time (Supplemental Tables S8 and S9). There was only the effect of the PPFD, with higher concentrations of pigments in the microgreens that received 20 μmol m−2 s−1 than in those that received 5 μmol m−2 s−1. However, regardless of the PPFD and irradiance time, the blue light promoted increases in the concentrations of chlorophyll a, chlorophyll b and carotenoids compared to the conditions in which the microgreens were grown only with white light (Table 3). The vitamin C and antioxidant power of the arugula microgreens responded positively to the blue light supplementation with the increases in both the supply period and PPFD, and, as observed for the chlorophylls and carotenoids, higher concentrations of vitamin C and antioxidant power were observed in the plants under the blue light compared with those that received only the white light (Table 3 and Supplemental Table S9).

Table 3.
Chlorophyll a (Chlor a), chlorophyll b (Chlor b), carotenoid (Car) and vitamin C (Vit C) contents and antioxidant power (AOP) of microgreen arugula as a function of photosynthetic photon flux density (PPFD), and blue light irradiation time and absence of blue light (control: white light).
Among the nutrients (Supplemental Tables S10–S12), there was an interaction between the factors only for nitrogen (N) and phosphorus (P). The N concentrations in the arugula microgreens cultivated with blue light did not differ from each other but were higher than the concentration observed when only blue light was supplied. As for the P, the differences detected between the treatments were of very small magnitude. Under 5 μmol m−2 s−1, a higher concentration was observed with 1 h of light, while under 20 μmol m−2 s−1, there was no effect of light time. With 1 h of light, the concentrations were similar under both irradiances, while with 2 h, a higher concentration was observed under 20 μmol m−2 s−1 (Table 4). For the other nutrients, there were no differences between the treatments with blue light application; however, all calcium concentrations in the microgreens cultivated with the supplemental blue light were higher than those of the microgreens cultivated only with white light. Under 1 h of blue light and with 5 μmol m−2 s−1, the Zn concentrations were higher than those observed in the arugula under the white light (Table 4).

Table 4.
Nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe) and zinc (Zn) contents of microgreen arugula as a function of photosynthetic photon flux density (PPFD), blue light irradiation time and absence of blue light (control: white light).
4. Discussion
Blue light has a proven beneficial effect on the morphogenesis, photosynthesis, growth and biosynthesis of phytocompounds, which play an important role in the prevention of chronic diseases in humans [13,14,15,16]. However, the fluency and duration of the light period can exceed the plant’s ability to prevent damage caused by photoinhibition and photo-oxidation, a hypothesis that was the subject of this study and deserves to be studied for each cultivated plant species.
According our results, with any PPFD evaluated, the blue light supplemental to the white light for 1 h did not impair the biometrics (Table 1) and yield (Table 2) of the arugula microgreens compared to the plants cultivated only with white light. However, increasing the duration of the period from 1 to 2 h led to lower hypocotyl lengths (HLs) and cotyledon areas (CAs) and yields, with greater reductions observed with 20 μmol m−2 s−1, which reached 14.7%, 53.2% and 26.9% for HL, CA and yield, respectively, compared with the values observed in the microgreens cultivated only with white light (Table 1 and Table 2). The result observed for the HLs corroborates that reported by Johnson et al. [24], who evaluated fluencies from 20 to 650 μmol m−2 s−1 and also found reduction in the arugula HL as the PPFD increased. The greater damage observed in the HL, CA and yield with the highest PPFD and exposure time are indicative of photostress, which led to a decrease in the carbon fixation rate, resulting in lower growth and biomass gain, contributing to the strong reduction in the yield observed (Table 2). Snowden et al. [32] and Noguchi and Amaki [33] also found reduced growth when Solanum lycopersicum, Cucumis sativus and Capsicum annum plants were exposed to blue LEDs. However, the results obtained by those authors and those found in our study either diverge (when the light time was shorter) or agree (longer time and higher PPFD), corroborating the need to evaluate the light characteristics for each species.
