Biotization with Plant Growth-Promoting Bacteria Benefits the Survival and Production of Potato (Solanum tuberosum L.) In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Biochemical Characterization of Bacteria
2.2.1. Indole-3-Acetic Acid (IAA) Biosynthesis
2.2.2. Gibberellic Acid (GA3) Biosynthesis
2.2.3. Quantification of Bacterial Salicylic Acid (SA)
2.2.4. ACC Deaminase Activity (ACCd)
2.3. Plant Material, Inoculation, and In Vitro Growth Conditions
2.4. Acclimatization and Re-Inoculation of Potato Seedlings of cv. Duvira and cv. Ágata in a Greenhouse
2.5. Statistical Analysis
3. Results
3.1. Biosynthesis of Indol-3-Acetic Acid (IAA), Gibberellic Acid (GA3), and Salicylic Acid (SA) in PGPB
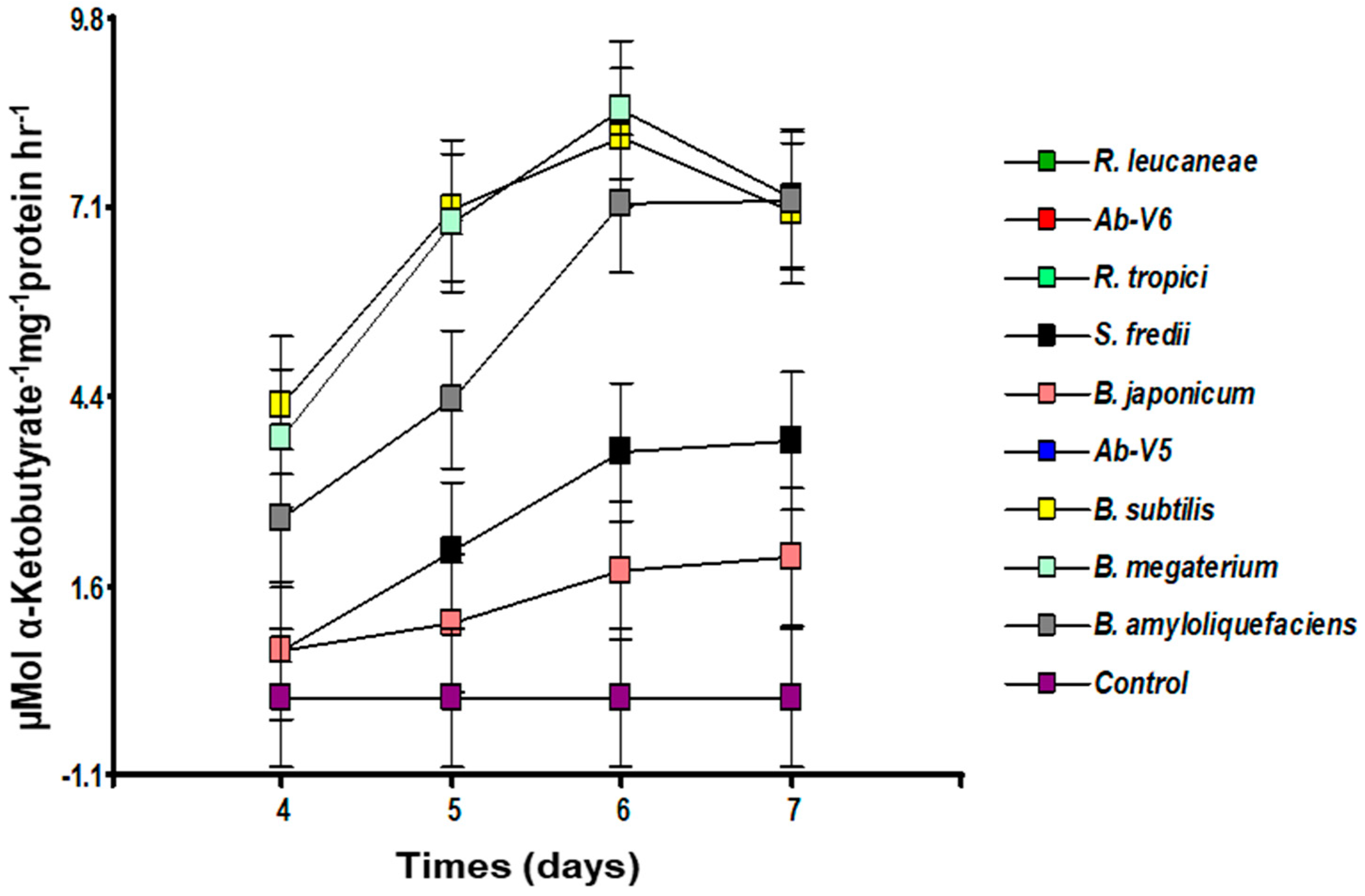
3.2. Determination of ACC Deaminase Activity (ACCd) in PGPB
3.3. Effect of Biotization on Growth Parameters of Potato cv. Duvira and cv. Ágata Propagated In Vitro
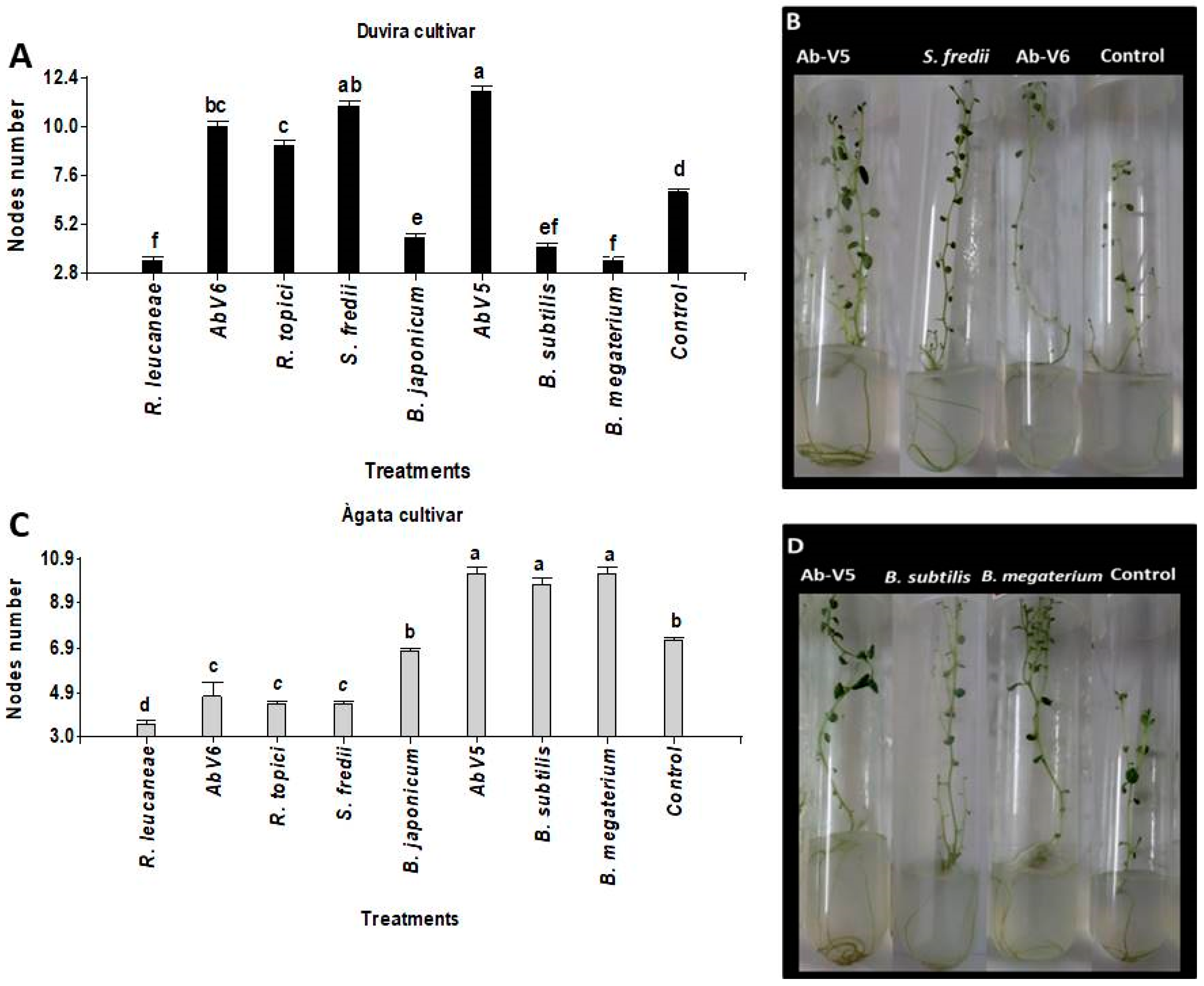
3.3.1. Influence of Biotization on the Development of the Number of Nodes in Potato Micro-Plants cv. Duvira and cv. Ágata
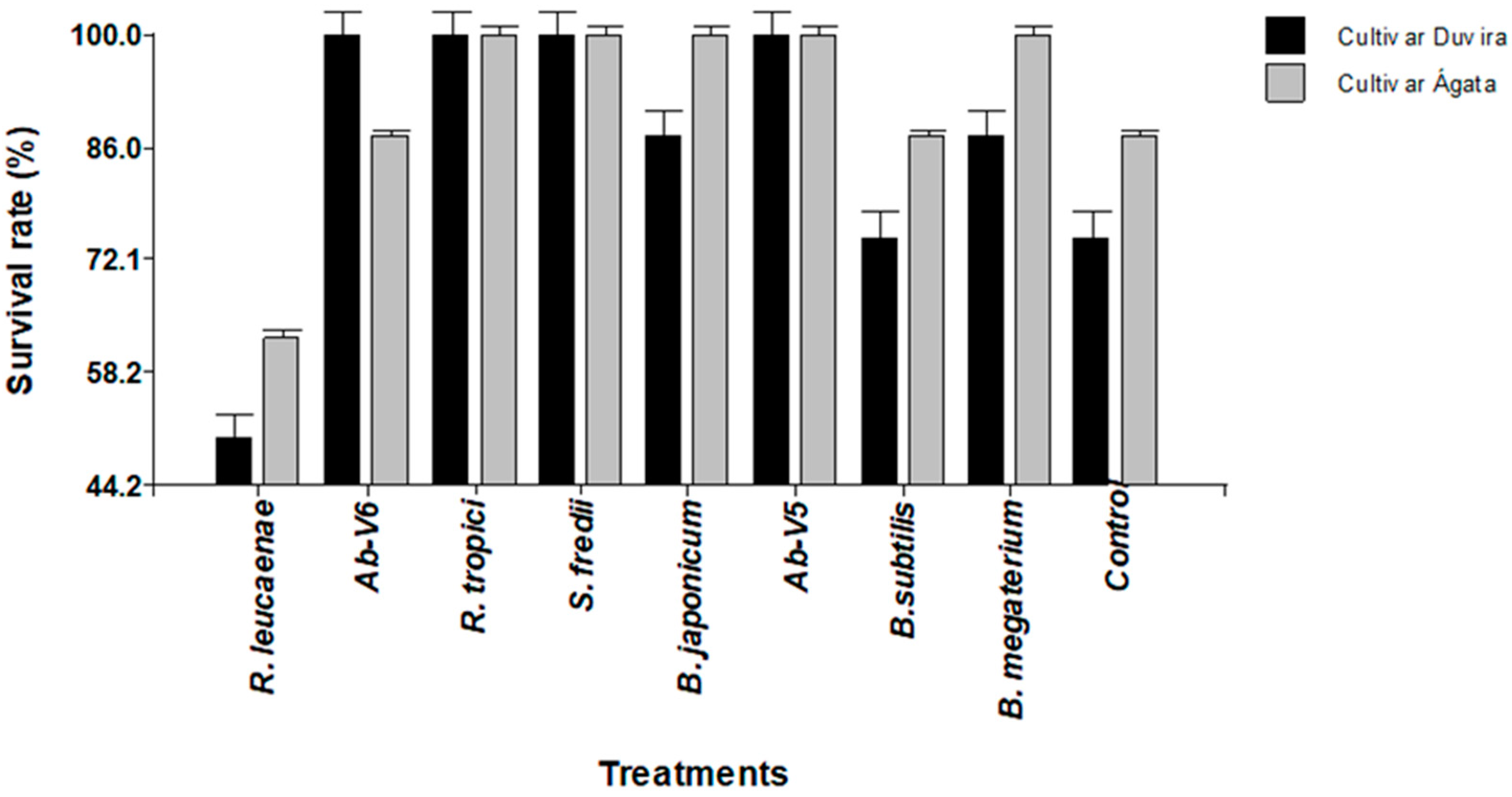
3.3.2. Effect of Biotization on Survival of Seedlings of cv. Duvira and cv. Ágata Under In Vivo Conditions
3.4. Evaluation of the Effect of Biotization on the Performance of Potato Seedlings cv. Duvira and Ágata in the Acclimatization and Re-Inoculation Phase
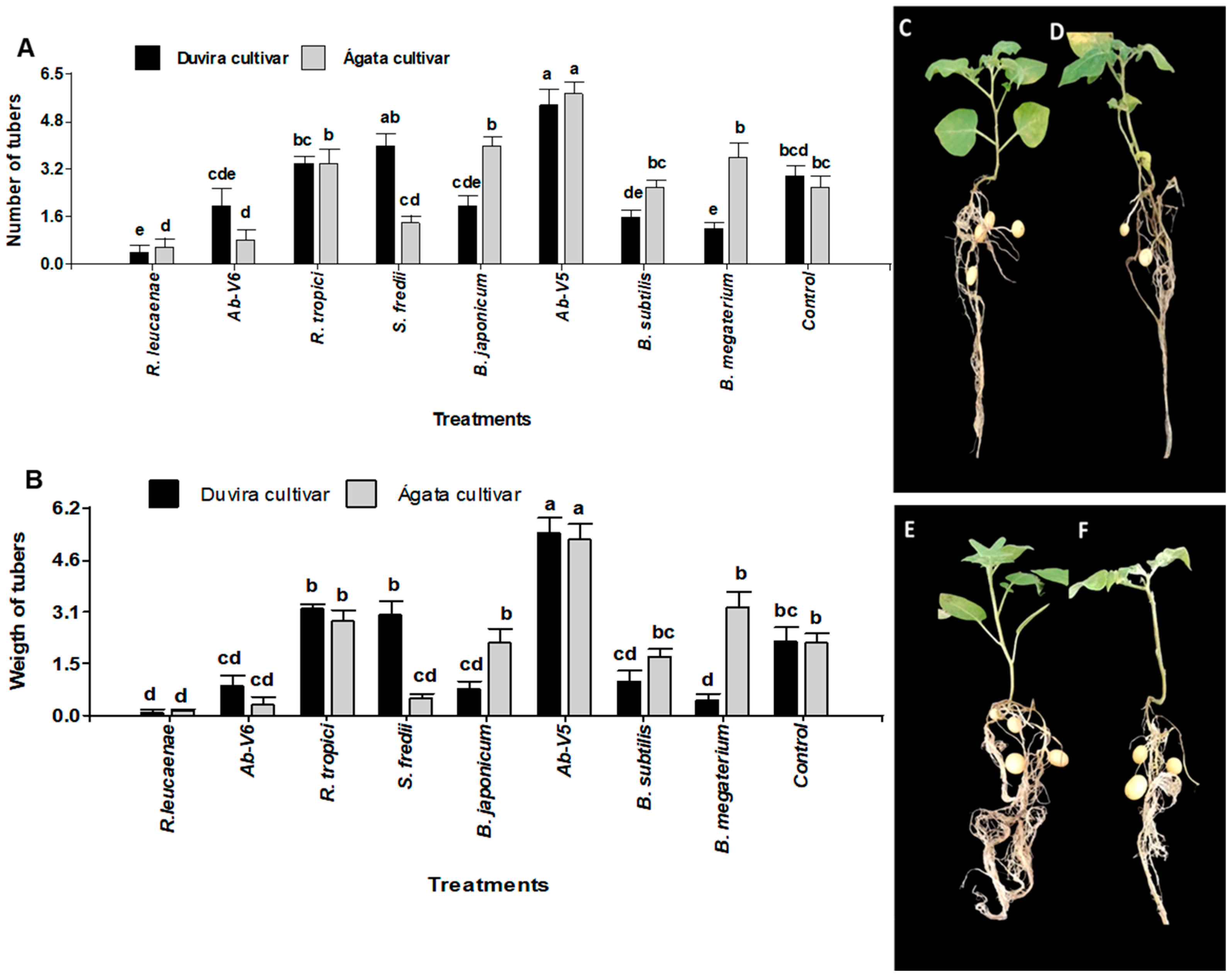
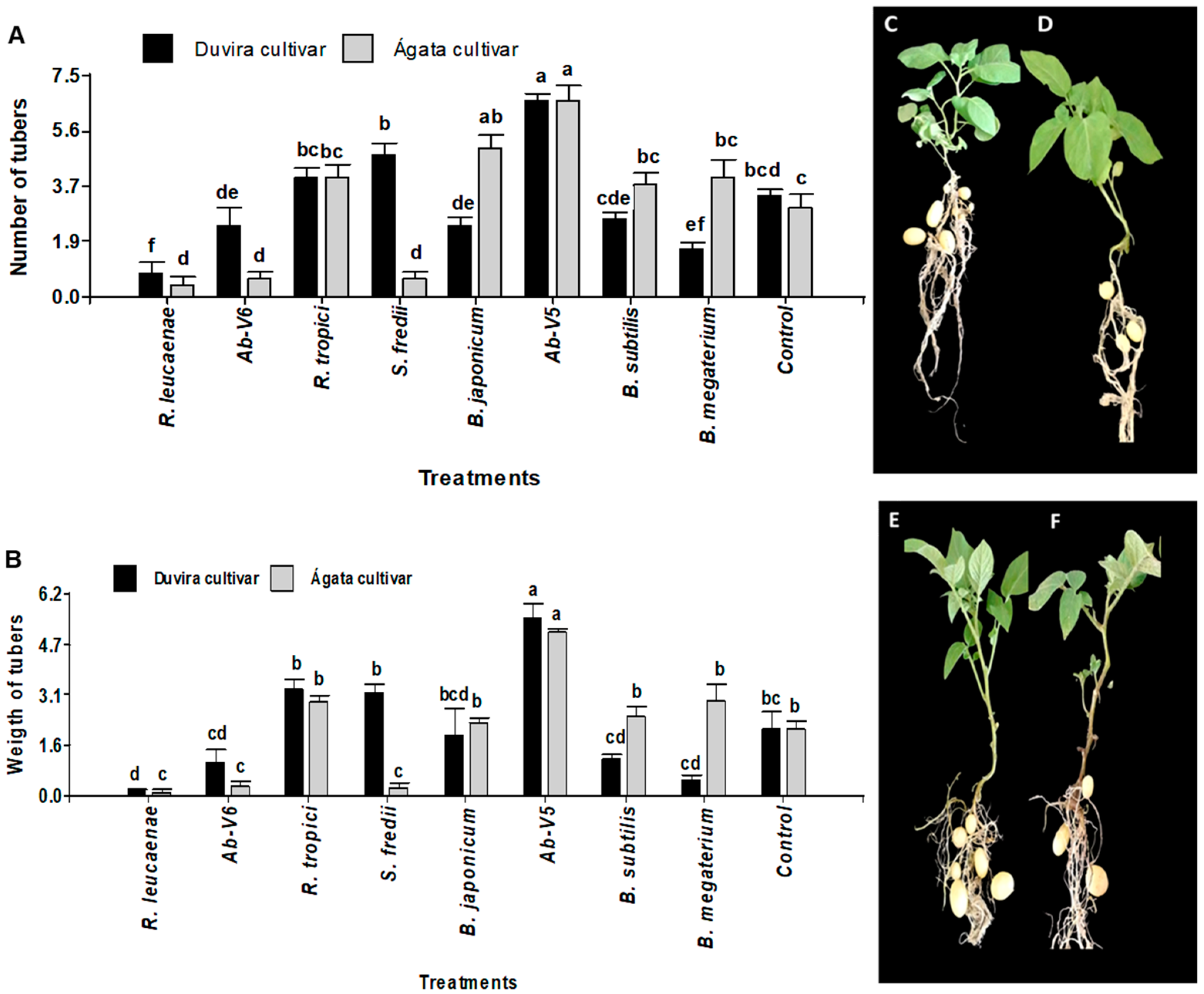
3.4.1. Effect of Biotization with PGPB on Tuber Formation and Weight in cv. Duvira and cv. Ágata
3.4.2. Effect of PGPB Re-Inoculation on Tuber Formation and Weight in cv. Duvira and cv. Ágata
4. Discussion
4.1. Growth-Promoting Functions of Bacteria
4.2. Influence of Biotization on Potato In Vitro Growth
4.3. Impact of Biotization on In Vivo Adaptation and Tuber Formation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castro-Restrepo, D.; Dominguez, M.I.; Gaviria-Gutiérrez, B.; Osorio, E.; Sierra, K. Biotization of Endophytes Trichoderma asperellum and Bacillus subtilis in Mentha spicata Micro Plants to Promote Growth, Pathogen Tolerance and Specialized Plant Metabolites. Plants 2022, 11, 1474. [Google Scholar] [CrossRef]
- Liang, C.; Wu, R.; Han, Y.; Wan, T.; Cai, Y. Optimizing Suitable Antibiotics for Bacterium Control in Micropropagation of Cherry Rootstock Using a Modified Leaf Disk Diffusion Method and E Test. Plants 2019, 8, 66. [Google Scholar] [CrossRef]
