Antifungal and Toxicological Evaluation of Natural Compounds Such as Chitosan, Citral, and Hexanal Against Colletotrichum asianum
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactivation of Colletotrichum Asianum Strain
2.2. Preparation and Application of Treatments
2.3. In Vitro Test
2.3.1. Mycelial Inhibition
Sporulation
2.3.2. Fungal Biomass
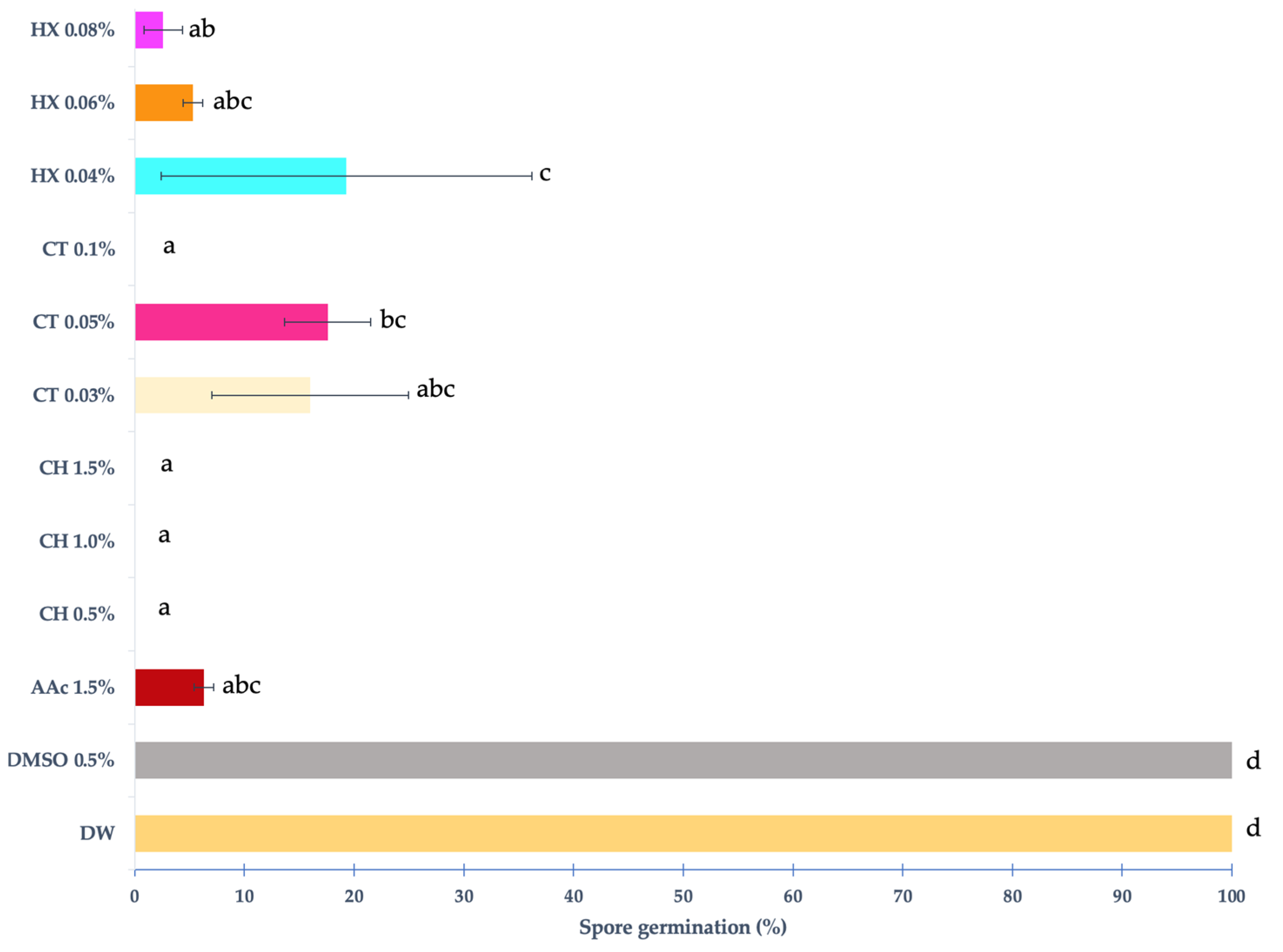
2.3.3. Percentage of Spore Germination
2.4. Cytotoxicological Tests
2.4.1. Seed Pre-Treatment
2.4.2. Seed Treatment Application
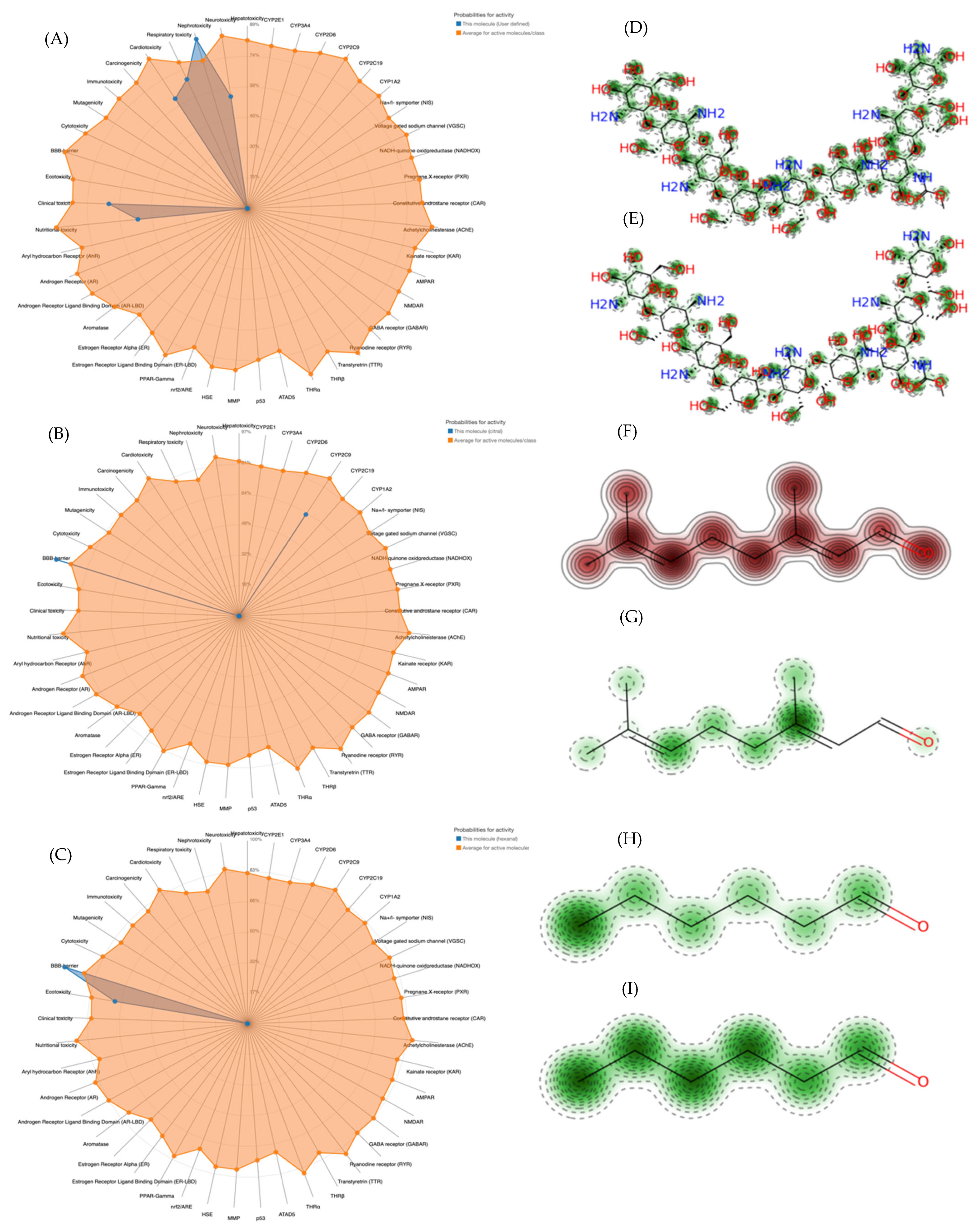
2.4.3. In Silico Analysis of Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET)
2.5. Statistical Analysis
3. Results
3.1. In Vitro Effect on the Development of Colletotrichum asianum
3.1.1. Inhibition of Radial Growth, Sporulation, and Fungal Biomass
3.1.2. Germination Percentage
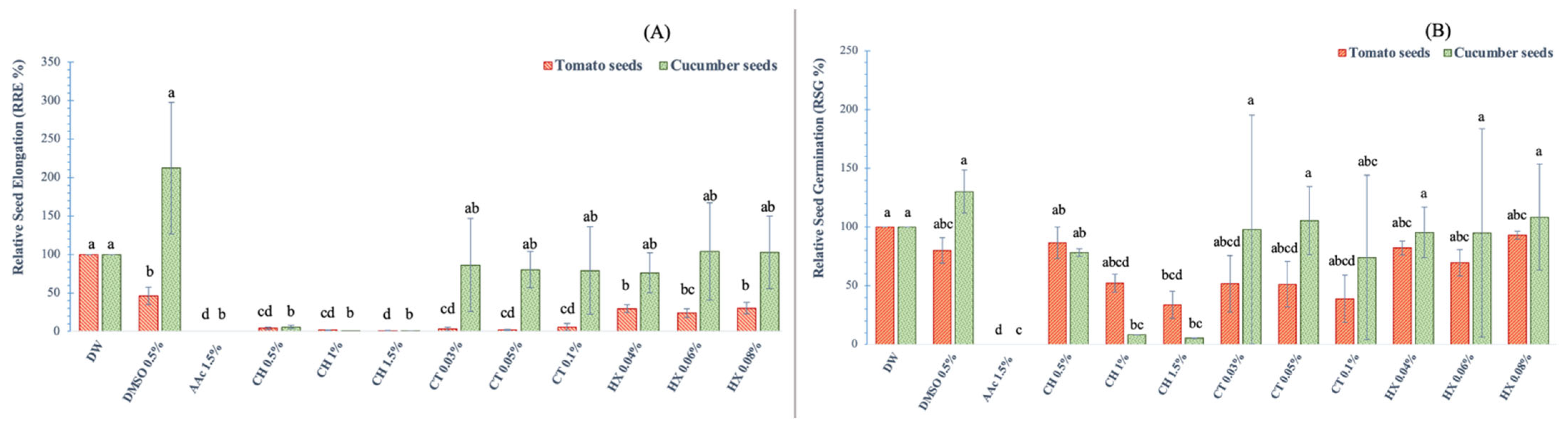
3.2. Cytotoxicological Effect on Seed Germination
3.2.1. Radicle Development in Tomato and Cucumber Seeds
3.2.2. Effect on Root Tissue
3.2.3. Toxicokinetic Predictions of Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. El Mango y Sus Componentes Bioactivos: Agregando Variedad al Platillo de Fruta de La Salud. Mango.org 2018, 3, 65. [Google Scholar]
- Rosman, N.F.; Malek, N.S.A.; Omar, H.; Hajar, N.; Buniyamin, I.; Abdullah, S.; Razzif, A.R.A.; Rusop, M.; Asli, N.A. Preserving Mango Quality: Assessing the Influence of Zinc Oxide Nanocomposite-Corn Starch Coating Concentrations on Postharvest Attributes. Food Biophys. 2024. [Google Scholar] [CrossRef]
- Noriega-Cantú, D.H.; Martínez-Bolaños, M.; Garrido-Ramirez, E.R.; Palacio-Martínez, V.; Ariza-Flores, R. Antracnosis Del Mango: Tecnología de Manejo Integrado, Agricultura-Inifap; Noriega-Cantú, D.H., Martínez-Bolaños, M., Garrido-Ramírez, E.R., Ariza-Flores, R., Eds.; AGRICULTURA-INIFAP-CIRPAS: Iguala de la Independencia, Mexico, 2024; Volume 1. [Google Scholar]
- Tovar-Pedraza, J.M.; Mora-Aguilera, J.A.; Nava-Díaz, C.; Lima, N.B.; Michereff, S.J.; Sandoval-Islas, J.S.; Câmara, M.P.S.; Téliz-Ortiz, D.; Leyva-Mir, S.G. Distribution and Pathogenicity of Colletotrichum species Associated with Mango Anthracnose in Mexico. Plant Dis. 2020, 104, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hio, J.C.; Martínez Lemus, E.P.; Rojas Zambrano, E.D.; Osorio Cardona, J.A.; Cruz Castiblanco, G.N.; Bustos Rodríguez, H.A. An Integrated Anthracnose Management Approach in Tommy Atkins Mango Cultivars in Cundinamarca—Colombia. Univ. Sci. 2024, 29, 253–273. [Google Scholar] [CrossRef]