Blue light is not just involved in growth characteristics; it can also be used as a cultural management factor in order to cause mild stress and stimulate plants to produce a greater amount of biocompounds for cell defense, a strategy employed to add greater nutraceutical value to food [34,35,36]. Thus, it can be noted that the greater the damage of blue light to arugula growth (Table 1 and Table 2), the higher the concentrations of phenolic compounds (Table 2), chlorophylls, carotenoids, vitamin C and antioxidant power (Table 3). Greater biosynthesis of compounds that protect the photosynthetic apparatus and the cell was promoted by the stimulation of the PPFD and the duration of the blue light period. Under these conditions, both the increments of the pigments to account for the excitation of the photosystems and the increments of the antioxidant compounds were triggered, which was portrayed by combining the results of the growth (Table 1 and Table 2) and the pigments and antioxidants (Table 2 and Table 3).
It is known that monochromatic blue light causes increases in chlorophyll a, chlorophyll b and carotenoid contents compared with those of plants under multispectral or red light [37,38] due to the need to increase photoprotective capacity by avoiding cell and plant tissue damage [37,38,39,40,41]. The concentrations of chlorophyll a, chlorophyll b, carotenoids, vitamin C and antioxidant power responded similarly to the supply of blue light and were maximized with 20 μmol m−2 s−1 (Table 3), under which they increased by 227, 198, 273, 249 and 27%, respectively, when compared to the microgreens that did not receive blue light. The phenolic compounds responded to the interaction between the factors of PPFD and duration of the blue light period. At the lowest PPFD, the increase in the light period did not promote an increase in phenols. However, with both 1 and 2 h of light, the increment in the PPFD increased the concentration of phenols, which was maximized with the maximums of the two factors evaluated (Table 2). This result was consistent with those observed by Son and Oh [23], who observed increments of 1.4 and 2.4 times in the concentrations of phenols in ‘Sunmang’ lettuce with blue and red light compared to white light [19,23] and who found increases in the contents of phenolic compounds in mustard and cabbage microgreens. Therefore, as observed for vitamin C and total antioxidants, blue light stimulated the synthesis of these secondary metabolites, as recommended by Wang et al. [42,43], which have the function of inhibiting or reducing the damage caused by reactive oxygen species, resulting from the high energy value of blue light. The positive correlation between antioxidant protection and increased blue light intensity has also been found in mustard, tatsoi and kohlrabi microgreens by Samoulienė et al. [44]. The content of phenolic compounds increased with the increasing light intensity and exposure time, in agreement with the results observed by Brazaitytė et al. [19] in mustard (Brassica juncea, ‘Red Lace’) and kale (Brassica napus, ‘Red Russian’) microgreens.
The unfavorable effect of higher PPFD and longer duration of blue light supplementation on the growth of hypocotyl and cotyledons, associated with greater biosynthesis of plant defense biocompounds, may justify the increases in the concentrations of N and P (Table 4). However, the times of exposure to blue light and its intensity did not influence the concentrations of potassium, magnesium, sulfur and iron, and these did not differ from the concentrations observed in the microgreens that did not receive blue light (control). The calcium and zinc concentrations were respectively higher and lower than those observed with the microgreens grown only with white light (Table 4). These results partially differ from those reported by Brazaitytė et al. [19], who evaluated blue and red light ratios and observed an increase in nutrient contents in mustard (Brassica juncea, ‘Red Lace’) and kale (Brassica napus, ‘Red Russian’) microgreens with a higher proportion of blue light.
