Abstract
Plant architecture includes traits such as plant height, stem diameter, and branching pattern, which have significant impacts on yield and fruit quality. Polyploidization can bring changes in plant architectural traits in different crops along with other agronomic and biochemical attributes; however, the specific physiological and biochemical mechanisms are still unclear. In this study, we utilized five watermelon lines: ‘91E7’, ‘Zhengzhou No. 3’, ‘Fanzu No. 1’, ‘Shenlong’, and ‘Houlv’, along with their corresponding autopolyploid derivatives (diploid, autotriploid, and autotetraploid) to compare plant architecture differences in different polyploidy watermelon plants. The results showed that the growth habits of diploid, triploid, and tetraploid watermelon plants were noticeably different. Triploid and tetraploid watermelon plants had greater stem diameters and larger leaf sizes. The leaf angle was also larger in polyploid watermelons than in their diploid ancestor lines. Although vine length was significantly higher in diploid watermelon, there was no significant difference in node number, indicating that the short vine length was due to the short internodal length. The major differences between diploid and polyploid watermelon plants were found in the branching pattern, as diploid watermelon lines have more branching compared to their polyploid sister lines. Furthermore, we examined the phytohormone content of diploid, triploid, and tetraploid ‘Fanzu No. 1’. The reasons for the selection of this material are its robust growth and profuse branching habit, which cause visible differences among the ploidy levels. Hormone analysis showed distinct variations in abscisic acid in the nodal and stem regions, gibberellin in the auxiliary bud regions, and brassinosteroids in the apical meristematic regions. The correlation coefficient also strongly correlated these hormones with architecture-related traits. Our findings indicated that gibberellin, ABA, and brassinosteroids might be associated with variations in plant architectural traits like branching, vine length, internodal length, stem thickness, and leaf angle among different ploidy levels of watermelon. The exogenous application of GA3 showed a positive effect on branching, whereas ABA showed a negative effect on branching. The application of brassinosteroid at the apical meristem demonstrated its effect on leaf angle, leaf size, and internodal length. The results of this study can provide a theoretical reference and valuable insights into the link between plant architecture and ploidy levels.
1. Introduction
Watermelon (Citrullus lanatus (Thumb) Matsum and Nakai) is a popular and widely cultivated fruit known for its sweet, juicy flesh. It is a member of the Cucurbitaceae family. Watermelon plants are typically diploid, meaning they have two sets of chromosomes (2n). Polyploid watermelons, specifically tetraploid watermelons, are created by the chromosome doubling of diploid watermelon through colchicine treatment. Triploid watermelons are sterile and produced by crossing a diploid (2n) watermelon with a tetraploid (4n) watermelon [1]. Triploid watermelon is distinguished by its odd number of chromosome sets and exhibits features such as seedlessness, larger fruit size, and enhanced sweetness, catering to consumer preferences and market demands. However, polyploidization not only causes seedlessness but also brings other physiological and morphological changes to plants, including the plant architecture.
Plant architecture, the arrangement and organization of plant organs such as leaves, stems, flowers, and roots, plays a crucial role in determining a plant’s overall growth, development, and interaction with its environment. These traits collectively determine how efficiently a plant captures sunlight, allocates resources, and interacts with its environment. Architectural traits also impact crop yield, quality, and resistance to biotic and abiotic stresses. Understanding plant architecture is crucial for designing efficient cultivation practices for improving crop yield, resource utilization, and overall plant performance. The arrangement of leaves on stems and their orientation and distribution within the canopy determine the plant’s efficiency in harvesting sunlight for photosynthesis [2,3]. This intricate interplay between light interception and the allocation of resources, including water, nutrients, and carbohydrates, is crucial for determining the overall growth and biomass accumulation in plants. The arrangement of stems and roots plays a pivotal role in determining how plants access and distribute these resources. Root architecture, in particular, is a subject of extensive research for its impact on nutrient uptake and water absorption [4]. Plant architecture is also a key player in shaping a plant’s response to environmental stresses, both biotic and abiotic. The spatial arrangement of leaves and stems can influence a plant’s susceptibility to diseases and pests. Additionally, certain architectural traits contribute to a plant’s ability to withstand abiotic stresses such as drought or high temperatures. An understanding of these traits aids in the development of stress-tolerant cultivars, contributing to sustainable and resilient agriculture [5]. Plant architecture also determines the spatial arrangement of plants in the field, plant density, and different management practices, which ultimately affect crop yield and production cost. Developing breeding programs designed to produce ideal plant architecture with desirable traits that generate economic benefits has become a crucial integrated goal of plant breeding [6]. Plant architecture has an important effect on watermelon production. Plants with long vines and dense branching need more space, resulting in fewer plants per unit area, and subsequently, a low yield of fruit. Dense plant growth also hinders the mechanization of crop production. The removal of lateral branches is an important management practice for watermelon production, which is completely dependent on manual labor. The development of low-branched varieties can reduce this labor cost. Hormonal regulation plays a crucial role in shaping the architecture of plants [7]. Plants have the ability to respond and adapt to their environment, and hormones serve as chemical messengers that coordinate various physiological processes, including growth, development, and responses to external stimuli [8]. Plant hormones, or growth regulators, contribute to the development of plant architecture. Hormones like auxins (IAA), gibberellins (GA3), cytokinins (Zr), abscisic acid (ABA), brassinosteroids (Br), etc., influence cell elongation, division, and differentiation, thus affecting how plants grow and branch. Hormones do not act in isolation; rather, they engage in crosstalk, forming a network of regulatory pathways [9].