- El-Banna, A.N.; El-Mahrouk, M.E.; Dewir, Y.H.; Farid, M.A.; Abou Elyazid, D.M.; Schumacher, H.M. Endophytic Bacteria in Banana In Vitro Cultures: Molecular Identification, Antibiotic Susceptibility, and Plant Survival. Horticulturae 2021, 7, 526. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Tolegen, A.B.; Kushnarenko, S.V.; Zholdybayeva, E.V.; Bettoni, J.C. Effect of Plant Preservative MixtureTM on Endophytic Bacteria Eradication from in Vitro-Grown Apple Shoots. Plants 2022, 11, 2624. [Google Scholar] [CrossRef]
- Soumare, A.; Diédhiou, A.G.; Arora, N.K.; Tawfeeq Al-Ani, L.K.; Ngom, M.; Fall, S.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L.; Sy, M.O. Potential Role and Utilization of Plant Growth Promoting Microbes in Plant Tissue Culture. Front. Microbiol. 2021, 12, 649878. [Google Scholar] [CrossRef]
- Herman, E.B. Beneficial effects of bacteria and fungi on plant tissue cultures. Agricell Rep. 1996, 27, 26–27. [Google Scholar]
- Herman, E.B. Contaminants promote potato micropropagation. Agricell Rep. 1987, 9, 38. [Google Scholar]
- Kanani, P.; Modi, A.; Kumar, A. Biotization of Endophytes in Micropropagation: A Helpful Enemy; Kumar, E.A., Singh, V.K., Eds.; Microbial Endophytes; Woodhead Publishing: Cambridge, UK, 2020; pp. 357–379. [Google Scholar] [CrossRef]
- Tkachenko, O.V.; Evseeva, N.V.; Terentyeva, E.V.; Burygin, G.L.; Shirokov, A.A.; Burov, A.M.; Matora, L.Y.; Shchyogolev, S.Y. Improved Production of High-Quality Potato Seeds in Aeroponics with Plant-Growth-Promoting Rhizobacteria. Potato Res. 2021, 64, 55–66. [Google Scholar] [CrossRef]
- Tkachenko, O.V.; Evseeva, N.V.; Boikova, N.V.; Matora LYu Burygin, G.L.; Lobachev, Y.V.; Shchyogolev, S.Y. Improved potato microclonal reproduction with the plant growth-promoting rhizobacteria Azospirillum. Agron. Sustain. Dev. 2015, 35, 1167–1174. [Google Scholar] [CrossRef]
- Maggini, V.; Mengoni, A.; Gallo, E.R.; Biffi, S.; Fani, R.; Firenzuoli, F.; Bogani, P. Tissue specificity and differential effects on in vitro plant growth of single bacterial endophytes isolated from the roots, leaves and rhizospheric soil of Echinacea purpurea. BMC Plant Biol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Souza, J.A.; Bettoni, J.C.; Dalla Costa, M.; Baldissera, T.C.; dos Passos, J.M.; Primieri, S. In vitro rooting and acclimatization of ‘Marubakaido’ apple rootstock using indole-3-acetic acid from rhizobacteria. Commun. Plant Sci. 2022, 12, 16–23. [Google Scholar]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of Plant Growth Promoting Rhizobacteria on the Content of Abscisic Acid and Salt Resistance of Wheat Plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of Indoleacetic Acid, Gibberellic Acid and ACC-Deaminase by Mortierella Strains Promote Winter Wheat Seedlings Growth under Different Conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef] [PubMed]