The present study ratified the importance of evaluating PPFD and the time of exposure of arugula microgreens to blue light because divergent effects were obtained depending on the intensity and duration of the supplementation offered to the arugula. The results show that under a combination of PPFD and irradiance duration, the commercial cultivation of arugula microgreens failed because there were a strong reduction in the yield when the blue light was applied for 2 h and aggravated damage with 20 μmol m−2 s−1 (Table 2). However, under lower, shorter time, the blue light supplementation did not cause reductions in the growth (Table 1) and yield parameters (Table 2) and promoted the agronomic biofortification of the arugula microgreens, bringing nutraceutical and, therefore, commercial benefits to the producer and consumer (Table 3 and Table 4). Therefore, since there was an increase in the phenols with the application of 20 μmol m−2 s−1 by 1 or 2 h (Table 2) and increases in chlorophylls, carotenoids, vitamin C, total antioxidants and some nutrients with both PPFD and blue light times (Table 3 and Table 4), the hypothesis proposed for the biofortification of arugula microgreens through the supplementation of blue light to white light was proven, and 20 μmol m−2 s−1 for 1 h is recommended, since with 2 h of light, there was a reduction in productivity, which is not acceptable when seeking to biofortify food.
5. Conclusions
Establishing a protocol for the use of light in the production of microgreens in a plant factory is essential. Variations in the light time and flux density of the blue light photons showed that it is possible to go from strong damage to the growth of arugula microgreens and to success in the enterprise, as the supplementation of white light with 20 μmol m−2 s−1 for 1 h day−1 of blue light, in addition to not negatively affecting the growth parameters (hypocotyl length and cotyledon area), promoted the biofortification of the arugula microgreens by increasing the concentrations of chlorophylls, carotenoids, phenols, vitamin C, total antioxidants, nitrogen, phosphorus and calcium.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11040412/s1. Table S1. Summary of the joint analysis of the experiments for hypocotyl length, cotyledon area and yield of the microgreen arugula. Table S2. Summary of the joint analysis of the experiments for phenols, chlorophyll a and chlorophyll b of the microgreen arugula. Table S3. Summary of the joint analysis of the experiments for carotenoids, vitamin C contents and antioxidant power of the microgreen arugula. Table S4. Summary of the joint analysis of the experiments for nitrogen, phosphorus and potassium contents of the microgreen arugula. Table S5. Summary of the joint analysis of the experiments for calcium, magnesium and sulfur contents of the microgreen arugula. Table S6. Summary of the joint analysis of the experiments for iron and zinc contents of the microgreen arugula. Table S7. Summary of the analysis of variance of the mean data of the experiments for hypocotyl length, cotyledon area and yield of the arugula microgreen as a function of photosynthetic photon flux density (PPFD) and blue light irradiation time (IT), and absence of blue light (control, white light). Table S8. Summary of the analysis of variance of the mean data of the experiments for phenols, chlorophyll a and chlorophyll b of the arugula microgreen as a function of photosynthetic photon flux density (PPFD) and blue light irradiation time (IT), and absence of blue light (control, white light). Table S9. Summary of the analysis of variance of the mean data of the experiments for carotenoids, vitamin C contents and antioxidant power of the arugula microgreen as a function of photosynthetic photon flux density (PPFD) and blue light irradiation time (IT), and absence of blue light (control, white light). Table S10. Summary of the analysis of variance of the mean data of the experiments for nitrogen, phosphorus and potassium contents of the arugula microgreen as a function of photosynthetic photon flux density (PPFD) and blue light irradiation time (IT), and absence of blue light (control, white light). Table S11. Summary of the analysis of variance of the mean data of the experiments for calcium, magnesium and sulfur contents of the arugula microgreen as a function of photosynthetic photon flux density (PPFD) and blue light irradiation time (IT), and absence of blue light (control, white light). Table S12. Summary of the analysis of variance of the mean data of the experiments for iron and zinc contents of the arugula microgreen as a function of photosynthetic photon flux density (PPFD) and blue light irradiation time (IT), and absence of blue light (control, white light).
Author Contributions
Conceptualization, A.B.C.F. and R.F.C.; methodology, A.B.C.F. and R.F.C.; formal analysis, A.B.C.F.; investigation, F.Q.M.; data curation, F.Q.M.; writing—original draft preparation, F.Q.M., R.F.C. and M.O.d.S.; writing—review and editing, A.B.C.F.; supervision, A.B.C.F. and R.F.C.; project administration, A.B.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are available on request from the corresponding author.