- Bautista Toro, A.M.; Leiva Piedra, J.L. Obtención de aceite esencial de molle (Schinus molle L.) y su evaluación antifúngica sobre Colletotrichum spp. in vitro. TZHOECOEN 2019, 11, 101–109. [Google Scholar] [CrossRef]
- Mhya, D.H.; Muhammad, J.S.; Urmar, N.S.; Mohammed, A. Impact of Chemical Pesticides on Antioxidant Constituents and Free Radical Scavenging Capacity of Pesticide-Treated Tomato (Solanum lycopersicum, L.) Fruits. J. Agric. Environ. 2024, 20, 187–199. [Google Scholar] [CrossRef]
- Gelaye, Y.; Negash, B. Residue of Pesticides in Fruits, Vegetables, and Their Management in Ethiopia. J. Chem. 2024, 1, 9948714. [Google Scholar] [CrossRef]
- Abobatta, W.F. Chitosan: A Promising Plant Stimulant. Int. J. Agric. Sci. Food Technol. 2023, 9, 098–103. [Google Scholar] [CrossRef]
- Usall, J.; Casals, C.; Sisquella, M.; Palou, L.; De Cal, A. Alternative Technologies to Control Postharvest Diseases of Stone Fruits. Stewart Postharvest Rev. 2015, 11, 1–6. [Google Scholar] [CrossRef]
- Berumen Varela, G.; Coronado-Partida, L.; Ochoa Jimenez, V.A.; Chacon Lopez, A.M.; Gutiérrez-Martínez, P. Efecto Del Quitosano En La Inducción de Resistencia Contra Colletotrichum sp. En Mango (Mangifera indica L.) Cv. Tommy Atkins. Investig. Cienc. 2015, 23, 16–21. [Google Scholar] [CrossRef]
- Ramos-Guerrero, A.; González-Estrada, R.R.; Hanako-Rosas, G.; Bautista-Baños, S.; Acevedo-Hernández, G.; Tiznado-Hernández, M.E.; Gutiérrez-Martínez, P. Use of Inductors in the Control of Colletotrichum gloeosporioides and Rhizopus stolonifer Isolated from Soursop Fruits: In Vitro Tests. Food Sci. Biotechnol. 2018, 27, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Herrera-González, J.A.; Hernindez-Sincliez, D.A.; Bueno-Rojas, D.A.; Ramos-Bell, S.; Velizquez-Estrada, R.M.; Bautista-Rosales, R.U.; Gutiérrez-Martinez, P. Effect of Commercial Chitosan on in vitro Inhibition of Colletotrichum siamense, Fruit Quality and Elicitor Effect on Postharvest Avocado Fruit. Rev. Mex. Ing. Química 2022, 21, Bio2706. [Google Scholar] [CrossRef]