- Orlikowska, T.; Nowak, K.; Reed, B. Bacteria in the plant tissue culture environment. Plant Cell Tissue Organ Cult. 2017, 128, 487–508. [Google Scholar] [CrossRef]
- Trdan, S.; Vučajnk, F.; Bohinc, T.; Vidrih, M. The effect of a mixture of two plant growth-promoting bacteria from Argentina on the yield of potato, and occurrence of primary potato diseases and pest—Short communication. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 89–94. [Google Scholar] [CrossRef]
- Naqqash, T.; Hameed, S.; Imran, A.; Hanif, M.K.; Majeed, A.; Van Elsas, J.D. Differential Response of Potato Toward Inoculation with Taxonomically Diverse Plant Growth Promoting Rhizobacteria. Front. Plant Sci. 2016, 7, 144. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2016.00144 (accessed on 15 March 2023).
- Quambusch, M.; Winkelmann, T. Bacterial endophytes in plant tissue culture: Mode of action, detection, and control. Methods Mol. Biol 2018, 1815, 69–88. [Google Scholar] [CrossRef]
- Kargapolova, K.; Burygin, G.L.; Tkachenko, O.V.; Evseeva, N.V.; Pukhalskiy, V.; Belimov, A.A. Effectiveness of inoculation of in vitro-grown potato micro plants with rhizosphere bacteria of the genus Azospirillum. Plant Cell Tissue Organ Cult. 2020, 141, 351–359. [Google Scholar] [CrossRef]
- Blanco Carrero, E.L.; Castro Molina, Y. Antagonismo de rizobacterias sobre hongos fitopatógenos, y su actividad microbiana con potencial biofertilizante, bioestimulante y biocontrolador. Rev. Colomb. Biotecnol. 2021, 23, 6–16. [Google Scholar] [CrossRef]
- Graham, H.D.; Thomas, L.B. Rapid, simple colorimetric method for the determination of micro quantities of gibberellic acid. J. Pharm. Sci. 1961, 50, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Sagar A Desai Isolation and characterization of gibberellic acid (GA3) producing rhizobacteria from sugarcane roots. Biosci. Discov. 2017, 8, 488–494. Available online: http://biosciencediscovery.com (accessed on 4 April 2023).
- De Meyer, G.; Höfte, M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology 1997, 87, 588–593. [Google Scholar] [PubMed]
- Islam, M.N.; Ali, M.S.; Choi, S.J.; Park, Y.I.; Baek, K.H. Salicylic Acid-Producing Endophytic Bacteria Increase Nicotine Accumulation and Resistance Against Wildfire Disease in Tobacco Plants. Microorganisms 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.; Foster, J.W. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–603. [Google Scholar] [CrossRef]
- Honma, M.; Shimomura, T. Metabolism of 1-Aminocyclopropane-1-carboxylic Acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar]
- Hoagland, D.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 39. [Google Scholar]