Polyploidy, a condition where an organism possesses more than two sets of chromosomes, is an intriguing phenomenon that has captured the attention of researchers for decades. In plants, polyploidy can arise through natural processes or be induced through breeding techniques [10]. Polyploid plants often exhibit altered traits, such as larger cell sizes, increased biomass, and changed morphology, which can have significant implications for agricultural production [11,12,13,14,15]. The modifications in plant architecture induced by polyploidy have significant implications for agricultural practices. Larger fruits, improved biomass production, and altered branching patterns can enhance crop yield and profitability [16]. Furthermore, polyploid plants may exhibit increased tolerance to environmental stresses, making them valuable resources for sustainable agriculture [17,18,19,20]. In watermelon, an increased ploidy level was reported to have a positive effect on quality-related traits by Davis et al. [21]. Polyploid watermelon also shows increased tolerance to abiotic stress like salinity and flooding [17,18].
As polyploidization is known to induce diversity in plant species, the study of polyploids alongside their diploid counterparts is a valuable approach to understanding the underlying mechanisms behind those specific traits of watermelon. Many studies on watermelon plant architectural traits like leaf morphology [22], root morphology [23], dwarfism [24,25], and branching [26] were conducted by different researchers, but all the works were based on diploid watermelon. The utilization of polyploidy in watermelon has a long history, primarily focused on generating seedless fruits. Some researchers worked on polyploid watermelon, but their research was predominantly focused on flower and fruit anatomy in diploid and tetraploid watermelon cultivars, as explored in studies by Khan et al. [27], Zhang et al. [28], Jaskani et al. [29], and Feng et al. [30]. However, the broader benefits of polyploidization, especially concerning plant architecture in watermelon, have been largely overlooked. Despite its significant impact on yield, fruit quality, and profitability, this aspect has not received due attention. Therefore, our study aims to fill this gap by documenting and understanding these changes, with a particular focus on hormonal regulation, which plays a key role in plant growth.
2. Materials and Methods
2.1. Plant Materials
Five watermelon lines ‘91E7’, ‘Zhengzhou No. 3’, ‘Fanzu No. 1’, ‘Shenlong’, and ‘Houlv’ and their autopolyploidy derivatives of the same genotype (diploid, autotriploid, and autotetraploid) were collected from the polyploidy watermelon breeding team, Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences, Zhengzhou, Henan, China. Autotetraploid watermelon was induced artificially by 0.1% colchicine from homozygous diploid watermelon; 0.1% (w/v) aqueous solution of colchicine was applied to the young watermelon seedling in the filed condition following Zhu et al. (2018) [18]. The success of tetraploid mutagenesis was confirmed through physiological phenotype and chromosome observation following Zhu et al. (2018) [18] (Figure S1). The plants were then self-crossed for 7 generations. They were then crossbred with their diploid counterpart to generate autotriploid watermelon.
2.2. Experimental Location and Climatic Condition
The field experiment was conducted at the Xinxiang experimental base of the Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Science, Henan, China. The geographical location is 35°7′40″ N and 113°45′57″ E and the altitude is 80 m above sea level. Characterized by a temperate continental monsoon climate, the area experiences an average annual temperature of 15.1 °C and receives approximately 568.3 mm of rainfall each year (Source: China Meteorological Administration).
The materials were grown under a polytunnel for the evaluation of the structural traits of the lines. The trial was performed in two consecutive spring and autumn seasons. The seedlings were grown in a seed bed and transplanted to the main field at four to six leaf stages 15 days after sowing. Plants were grown in three replications following a randomized complete block design (RCBD), where each replication contained 30 plants. A plant spacing of 50 cm and a row spacing of 2.5 m were maintained throughout the experiment. Without clipping off side branches, the other cultivation management measures were the same as the normal growth of watermelon management.