- Duan, B.; Reymick, O.O.; Liu, Z.; Zhou, Y.; Wang, X.; Feng, Z.; Tao, N. Citral Enhances Disease Resistance in Postharvest Citrus Fruit through Inducing Jasmonic Acid Pathway and Accumulating Phenylpropanoid Compounds. Postharvest Biol. Technol. 2024, 207, 1–13. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Able, A.J.; Ford, C.M.; Scott, E.S. Effect of Volatile Citral on the Development of Blue Mould, Green Mould and Sour Rot on Navel Orange. Australas. Plant Pathol. 2014, 43, 403–411. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Chen, J.; Lin, L.; Wan, C. Possible Fungicidal Effect of Citral on Kiwifruit Pathogens and Their Mechanisms of Actions. Physiol. Mol. Plant Pathol. 2021, 114, 101631. [Google Scholar] [CrossRef]
- Abo El-Ela, F.I.; Hassan, W.H.; Amer, A.M.; El-Dek, S.I. Antifungal Activity of Chitosan Polymeric Nanoparticles and Correlation with Their PH Against Mucor circinelloides Causing Mucormycosis, Along with Penicillium notatum and Aspergillus species. Curr. Microbiol. 2024, 81, 1–15. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Q.; Zhou, A.; Wen, X.; Li, M.; Li, R.; Liao, X.; Xu, T. The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis. J. Fungi 2021, 7, 1023. [Google Scholar] [CrossRef]
- Blancas-Benitez, F.J.; González-Estrada, R.R.; Rivas-García, T.; Moreno-Hernández, C.; Herrera-González, J.A.; Sánchez-Burgos, J.A.; Ramos-Bell, S.; Gutierrez-Martinez, P. Chapter 14—Natural Compound/Green Nanoemulsions for Disease Control at Postharvest Stage in Fruits. In Bio-Based Nanoemulsions for Agri-Food Applications; Abd-Elsalam, K.A., Murugan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–243. [Google Scholar] [CrossRef]
- Montes-Ramírez, P.; Montaño-Leyva, B.; Blancas-Benitez, F.J.; Bautista-Rosales, P.U.; Ruelas-Hernández, N.D.; Martínez-Robinson, K.; González-Estrada, R.R. Active Films and Coatings Based on Commercial Chitosan with Natural Extracts Addition from Coconut By-Products: Physicochemical Characterization and Antifungal Protection on Tomato Fruits. Food Control 2024, 155, 1–15. [Google Scholar] [CrossRef]
- Oliveira, T.A.; Paiva, C.A.; Silva, A.C.; Nascimento, L.V.; Leite, R.H.L.; Aroucha, E.M.M. Postharvest Quality of Tommy Atkins Mangoes Coated with Cassava Starch and Chitosan-Based Coatings. J. Agric. Sci. 2018, 10, 401. [Google Scholar] [CrossRef][Green Version]