- Jarstfer, A.G.; Sylvia, D.M. Inoculum production and inoculation strategies for vesicular-arbuscular mycorrhizal fungi. In Soil Microbial Ecology: Applications in Agricultural and Environmental Management; Metting, F.B., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1992; p. e377. [Google Scholar]
- Ormeño-Orrillo, E.; Menna, P.; Almeida, L.G.P.; Ollero, F.J.; Nicolás, M.F.; Rodrigues, E.P.; Nakatani, A.S.; Batista, J.S.S.; Chueire, L.M.O.; Souza, R.C.; et al. Genomic basis of broad host range and environmental adaptability of Rhizobium tropici CIAT 899 and Rhizobium sp. PRF 81 which are used in inoculants for common bean (Phaseolus vulgaris L.). BMC Genom. 1992, 13, 735. [Google Scholar] [CrossRef]
- Velandia, K.; Reid, J.B.; Foo, E. Right time, right place: The dynamic role of hormones in rhizobial infection and nodulation of legumes. Plant Commun. 2022, 3, 100327. [Google Scholar] [CrossRef] [PubMed]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant-stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Wagi, S.; Ahmed, A. Bacillus spp.: Potent microfactories of bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef] [PubMed]
- Khianngam, S.; Meetum, P.; Chiangmai, P.N.; Tanasupawat, S. Identification and optimisation of indole-3-acetic acid production of endophytic bacteria and their effects on plant growth. Trop. Life Sci. Res. 2023, 34, 219. [Google Scholar]
- Lebrazi, S.; Niehaus, K.; Bednarz, H.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and optimization of indole-3-acetic acid production and phosphate solubilization by rhizobacterial strains isolated from Acacia cyanophylla root nodules and their effects on its plant growth. J. Genet. Eng. Biotechnol. 2020, 18, 71. [Google Scholar] [CrossRef]
- Donati, A.J.; Lee, H.-I.; Leveau, J.H.J. Chang W-S Effects of indole-3-acetic acid on the transcriptional activities and stress tolerance of Bradyrhizobium japonicum. PLoS ONE 2013, 8, 76559. [Google Scholar] [CrossRef]
- Shani, E.; Salehin, M.; Zhang, Y.; Sanchez, S.E.; Doherty Wang, R. Plant stress tolerance requires auxin-sensitive Aux/IAA transcriptional repressors. Curr. Biol. 2017, 27, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Singh, D.; Saksena, H.B.; Sharma, M.; Tiwari, A.; Awasthi, P.; Botta, H.K.; Shukla, B.N.; Laxmi, A. Understanding the intricate web of phytohormone signalling in modulating root system architecture. Int. J. Mol. Sci. 2021, 22, 5508. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that Go Far beyond Biological Nitrogen Fixation. AMB Expr. 2018, 8, 73. [Google Scholar] [CrossRef]