Acknowledgments
We would like to thank CAPES and CNPq.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ampim, P.A.; Obeng, E.; Olvera-Gonzalez, E. Indoor vegetable production: An alternative approach to increasing cultivation. Plants 2022, 11, 2843. [Google Scholar] [CrossRef] [PubMed]
- United Nations (UN). End Hunger, Achieve Food Security and Improved Nutrition and Promote Sustainable Agriculture. Available online: https://sustainabledevelopment.un.org/sdg2 (accessed on 22 November 2022).
- Shibaeva, T.G.; Sherudilo, E.G.; Rubaeva, A.A.; Titov, A.F. Continuous LED lighting enhances yield and nutritional value of four genotypes of Brassicaceae microgreens. Plants 2022, 11, 176. [Google Scholar] [CrossRef]
- Horizon Grand View Research. China Microgreens Market Size & Outlook, 2023–2030. 2025. Available online: https://www.grandviewresearch.com/horizon/outlook/microgreens-market/china (accessed on 6 February 2025).
- Mendes, F.Q.; Purquerio, L.F.V.; Carvalho, R.F.; Cecílio Filho, A.B. White light intensities for maximum yield and quality of arugula microgreens. Pesqui. Agropecu. Trop. 2024, 54, e79951. [Google Scholar] [CrossRef]
- Xiao, Z.; Rausch, S.; Luo, Y.; Sun, J.; Yu, L.; Wang, Q.; Chein, P.; Stommel, J. Microgreens of Brassicaceae: Genetic diversity of phytochemical concentrations and antioxidant capacity. LWT 2019, 101, 731–737. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Ager, E.; Kong, L.; Tan, L. Nutritional quality and health benefits of microgreens, a crop of modern agriculture. J. Future Foods 2021, 1, 58–66. [Google Scholar] [CrossRef]
- Lanoue, J.; Louis, S.; Little, C.; Hao, X. Continuous lighting can improve yield and reduce energy costs while increasing or maintaining nutritional contents of microgreens. Front. Plant Sci. 2022, 13, 983222. [Google Scholar] [CrossRef]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 56. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Shao, L.; Li, T.; Wang, Y.; Wang, R. Response of photosynthetic capacity of tomato leaves to different LED light wavelength. Environ. Exp. Bot. 2018, 150, 161–171. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Cho, K.M.; Lee, H.Y.; Cho, D.Y.; Lee, G.O.; Jang, S.N.; Lee, Y.; Kim, D.; Son, K.-H. Effects of white LED lighting with specific shorter blue and/or green wavelength on the growth and quality of two lettuce cultivars in a vertical farming system. Agronomy 2021, 11, 2111. [Google Scholar] [CrossRef]
- Medelo, M.J.Y.; Alves, T.N.; Ribera, L.M.; Silva, L.N.C.; Carvalho, R.F.; Calori, A.H.; Cecilio Filho, A.B. Harvest time, photoperiod and white light irradiance on yield of red cabbage microgreens in plant factory. Chil. J. Agric. Res. 2025, 85, 181–192. [Google Scholar] [CrossRef]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Morrow, R.C. Blue wavelengths from LED lighting increase nutritionally important metabolites in specialty crops. HortScience 2015, 50, 1285–1288. [Google Scholar] [CrossRef]
- Kang, W.H.; Park, J.S.; Park, K.S.; Son, J.E. Leaf photosynthetic rate, growth, and morphology of lettuce under different fractions of red, blue, and green light from light-emitting diodes (LEDs). Hortic. Environ. Biote 2016, 57, 573–579. [Google Scholar] [CrossRef]