- Sivakumar, D.; Tuna Gunes, N.; Romanazzi, G. A Comprehensive Review on the Impact of Edible Coatings, Essential Oils, and Their Nano Formulations on Postharvest Decay Anthracnose of Avocados, Mangoes, and Papayas. Front. Microbiol. 2021, 12, 711092. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Ortiz, G.; Gaxiola-Camacho, S.M.; San-Martín-hernández, C.; Martínez-Téllez, M.Á.; Aispuro-Hernández, E.; Lizardi-Mendoza, J.; Quintana-Obregón, E.A. Chitosan Sensitivity of Fungi Isolated from Mango (Mangifera indica L.) with Anthracnose. Molecules 2022, 27, 1244. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Hernández, C.L.; Zambrano-Zaragoza, M.L.; Velázquez-Estrada, R.M.; Sánchez-Burgos, J.A.; Gutiérrez-Martinez, P. Identification of a Colletotrichum sp. from Mango Fruit and Its in Vitro Control by GRAS Compounds. Rev. Mex. Ing. Quimica 2022, 21, Bio2777. [Google Scholar] [CrossRef]
- Pinheiro de-Menezes, C.; Soares-Medeiros, C.I.; Alves de Lima-Perez, A.L.; Pereira-de Sousa, J.; Sousa-Pinheiro, L.; Alves-de Oliveira Filho, A.; de Oliveira-Lima, E. Citral: Antifungal Activity and Mode of Action, against Cladosporium oxysporum. Ciência E Natura 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Ortega-Sánchez, E.; Loera, O.; Viniegra-González, G. The Effect of the Ratio between Substrate Concentration and Specific Area of the Support on the Biomass Yield of Fungal Surface Cultures. Rev. Mex. Ing. Química 2012, 11, 485–494. [Google Scholar]
- Ramos-Bell, S.; Hernández-Montiel, L.G.; Velázquez-Estrada, R.M.; Moreno-Hernández, C.L.; Gutiérrez-Martínez, P. Chitosan and Salicylic Acid as Alternatives for the Control of Postharvest Fungal Diseases in Blueberries (Vaccinium corymbosum). Int. Food Res. J. 2023, 30, 992–1000. [Google Scholar] [CrossRef]
- Gálvez-Iriqui, A.C.; García-Romo, J.S.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Burboa-Zazueta, M.G.; Luque-Alcaraz, A.G.; Calderón-Santoyo, M.; Argüelles-Monal, W.M.; Plascencia-Jatomea, M. Phytotoxicity, Cytotoxicity, and in Vivo Antifungal Efficacy of Chitosan Nanobiocomposites on Prokaryotic and Eukaryotic Cells. Environ. Sci. Pollut. Res. 2021, 28, 3051–3065. [Google Scholar] [CrossRef]
- Bagur-González, M.G.; Estepa-Molina, C.; Martín-Peinado, F.; Morales-Ruano, S. Toxicity Assessment Using Lactuca sativa L. Bioassay of the Metal(Loid)s As, Cu, Mn, Pb and Zn in Soluble-in-Water Saturated Soil Extracts from an Abandoned Mining Site. J. Soils Sediments 2011, 11, 281–289. [Google Scholar] [CrossRef]
- Megías, M.; Molist, P.; Pombal, M.A. Técnicas Histológicas: Protocolos Safranina—Azul alcián o Verde rápido. Available online: https://mmegias.webs.uvigo.es/6-tecnicas/protocolos/p-tincion-safranina-a-v.php (accessed on 12 November 2024).