- Costacurta, A.; Vanderleyden, J. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 1995, 21, 1–18. [Google Scholar] [CrossRef]
- Keswani, C.; Singh, H.B.; García-Estrada, C.; Caradus, J.; He, Y.-W.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Sansinenea, E. Antimicrobial secondary metabolites from agriculturally important bacteria as next-generation pesticides. Appl. Microbiol. Biotechnol. 2020, 104, 1013–1034. [Google Scholar] [CrossRef]
- Mekonnen, H.; Kibret, M. The roles of plant growth promoting rhizobacteria in sustainable vegetable production in Ethiopia. Chem. Biol. Technol. Agric. 2021, 8, 15. [Google Scholar]
- Tanaka, S.; Han, X.; Kahmann, R. Microbial effectors target multiple steps in the salicylic acid production and signaling pathway. Front. Plant Sci. 2015, 6, 349. [Google Scholar]
- Shanmugam, P.; Narayanasamy, M. Optimization and production of salicylic acid by rhizobacterial strain Bacillus licheniformis MML2501. Int. J. Microbiol. 2008, 6, 94–98. [Google Scholar]
- Bakker, P.A.H.M.; Ran, L.; Mercado-Blanco, J. Rhizobacterial salicylate production provokes headaches! Plant Soil 2014, 382, 1–16. [Google Scholar] [CrossRef]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Izaguirre, M.J.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria improve seedling growth of sunflower under water stress, produce salicylic acid, and inhibit growth of pathogenic fungi. Curr. Microbiol. 2010, 61, 485–493. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can. J. Microbiol. 2001, 47, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.M.; Benso, A.; Walitang, D.I.; Sa, T. Development of ACCd producer A. brasilense mutant and the effect of inoculation on red pepper plants. 3 Biotech 2022, 12, 252. [Google Scholar] [CrossRef]
- Tiwari, G.; Duraivadivel, P.; Sharma, S.P.H. 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. By mitigating drought and salt stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef] [PubMed]
- Weinert, N.; Meincke, R.; Gottwald, C.; Heuer, H.; Schloter, M.; Berg, G.; Smalla, K. Bacterial diversity on the surface of potato tubers in soil and the influence of the plant genotype. FEMS Microbiol. Ecol. 2010, 74, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Balliu, A.; Zheng, Y.; Sallaku, G.; Fernández, J.A.; Gruda, N.S.; Tuzel, Y. Environmental and Cultivation Factors Affect the Morphology, Architecture and Performance of Root Systems in Soilless Grown Plants. Horticulturae 2021, 7, 243. [Google Scholar] [CrossRef]