- Kang, L.K.; Huang, Y.J.; Lim, W.T.; Hsu, P.H.; Hwang, P.A. Growth, pigment content, antioxidant activity, and phytoene desaturase gene expression in Caulerpa lentillifera grown under different combinations of blue and red light-emitting diodes. J. Appl. Phycol. 2020, 32, 1971–1982. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red led light on brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Gödecke, T.; Stein, A.J.; Qaim, M. The global burden of chronic and hidden hunger: Trend and determinants. Glob. Food Sec. 2018, 18, 21–29. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral biofortification of vegetables as a tool to improve human diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Kong, Y.; Masabni, J.; Niu, G. Effect of temperature variation and blue and red leds on the elongation of arugula and mustard microgreens. Horticulturae 2023, 9, 608. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Johnson, R.E.; Kong, Y.; Zheng, Y. Elongation growth mediated by blue light varies with light intensities and plant species: A comparison with red light in arugula and mustard seedlings. Environ. Exp. Bot. 2020, 169, 103898. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Ottosen, C.O.; Rosenqvist, E. Spectral effects of LEDs on chlorophyll fluorescence and pigmentation in Phalaenopsis ‘Vivien’and ‘Purple Star’. Physiol. Plant 2015, 154, 314–327. [Google Scholar] [CrossRef]
- Furlani, P.R.; Bolonhezi, D.; Silveira, L.C.P.; Faquin, V. Nutrição mineral de hortaliças, preparo e manejo de soluções nutritivas. Inf. Agropecu. 1999, 20, 90–98. [Google Scholar]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Strohecker, R.; Henining, H.M. Análisis de Vitaminas: Métodos Comprobados; Paz Montalvo: Madrid, Spain, 1967; 428p. [Google Scholar]
- Larrauri, J.A.; Rupérez, P.; Saura-Calixto, F. Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J. Agric. Food Chem. 1997, 45, 1390–1393. [Google Scholar] [CrossRef]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; Carmo, C.A.F.S.; Mello, W.J. Análises químicas de tecido vegetal. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Silva, F.C., Ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2009; pp. 193–233. [Google Scholar]
- Barbosa, J.C.; Maldonado Júnior, W. Experimentação Agronômica e AgroEstat: Sistema para Análises Estatísticas de Ensaios Agronômicos; Multipress: Jaboticabal, Brazil, 2015. [Google Scholar]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of seven diverse species to blue and green light: Interactions with photon flux. PLoS ONE 2016, 11, e0163121. [Google Scholar] [CrossRef]
- Noguchi, A.; Amaki, W. Effects of light quality on the growth and essential oil production in Mexican mint. In Proceedings of the VIII International Symposium on Light in Horticulture, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Kalaji, H.M.; Govindjee. Photosynthetic responses of sun-and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Petroutsos, D.; Tokutsu, R.; Maruyama, S.; Flori, S.; Greiner, A.; Magneschi, L.; Cusant, L.; Kottke, T.; Mittag, M.; Hegemann, P.; et al. A blue-light photoreceptor mediates the feedback regulation of photosynthesis. Nature 2016, 537, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Botyanszka, L.; Chen, Y.E.; Allakhverdiev, S.I. Phenotyping of isogenic chlorophyll-less bread and durum wheat mutant lines in relation to photoprotection and photosynthetic capacity. Photosynth. Res. 2019, 139, 239–251. [Google Scholar] [CrossRef]
- Hamdani, S.; Khan, N.; Perveen, S.; Qu, M.; Jiang, J.; Govindjee; Zhu, X.G. Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth. Res. 2019, 139, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, Z.; Wang, X.; Gu, L.; Yoshizumi, T.; Yang, Z.; Yang, L.; Liu, Q.; Han, Y.-J.; Kim, J.-I.; et al. Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 2016, 354, 343–347. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Q.; Wang, X.; Zuo, Z.; Oka, Y.; Lin, C. New insights into the mechanisms of phytochrome–cryptochrome coaction. New Phytol. 2018, 217, 547–551. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskienė, J.; Duchovskis, P. LED irradiance level affects growth and nutritional quality of Brassica microgreens. Cent. Eur. J. Biol. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).