- Islam, S.; Hussain, E.A.; Shujaat, S.; Khan, M.U.; Ali, Q.; Malook, S.U.; Ali, D. Antibacterial Potential of Propolis: Molecular Docking, Simulation and Toxicity Analysis. AMB Express 2024, 14, 81. [Google Scholar] [CrossRef]
- Gowda, S.; Sriram, S. Green Synthesis of Chitosan Silver Nanocomposites and Their Antifungal Activity against Colletotrichum truncatum Causing Anthracnose in Chillies. Plant Nano Biol. 2023, 5, 100041. [Google Scholar] [CrossRef]
- El-araby, A.; Janati, W.; Ullah, R.; Uddin, N.; Bari, A. Antifungal Efficacy of Chitosan Extracted from Shrimp Shell on Strawberry (Fragaria × ananassa) Postharvest Spoilage Fungi. Heliyon 2024, 10, e29286. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Coviello, L.; Triunfo, M.; Guarnieri, A.; Scieuzo, C.; Salvia, R.; Falabella, P.; Nuzzaci, M. In Vitro Antifungal Activity and in Vivo Edible Coating Efficacy of Insect-Derived Chitosan against Botrytis cinerea in Strawberry. Int. J. Biol. Macromol. 2024, 279, 135158. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Batista, E.; González-Arias, C.A.; Velázquez-Estrada, R.M.; Herrera-González, J.A.; Gutiérrez-Martínez, P. In Vitro and in Vivo Antifungal Activity of Chitosan and Identification of Potentially Toxigenic Fungi in Stored Maize of Nayarit, Mexico. Rev. Mex. Ing. Quimica 2024, 23, Bio24223. [Google Scholar] [CrossRef]
- Rayón-Díaz, E.; Birke-Biewendt, A.B.; Velázquez-Estrada, R.M.; González-Estrada, R.R.; Ramírez-Vázquez, M.; Rosas-Saito, G.H.; Gutierrez-Martinez, P. Sodium Silicate and Chitosan: An Alternative for the in Vitro Control of Colletotrichum gloeosporioides Isolated from Papaya (Carica papaya L.). Rev. Bio Cienc. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Amador-Alférez, K.A.; Na Díaz-González, J.; Loza-Cornejo, S.; Yareth Bivián-Castro, E. Efecto de Diferentes Reguladores de Crecimiento Vegetal Sobre La Germinación de Semillas y Desarrollo de Plántulas de Dos Especies de Ferocactus (Cactaceae). Polibotanica 2013, 35, 109–131. [Google Scholar]
- Chirinos-Álvarez, A.B. Efecto Del Quitosano Sobre la Germinación Y Preservación de la Semilla de Calabacín (Curcubita Pepo). Tesis de Maestría, Universidad de Los Andes, Mérida, Venezuela, 2013. [Google Scholar] [CrossRef]
- Tran, L.-D.; Nguyen, B.X.; Phan, L.T.; Nguyen, T.Q.; Nguyen, T.D.; Tran, T.T.; Tran, T.V. In-Vitro Antimicrobial Activity of Chitosan Derived from Shrimp Co-Products against Pathogenic Microorganism Isolated in Vietnam. Authorea 2024, 1–15. [Google Scholar] [CrossRef]
- Anusha, B.; Sathya, K.; Parthasarathy, S.; Prabakar, K.; Raghu, D.; Thiribhuvanamala, G.; Ramjegathesh, R.; Subramanian, K.S.; Paliyath, G.; Jayasankar, S. Effect of Hexanal on Mycelial Growth and Spore Germination of Colletotrichum gloeosporioides and Lasiodiplodia theobromae of Mango. Trop. Agric. 2016, 93, 312–322. [Google Scholar]
- dos Passos-Braga, S.; Alencar-Lundgren, G.; Alves-Macedo, S.; Fechine-Tavares, J.; dos Santos-Vieira, W.A.; Saraiva-Câmara, M.P.; Leite-de Souza, E. Application of Coatings Formed by Chitosan and Mentha Essential Oils to Control Anthracnose Caused by Colletotrichum gloesporioides and C. brevisporum in Papaya (Carica papaya L.) Fruit. Int. J. Biol. Macromol. 2019, 139, 631–639. [Google Scholar] [CrossRef]
- Benatar, G.V.; Nurhayati, Y.; Kulsum, U. Biological Agent Trichoderma asperellum and Its in Vitro Inhibitory Activity Against Mango Fruit Rot Pathogens. J. Biol. Trop. 2023, 23, 70–75. [Google Scholar] [CrossRef]
- de Oliveira, B.F.; Reis, A.; da Costa, C.A.; Moita, A.W.; Pilon, L. Evaluation of Chitosan for in Vitro Control of Colletotrichum tamarilloi and Anthracnose on Scarlet Eggplant Fruit. Hortic. Bras. 2023, 41, e2621. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of Acetylation Degree and Molecular Weight of Homogeneous Chitosans on Antibacterial and Antifungal Activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Xavier Giacomini, G.; de Figueiredo Nachtigal, G.; Roberto Martins, C.; Roedel Hirdes, A.; Alexandre Valgas, R.; Wohlmuth Alves dos Santos, A.J.R. Eco-Friendly Fungicide Based on Chitosan and Pecan Nut Oil: Development and Evaluation in Anthracnose Control. Acta Sci. Biol. Sci. 2023, 45, e62090. [Google Scholar] [CrossRef]
- Heras-Mozos, R. Envases Activos Basados En El Anclaje Covalente Reversible de Compuestos Antimicrobianos En Quitosano. Tesis Doctoral, Universitat Politècnica de Valencia, Valencia, Spain, 2022. [Google Scholar]