- Vishnupradeep, R.; Bruno, L.B.; Taj, Z.; Karthik, C.; Challabathula, D.; Kumar, A.; Rajkumar, M. Plant growth promoting bacteria improve growth and phytostabilization potential of Zea mays under chromium and drought stress by altering photosynthetic and antioxidant responses. Environ. Technol. Innov. 2022, 25, 102154. [Google Scholar]

| Strain | Species | Geographic Origin |
|---|---|---|
| BR 935 | Rhizobium leucaneae | Cipó-BA, Brazil |
| Ab-V6 (= CNPSo2084) | Azospirillum brasilense | Curitiba-PR, Brazil |
| Ab-V5 (= CCMA1291) | Azospirillum brasilense | Curitiba-PR, Brazil |
| CCMA0088 | Bradyrhizobium japonicum | Arcos-MG, Brazil |
| CCMA0122 | Sinorhizobium fredii | Luminárias-MG, Brazil |
| CIAT899 | Rhizobium tropici | Colombia |
| CCMA0401 | Bacillus subtilis | Alto Garças-MT, Brazil |
| CCMA0004 | Bacillus megaterium | Confresa-MT, Brazil |
| CCMA0112 | Bacillus amyloliquefaciens | Luminárias-MG, Brazil |
| In Vitro Duvira Cultivar | Length (cm) | Dry Weight (mg) | |||
|---|---|---|---|---|---|
| Treatments | Shoot | Root | Shoot | Root | Total Biomass |
| R. leucaneae | 3.6 ± 0.38 e | 1.3 ± 0.36 ef | 10.0 ± 1.22 d | 0.00 ± 0.00 d | 10.00 d |
| Ab-V6 | 8.3 ± 0.43 c | 4.1 ± 0.24 a | 30.0 ± 2.25 c | 10.00 ± 1.66 de | 40.00 cd |
| R. tropici | 7.7 ± 0.60 c | 3.3 ± 0.22 bc | 110.0 ± 3.24 b | 50.10 ± 2.94 b | 160.10 b |
| S. fredii | 10.8 ± 0.34 b | 4.3 ± 0.31 a | 130.0 ± 3.42 ab | 110.0 ± 4.10 a | 240.00 ab |
| B. japonicum | 5.7 ± 0.12 d | 3.3 ± 0.20 bc | 20.0 ± 1.53 c | 10.0 ± 0.33 d | 30.00 c |
| Ab-V5 | 12.3 ± 0.28 a | 4.1 ± 0.22 a | 150.0 ± 2.19 a | 110.0 ± 3.51 a | 260.00 a |
| B. subtilis | 3.8 ± 0.16 e | 2.0 ± 0.26 de | 10.0 ± 0.86 d | 10.0 ± 0.70 d | 20.00 d |
| B. megaterium | 3.3 ± 0.20 e | 2.1 ± 0.25 d | 30.0 ± 2.68 c | 0.00 ± 0.00 d | 30.00 c |
| Control | 7.2 ± 0.41 d | 3.1 ± 0.30 c | 40.0 ± 2.34 c | 30.1 ± 1.52 c | 70.10 c |
| In Vitro Ágata Cultivar | Length (cm) | Dry Weight (mg) | |||
|---|---|---|---|---|---|
| Treatments | Shoot | Root | Shoot | Root | Total Biomass |
| R. leucaneae | 3.8 ± 0.26 f | 1.5 ± 0.23 e | 20.0 ± 1.58 c | 0.0 ± 0.00 de | 20.0 c |
| Ab-V6 | 3.3 ± 0.24 f | 1.9 ± 0.21 e | 10.0 ± 0.71 c | 0.0 ± 0.00 cde | 10.0 c |
| R. tropici | 6.8 ± 0.36 d | 3.9 ± 0.30 c | 90.0 ± 1.66 b | 50.00 ± 1.01 b | 140.0 b |
| S. fredii | 6.5 ± 0.28 d | 3.9 ± 0.32 c | 10.0 ± 0.59 c | 0.0 ± 0.00 e | 10.0 c |
| B. japonicum | 10.6 ± 0.40 c | 5.1 ± 0.25 b | 110.0 ± 4.23 b | 50.0 ± 2.29 b | 160.0 b |
| Ab-V5 | 12.5 ± 0.32 a | 6.9 ± 0.18 a | 140.0 ± 3.85 a | 110.0 ± 3.55 a | 250.0 a |
| B. subtilis | 11.8 ± 0.37 b | 3.6 ± 0.16 c | 110.0 ± 2.45 ab | 50.0 ± 2.82 b | 160.0 ab |
| B. megaterium | 12.0 ± 0.34 ab | 6.4 ± 0.16 a | 120.0 ± 3.04 ab | 80.0 ± 3.07 ab | 200.0 ab |
| Control | 10.1 ± 0.69 c | 3.6 ± 0.23 c | 30.0 ± 1.85 c | 30.0 ± 1.76 cde | 60 c |
| (A) | In Vivo | Duvira Cultivar | ||||
| Acclimatization | Treatments | Length (cm) | Dry Weight (g) | |||
| Shoot | Root | Shoot | Root | Total Biomass | ||
| R. leucaenae | 3.8 ± 0.37 g | 2.0 ± 0.40 fg | 0.3 ± 0.03 c | 0.08 ± 0.10 c | 0.3 ± 0.10 c | |
| Ab-V6 | 13.7 ± 0.39 c | 14.5 ± 0.32 c | 1.9 ± 0.14 a | 1.2 ± 0.08 a | 3.1 ± 0.15 a | |
| R. tropici | 16.8 ± 0.41 ab | 13.7 ± 0.26 bc | 1.2 ± 0.20 b | 1.0 ± 0.26 a | 2.2 ± 0.21 b | |
| S. fredii | 14.7 ± 0.28 bc | 12.4 ± 0.35 c | 1.3 ± 0.24 b | 1.2 ± 0.11 a | 2.5 ± 0.11 ab | |
| B. japonicum | 9.5 ± 0.51 c | 8.8 ± 0.22 de | 0.4 ± 0.07 c | 0.3 ± 0.08 bc | 0.7 ± 0.20 c | |
| Ab-V5 | 17.6 ± 0.38 a | 17.0 ± 0.40 a | 1.3 ± 0.26 b | 0.8 ± 0.21 ab | 2.1 ± 0.13 b | |
| B. subtilis | 8.4 ± 0.38 ef | 7.0 ± 0.19 ef | 0.3 ± 0.03 c | 0.2 ± 0.15 c | 0.5 ± 0.02 c | |
| B. megaterium | 8.5 ± 0.39 ef | 8.3 ± 0.26 de | 0.4 ± 0.03 c | 0.2 ± 0.13 c | 0.6 ± 0.17 c | |
| Control | 11.7 ± 0.46 d | 10.3 ± 0.38 d | 0.5 ± 0.02 c | 0.3 ± 0.15 c | 0.8 ± 0.19 c | |
| (B) | Length (cm) | Dry Weight (g) | ||||
| Re-inoculation | Treatments | Shoot | Root | Shoot | Root | Total Biomass |
| R. leucaenae | 4.0 ± 0.05 f | 2.2 ± 0.03 ef | 0.4 ± 0.20 c | 0.1 ± 0.10 c | 0.5 ± 0.03 c | |
| Ab-V6 | 15.2 ± 0.16 b | 15.1 ± 0.05 b | 2.1 ± 0.16 a | 2.0 ± 0.26 a | 4.1 ± 0.11 a | |
| R. tropici | 17.7 ± 0.20 a | 15.0 ± 0.02 b | 1.5 ± 0.33 b | 1.1 ± 0.37 b | 2.6 ± 0.05 b | |
| S. fredii | 15.3 ± 0.11 b | 13.6 ± 0.11 bc | 1.2 ± 0.11 b | 1.0 ± 0.21 b | 2.2 ± 0.05 b | |
| B. japonicum | 10.2 ± 0.31 cd | 8.2 ± 0.25 d | 0.3 ± 0.10 c | 0.2 ± 0.27 c | 0.5 ± 0.14 c | |
| Ab-V5 | 19.0 ± 0.35 a | 18.3 ± 0.21 a | 2.1 ± 0.14 a | 1.1 ± 0.15 b | 3.2 ± 0.15 ab | |
| B. subtilis | 10.0 ± 0.12 cd | 8.1 ± 0.22 d | 0.5± 0.25 c | 0.3 ± 0.12 c | 0.8 ± 0.22 c | |
| B. megaterium | 7.1 ± 0.10 e | 8.1 ± 0.14 d | 0.3 ± 0.17 c | 0.2 ± 0.16 c | 0.5 ± 0.18 c | |
| Control | 11.1 ± 0.22 c | 9.8 ± 0.26 d | 0.4 ± 0.19 c | 0.3 ± 0.10 c | 0.7 ± 0.20 c | |
| (A) | In Vivo | Ágata Cultivar | ||||
| Acclimatization | Treatments | Length (cm) | Dry Weight (g) | |||
| Shoot | Root | Shoot | Root | Total Biomass | ||
| R. leucaenae | 4.0 ± 0.24 e | 2.1 ± 0.31 c | 0.1 ± 0.03 e | 0.1 ± 0.03 d | 0.2 ± 0.15 ed | |
| Ab-V6 | 7.0 ± 0.25 d | 5.0 ± 0.22 cd | 0.4 ± 0.12 ce | 0.2 ± 0.03 cd | 0.6 ± 0.05 cd | |
| R. tropici | 14.4 ± 0.30 b | 5.7 ± 0.16 bc | 1.6 ± 0.20 b | 0.9 ± 0.05 b | 2.5 ± 0.22 b | |
| S. fredii | 6.9 ± 0.38 d | 3.2± 0.30 e | 0.2 ± 0.08 de | 0.1 ± 0.05 d | 0.3 ± 0.04 de | |
| B. japonicum | 15.3 ± 0.49 b | 7.6 ± 0.15 a | 1.3 ± 0.10 b | 0.9 ± 0.16 b | 2.2 ± 0.18 b | |
| Ab-V5 | 17.0 ± 0.27 a | 8.2 ± 0.15 a | 2.1 ± 0.14 a | 2.0 ± 0.29 a | 4.1 ± 0.26 a | |