- Chávez-Magdaleno, M.E.; González-Estrada, R.R.; Ramos-Guerrero, A.; Plascencia-Jatomea, M.; Gutiérrez-Martínez, P. Effect of Pepper Tree (Schinus molle) Essential Oil-Loaded Chitosan Bio-Nanocomposites on Postharvest Control of Colletotrichum gloeosporioides and Quality Evaluations in Avocado (Persea americana) Cv. Hass. Food Sci. Biotechnol. 2018, 27, 1871–1875. [Google Scholar] [CrossRef]
- González-Estrada, R.R.; Vega-Arreguín, J.; Robles-Villanueva, B.A.; Velázquez-Estrada, R.M.; Ramos-Guerrero, A.; Gutiérrez-Martínez, P. In Vitro Evaluation of Non-Conventional Chemicals for Penicillium citrinum Control. Polibotanica 2020, 49, 161–172. [Google Scholar] [CrossRef]
- Ochoa-Jiménez, V.A.; Berumen-Varela, G.; Balois-Morales, R.; Bautista-Rosales, P.U.; Chacón-López, M.A.; Gutiérrez-Martínez, P. Chitosan inhibits the In Vitro development of Colletotrichum sp. from Banana (Musa × paradisiaca L.) fruits. Acta Biolo. Colomb. 2024, 29, 56–61. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral Inhibits Mycelial Growth of Penicillium italicum by a Membrane Damage Mechanism. Food Control. 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Guo, L.; Li, Y.; Mao, X.; Tao, R.; Tao, B.; Zhou, Z. Antifungal Activity of Polymethoxylated Flavonoids (PMFs)-Loaded Citral Nanoemulsion against Penicillium italicum by Causing Cell Membrane Damage. J. Fungi 2022, 8, 388. [Google Scholar] [CrossRef]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral Exerts Its Antifungal Activity against Penicillium digitatum by Affecting the Mitochondrial Morphology and Function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef]
- Tang, X.; Shao, Y.L.; Tang, Y.J.; Zhou, W.W. Antifungal Activity of Essential Oil Compounds (Geraniol and Citral) and Inhibitory Mechanisms on Grain Pathogens (Aspergillus flavus and Aspergillus ochraceus). Molecules 2018, 23, 2108. [Google Scholar] [CrossRef]
- Zulu, L.; Gao, H.; Zhu, Y.; Wu, H.; Xie, Y.; Liu, X.; Yao, H.; Rao, Q. Antifungal Effects of Seven Plant Essential Oils against Penicillium digitatum. Chem. Biol. Technol. Agric. 2023, 10, 82. [Google Scholar] [CrossRef]
- Barrado, N.M.; Alcaraz, M.L.; Dublan, M.A.; Nesprias, R.K. Efectos del quitosano sobre la germinación de semillas de Eruca versicaria ssp. sativa. Investig. Joven 2023, 10, 131. [Google Scholar]
- Rosabal-Ayan, L.; Martínez-González, L.; Reyes-Guerrero, Y.; DellÁmico-Rodríguez, J.; Núñez-Vázquez, M. Review Physiological, Biochemistry and Gene Expression Aspects in Water Stress. Influence in the Germination Process. Cultiv. Trop. 2014, 35, 24–35. [Google Scholar]
- López-Bermúdez, L.; Quintana-Obregón, E.; Rosas-Burgos, E.; Gálvez, A.; Gutierrez-Martinez, P.; Lizardi-Mendoza, J.; Plascencia-Jatomea, M. Acute Phytotoxicity and Antifungal Effect of Nanochitosan Particles on Colletotrichum fructicola with Low Susceptibility to Chitosan. Curr. Microbiol. 2024, 81, 445. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Niazi, H.; Azimi, R.; Amini, A.; Rasikh, Z.U.R.; Niazi, S.; Niazi, M. Effect of Different Concentrations of Chitosan on Germination and Growth of Sweet Thai Basil. Aust. J. Eng. Innov. Technol. 2023, 5, 255–263. [Google Scholar] [CrossRef]
- Steven, S.; Islam, M.S.; Ghimire, A.; Methela, N.J.; Kwon, E.H.; Yun, B.W.; Lee, I.J.; Kim, S.H.; Kim, Y. Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.). Plants 2024, 13, 1290. [Google Scholar] [CrossRef]
- Barros-Zacharias, M.; Forti, V.A.; da Silva, M.A. Physiological Potential and Health Quality of Corn Seeds Coated with Chitosan. Sci. Agric. 2024, 81, e20230121. [Google Scholar] [CrossRef]
- Graña, E.; Díaz-Tielas, C.; López-González, D.; Martínez-Peñalver, A.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The Plant Secondary Metabolite Citral Alters Water Status and Prevents Seed Formation in Arabidopsis thaliana. Plant Biol. 2016, 18, 423–432. [Google Scholar] [CrossRef]
- Graña, E.; Sotelo, T.; Díaz-Tielas, C.; Araniti, F.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Citral Induces Auxin and Ethylene-Mediated Malformations and Arrests Cell Division in Arabidopsis thaliana Roots. J. Chem. Ecol. 2013, 39, 271–282. [Google Scholar] [CrossRef]