| B. subtilis | 12.3 ± 0.35 c | 5.8 ± 0.20 bc | 0.6 ± 0.11 cd | 0.2 ± 0.11 cd | 0.8 ± 0.10 cd | |
| B. megaterium | 14.6 ± 0.39 b | 7.2 ± 0.33 ab | 1.2 ± 0.20 b | 1.0 ± 0.17 b | 2.2 ± 0.08 b | |
| Control | 12.5 ± 0.36 c | 5.1 ± 0.30 cd | 0.7 ± 0.11 c | 0.3 ± 0.08 c | 1.0 ± 0.11 c | |
| (B) | Length (cm) | Dry Weight (g) | ||||
| Re-inoculation | Treatments | Shoot | Root | Shoot | Root | Total Biomass |
| R. leucaenae | 3.8 ± 0.16 e | 3.1± 0.21 de | 0.17± 0.05 e | 0.1 ± 0.02 de | 0.2 ± 0.06 de | |
| Ab-V6 | 8.0 ± 0.11 d | 5.3 ± 0.24 c | 0.5 ± 0.01 c | 0.3 ± 0.08 cd | 0.8 ± 0.10 cd | |
| R. tropici | 16.0 ± 0.20 b | 6.4± 0.24 c | 1.2 ± 0.07 b | 0.8 ± 0.05 b | 2.0 ± 0.15 b | |
| S. fredii | 5.1 ± 0.04 e | 4.0 ± 0.31 cd | 0.3± 0.11 d | 0.2 ± 0.10 de | 0.5 ± 0.02 de | |
| B. japonicum | 14.8 ± 0.21 b | 6.9 ± 0.31 b | 1.0 ± 0.14 b | 0.7 ± 0.12 b | 1.7 ± 0.21 b | |
| Ab-V5 | 19.1 ± 0.18 a | 10.3± 0.27 a | 2.4 ± 0.20 a | 2.6± 0.15 a | 5.0 ± 0.26 a | |
| B. subtilis | 14.3 ± 0.11 c | 8.0 ± 0.33 ab | 1.0± 0.08 b | 0.7 ± 0.04 b | 1.7 ± 0.11 b | |
| B. megaterium | 15.1 ± 0.20 b | 7.8 ± 0.30 ab | 1.1 ± 0.10 b | 0.8 ± 0.10 b | 1.9 ± 0.11 b | |
| Control | 11.7 ± 0.13 cd | 5.2 ± 0.35 c | 0.5 ± 0.12 c | 0.4± 0.08 cd | 0.9 ± 0.08 cd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Molina, Y.; Dória, J.; Gómez Sepúlveda, A.M.; Carvalho, L.Q.; Pasqual, M.; Jesus, E.d.C. Biotization with Plant Growth-Promoting Bacteria Benefits the Survival and Production of Potato (Solanum tuberosum L.) In Vitro and In Vivo. Horticulturae 2025, 11, 393. https://doi.org/10.3390/horticulturae11040393
Castro Molina Y, Dória J, Gómez Sepúlveda AM, Carvalho LQ, Pasqual M, Jesus EdC. Biotization with Plant Growth-Promoting Bacteria Benefits the Survival and Production of Potato (Solanum tuberosum L.) In Vitro and In Vivo. Horticulturae. 2025; 11(4):393. https://doi.org/10.3390/horticulturae11040393
Chicago/Turabian StyleCastro Molina, Yulimar, Joyce Dória, Ana Milena Gómez Sepúlveda, Luna Queiroz Carvalho, Moacir Pasqual, and Ederson da Conceição Jesus. 2025. "Biotization with Plant Growth-Promoting Bacteria Benefits the Survival and Production of Potato (Solanum tuberosum L.) In Vitro and In Vivo" Horticulturae 11, no. 4: 393. https://doi.org/10.3390/horticulturae11040393
APA StyleCastro Molina, Y., Dória, J., Gómez Sepúlveda, A. M., Carvalho, L. Q., Pasqual, M., & Jesus, E. d. C. (2025). Biotization with Plant Growth-Promoting Bacteria Benefits the Survival and Production of Potato (Solanum tuberosum L.) In Vitro and In Vivo. Horticulturae, 11(4), 393. https://doi.org/10.3390/horticulturae11040393