- Torres-Pagán, N.; Muñoz, M.; Barbero, S.; Mamone, R.; Peiró, R.; Carrubba, A.; Sánchez-Moreiras, A.M.; Gómez de Barreda, D.; Verdeguer, M. Herbicidal Potential of the Natural Compounds Carvacrol, Thymol, Eugenol, p-Cymene, Citral and Pelargonic Acid in Field Conditions: Indications for Better Performance. Agronomy 2024, 14, 537. [Google Scholar] [CrossRef]
- Kurashov, E.A.; Fedorova, E.V.; Krylova, J.V.; Mitrukova, G.G. Assessment of the Potential Biological Activity of Low Molecular Weight Metabolites of Freshwater Macrophytes with QSAR. Scientifica 2016, 2016, 1205680. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, A.; Hamada Saud, D.; Rebiai, A.; Achouri, A.; Benabdesselam, S.; Mohamed Abd El-Mordy, F.; Pohl, P.; Ah-mad, S.F.; Attia, S.M.; Abulkhair, H.S.; et al. Introducing the antibacterial and photocatalytic degradation potentials of biosynthesized chitosan, chitosan–ZnO, and chitosan–ZnO/PVP nanoparticles. Sci. Rep. 2024, 14, 14753. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, A.; Shahwan, M.; Furkan, M.; Yadav, D.K.; Khan, R.H. Computational and Spectroscopic Insight into the Binding of Citral with Human Transferrin: Targeting Neurodegenerative Diseases. Heliyon 2024, 10, e32755. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Concentration (%) | Sporulation (106 Spores/mL) | Biomass Dry Weight (g) |
|---|---|---|---|
| DW | - | 8.28 ± 2.84 c | 0.612 ± 0.006 c |
| DMSO | 0.5 | 4.52 ± 2.79 abc | 0.630 ± 0.011 c |
| AAc | 1.5 | 0.00 ± 0.00 a | 0.000 ± 0.000 a |
| CH | 0.5 | 6.40 ± 2.17 b | 0.604 ± 0.021 c |
| 1.0 | 0.21 ± 0.21 a | 0.000 ± 0.000 a | |
| 1.5 | 0.00 ± 0.00 a | 0.000 ± 0.000 a | |
| CT | 0.03 | 0.00 ± 0.00 a | 0.000 ± 0.000 a |
| 0.05 | 0.00 ± 0.00 a | 0.000 ± 0.000 a | |
| 0.1 | 0.00 ± 0.00 a | 0.000 ± 0.000 a | |
| HX | 0.04 | 8.41 ± 5.81 c | 0.252 ± 0.123 b |
| 0.06 | 0.00 ± 0.00 a | 0.325 ± 0.123 b | |
| 0.08 | 0.00 ± 0.00 a | 0.318 ± 0.121 b |
| Treatment | GI %Ts | REI %Ts | NREI Ts | NRGI Ts |
| DW | 100 ± 0.00 a | 0.00 ± 0.00 d | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| DMSO 0.5% | 39.35 ± 14.30 b | 53.88 ± 11.26 c | −0.53 ± 0.11 b | −0.19 ± 0.10 abc |
| AAc 1.5% | 0.00 ± 0.00 d | 100.00 ± 0.00 a | −1.00 ± 0.00 d | −1.00 ± 0.00 d |
| CH 0.5% | 3.87 ± 1.65 cd | 95.77 ± 1.44 ab | −0.95 ± 0.01 cd | −0.13 ± 0.13 ab |
| CH 1% | 6.00 ± 5.41 cd | 98.26 ± 0.43 ab | −0.97 ± 0.00 cd | −0.47 ± 0.07 abcd |
| CH 1.5% | 0.42 ± 0.22 d | 99.02 ± 0.40 a | −0.98 ± 0.00 cd | −0.66 ± 0.11 bcd |
| CT 0.03% | 2.77 ± 2.59 cd | 96.91 ± 2.43 ab | −0.93 ± 0.02 cd | −0.48 ± 0.24 abcd |
| CT 0.05% | 1.11 ± 0.64 d | 98.05 ± 0.48 ab | −0.97 ± 0.00 cd | −0.48 ± 0.19 abcd |
| CT 0.1% | 3.56 ± 3.21 cd | 94.51 ± 0.40 ab | −0.94 ± 0.04 cd | −0.61 ± 0.20 abcd |
| HX 0.04% | 24.63 ± 5.46 bcd | 70.32 ± 5.32 c | 0.69 ± 0.05 b | 0.17 ± 0.06 abc |
| HX 0.06% | 15.39 ± 1.89 bcd | 75.88 ± 5.56 bc | −0.75 ± 0.05 bc | −0.30 ± 0.11 abc |
| HX 0.08% | 27.78 ± 6.25 cd | 69.72 ± 7.41 c | −0.69 ± 0.07 b | −0.07 ± 0.03 ab |
| Treatment | GI %Cs | REI %Cs | NREI Cs | NRGI Cs |
| DW | 100 ± 0.00 ab | 0.00 ± 0.00 b | 0.00 ± 0.00 ab | 0.00 ± 0.00 c |
| DMSO 0.5% | 278.73 ± 108.15 b | −112.26 ± 85.62 b | 1.12 ± 0.85 b | 0.30 ± 0.18 c |
| AAc 1.5% | 0.00 ± 0.00 a | 100.00 ± 0.00 a | −1.00 ± 0.00 a | −1.00 ± 0.00 a |
| CH 0.5% | 5.26 ± 3.20 a | 94.26 ± 2.19 a | −0.94 ± 0.02 a | −0.21 ± 0.19 bc |
| CH 1% | 0.02 ± 0.02 a | 99.90 ± 0.10 a | −1.00 ± 0.00 a | −0.91 ± 0.08 ab |
| CH 1.5% | 0.04 ± 0.04 a | 99.73 ± 0.76 a | −0.99 ± 0.00 a | −0.94 ± 0.05 ab |
| CT 0.03% | 115.55 ± 97.40 ab | 15.43 ± 58.49 ab | −0.13 ± 0.60 ab | −0.02 ± 0.27 c |
| CT 0.05% | 86.91 ± 28.98 ab | 19.63 ± 23.41 ab | −0.19 ± 0.23 ab | 0.05 ± 0.05 c |
| CT 0.1% | 82.68 ± 70.15 ab | 20.90 ± 57.06 ab | −0.20 ± 0.57 ab | −0.25 ± 0.21 abc |
| HX 0.04% | 71.18 ± 21.58 ab | 23.83 ± 26.05 ab | −0.24 ± 0.26 ab | −0.04 ± 0.14 c |
| HX 0.06% | 124.89 ± 88.82 ab | −4.03 ± 63.45 ab | 0.04 ± 0.63 ab | −0.05 ± 0.20 c |
| HX 0.08% | 108.01 ± 45.08 ab | −19.33 ± 39.55 ab | 0.02 ± 0.47 ab | 0.08 ± 0.08 c |
| Cross-section of root tissue |  |  |  |
| Radicle development |  | 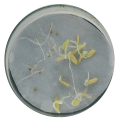 | 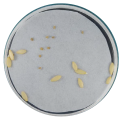 |
| Treatment | DW | DMSO 0.5% | AAc 1.5% |
| Cross-section of root tissue | 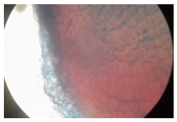 | 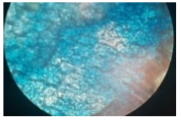 | 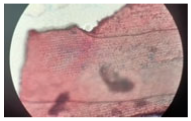 |
| Radicle development | 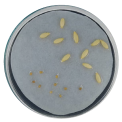 | 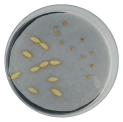 | 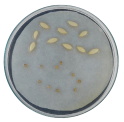 |
| Treatment | CH 0.5% | CH 1.0% | CH 1.5% |
| Cross-section of root tissue | 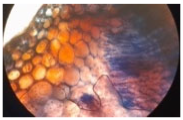 | 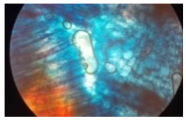 |  |
| Radicle development |  |  |  |
| Treatment | CT 0.03% | CT 0.05% | CT 0.1% |
| Cross-section of root tissue | 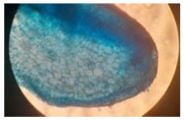 | 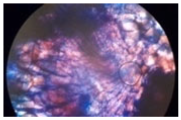 | 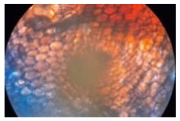 |
| Radicle development | 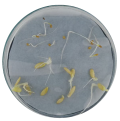 | 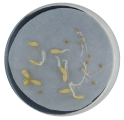 |  |
| Treatment | HX 0.04% | HX 0.06% | HX 0.08% |
| Treatment | Molecular Weight (g/mol) | Topological Polar Surface Area (TPSA) | Hydrogen Bond Donors Count | Hydrogen Bond Acceptors Count | Predicted Toxicity Class | LD50 (mg/kg) |
|---|---|---|---|---|---|---|
| CH | 1526.45 | 808 Å2 | 29 | 48 | 2 | 50 |
| CT | 152.23 | 17.07 Å2 | 0 | 1 | 4 | 500 |
| HX | 100.16 | 17.07 Å2 | 0 | 1 | 5 | 3240 |
| Classification | Target | CH | CT | HX | |||
|---|---|---|---|---|---|---|---|
| Prediction | Probability | Prediction | Probability | Prediction | Probability | ||
| Organ toxicity | Hepatotoxicity | Inactive | 0.74 | Inactive | 0.69 | Inactive | 0.73 |
| Toxicity endpoints | Carcinogenicity | Inactive | 0.69 | Inactive | 0.88 | Inactive | 0.59 |
| Immunotoxicity | Inactive | 0.83 | Inactive | 0.99 | Inactive | 0.97 | |
| Mutagenicity | Inactive | 0.62 | Inactive | 0.98 | Inactive | 0.97 | |
| Tox21-Nuclear receptor signaling pathways | Cytotoxicity | Inactive | 0.65 | Inactive | 0.82 | Inactive | 0.75 |
| Aryl hydrocarbon receptor (AhR) | Inactive | 0.97 | Inactive | 1.0 | Inactive | 1.0 | |
| Androgen receptor (AR) | Inactive | 0.92 | Inactive | 1.0 | Inactive | 1.0 | |
| Androgen receptor ligand binding domain (AR-LBD) | Inactive | 0.96 | Inactive | 0.95 | Inactive | 1.0 | |
| Aromatase | Inactive | 0.98 | Inactive | 1.0 | Inactive | 1.0 | |
| Estrogen receptor alpha (ER) | Inactive | 0.69 | Inactive | 0.94 | Inactive | 0.78 | |
| Estrogen receptor ligand binding domain (ER-LBD) | Inactive | 0.94 | Inactive | 0.96 | Inactive | 1.0 | |
| Peroxisome proliferator-activated receptor gamma (PPAR-Gamma) | Inactive | 0.99 | Inactive | 1.0 | Inactive | 0.99 | |
| Tox21-Stress response pathways | Nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element (nrf2/ARE) | Inactive | 0.98 | Inactive | 0.99 | Inactive | 1.0 |
| Heat shock factor response element (HSE) | Inactive | 0.98 | Inactive | 0.99 | Inactive | 1.0 | |
| Mitochondrial membrane potential (MMP) | Inactive | 0.95 | Inactive | 0.98 | Inactive | 0.95 | |
| Phosphoprotein (tumor suppressor) p53 | Inactive | 0.95 | Inactive | 1.0 | Inactive | 1.0 | |
| ATPase family AAA domain-containing protein 5 (ATAD5) | Inactive | 0.98 | Inactive | 1.0 | Inactive | 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayón-Díaz, E.; Hernández-Montiel, L.G.; Zamora-Gasga, V.M.; Sánchez-Burgos, J.A.; Ramos-Bell, S.; Velázquez-Estrada, R.M.; Herrera-González, J.A.; Gutiérrez-Martínez, P. Antifungal and Toxicological Evaluation of Natural Compounds Such as Chitosan, Citral, and Hexanal Against Colletotrichum asianum. Horticulturae 2025, 11, 474. https://doi.org/10.3390/horticulturae11050474
Rayón-Díaz E, Hernández-Montiel LG, Zamora-Gasga VM, Sánchez-Burgos JA, Ramos-Bell S, Velázquez-Estrada RM, Herrera-González JA, Gutiérrez-Martínez P. Antifungal and Toxicological Evaluation of Natural Compounds Such as Chitosan, Citral, and Hexanal Against Colletotrichum asianum. Horticulturae. 2025; 11(5):474. https://doi.org/10.3390/horticulturae11050474
Chicago/Turabian StyleRayón-Díaz, Edson, Luis G. Hernández-Montiel, Víctor Manuel Zamora-Gasga, Jorge A. Sánchez-Burgos, Surelys Ramos-Bell, Rita María Velázquez-Estrada, Juan Antonio Herrera-González, and Porfirio Gutiérrez-Martínez. 2025. "Antifungal and Toxicological Evaluation of Natural Compounds Such as Chitosan, Citral, and Hexanal Against Colletotrichum asianum" Horticulturae 11, no. 5: 474. https://doi.org/10.3390/horticulturae11050474
APA StyleRayón-Díaz, E., Hernández-Montiel, L. G., Zamora-Gasga, V. M., Sánchez-Burgos, J. A., Ramos-Bell, S., Velázquez-Estrada, R. M., Herrera-González, J. A., & Gutiérrez-Martínez, P. (2025). Antifungal and Toxicological Evaluation of Natural Compounds Such as Chitosan, Citral, and Hexanal Against Colletotrichum asianum. Horticulturae, 11(5), 474. https://doi.org/10.3390/horticulturae11050474