2.3. Phenotypic Data Collection
Data on different morphological traits were collected. Data were measured from at least five plants per accession and averages were taken. Vine length, number of primary branches, and number of nodes were counted at 35 days after transplanting (DAT) at the mid-growth stage and 55 DAT after the plant reached its maximum growth stage. Internodal length and stem thickness, leaf area, leaf angle, petiole length, and petiole thickness were measured at 35 DAT. Measurements were conducted on the 8th to 10th nodal sections from the top, as leaves and stems at these positions achieve their maximum size while remaining young and green. A measuring tape was used to measure the vine length, whereas Internodal length, stem thickness, petiole length, and petiole thickness were measured by Vernier calipers. A digital camera was used to photograph the leaf, and the leaf area and leaf angle were measured by the computer software Digimizer Version 6.3.0 using the digital image. All the measurements were performed following the International system of unit (SI).
2.4. Hormone Content Measurement
‘Fanzu No. 1’ was used for hormone quantification. The reason for the selection of this material is its profuse growth and branching, which show visible differences among the ploidy levels. In addition, the diploid of ‘Fanzu No. 1’ is the core parent of watermelon breeding in China. The tetraploid of ‘Fanzu No. 1’ is stable, easy to set fruit, and has a large number of seeds (which is important because most tetraploid watermelons are not easy to set fruit and have a small number of seeds). Apical meristem and nodal samples were collected at 15, 25, 35, and 55 DAT. Auxiliary buds were collected at 35 DAT. Three samples were collected for every stage in three replicates. Every sample contained plant tissue from three plants. All the materials were collected in liquid nitrogen and preserved in a refrigerator at −80 °C. Five important phytohormones, viz. IAA, Zr, GA, ABA, and Br, were quantified using the Enzyme Linked Immunosorbent Assay (ELISA) method (College of Agriculture and Biotechnology, China Agricultural University) following Mo et al. [31]. Every analysis was performed three times to obtain three technical replicates.
2.5. Exogenous Application of Plant Hormone
Three ploidy levels of ‘Fanzu No. 1’ (diploid, triploid, and tetraploid) were used for the exogenous hormone application. Three important plant growth regulatory hormones, GA3, ABA, and Br, were used in this experiment. For GA3, three hormone concentrations—100 µM/L, 200 µM/L, and 300 µM/L—were used. For ABA, the hormone concentrations were 50 µM/L, 100 µM/L, and 150 µM/L, and for Br, they were 1 µM/L, 10 µM/L, and 100 µM/L. In each case, a control treatment was also applied with double-distilled water. The experiment was plotted in RCBD with three replications, and each replication consisted of 30 plants. ABA and GA3 were applied to the nodal region with high care, avoiding the apical meristem. Br was sprayed only on the apical meristem. The hormone application started at 15 DAT when the plants started their vegetative growth. The hormone application was performed every alternate day, and it was continued up to 35 DAT. Data on the hormone application were taken at 35 DAT.
2.6. Histological Analysis
For the histology of stem tissue, 1 cm samples from the 8th to 10th nodal region were collected. Samples were preserved in FAA (4% paraformaldehyde) solution. Paraffin-embedded tissues were sliced into 10 μm thick sections with a sliding microtome. The tissue was stained with toluidine blue and observed with a NIKON ECLIPSE E 100 upright optical microscope, Nikon, Tokyo, Japan. Images were captured by the NIKON DS-U3, Nikon, Tokyo, Japan and cell measurement was performed using the computer software Digimizer Version 6.3.0.
2.7. Statistical Analysis
Data from two cropping seasons were combined for each accession. Analysis of variance (ANOVA) was performed using the Statistical Tool for Agricultural Research (STAR), Version: 2.0.1 developed by the International Rice Research Institute (IRRI). Excel for Microsoft 365 package version 16.0.14026.20246 was used to draw the graphs. Tukey’s honestly significant difference (HSD) test was performed to compare the means sets at a 5% probability level. Pearson’s correlation was performed using OriginPro, Version 2022. OriginLab Corporation, Northampton, MA, USA, whereas principal component analysis (PCA) was performed using GraphPad Prism version 9.0.0 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com Computer software.
3. Results
3.1. Comparison of Plant Architectural Traits among Diploid, Triploid, and Tetraploid Watermelon Plants
3.1.1. Ploidy Induced Variations in Branching
Branching is a fundamental aspect of plant architecture that plays a crucial role in determining the overall structure, growth, and adaptability of plants. In our study, we found significant impacts of both genotype and ploidy level on branch number (Figure 1A). Among the genotypes, ‘Fanzu No. 1’ had the highest branch numbers across all ploidy levels, particularly at Diploid (10.43). ‘Houlv’ consistently showed the lowest branch numbers among the genotypes (3.88, 2.45, and 1.9 branches in diploid, triploid, and tetraploid, respectively).
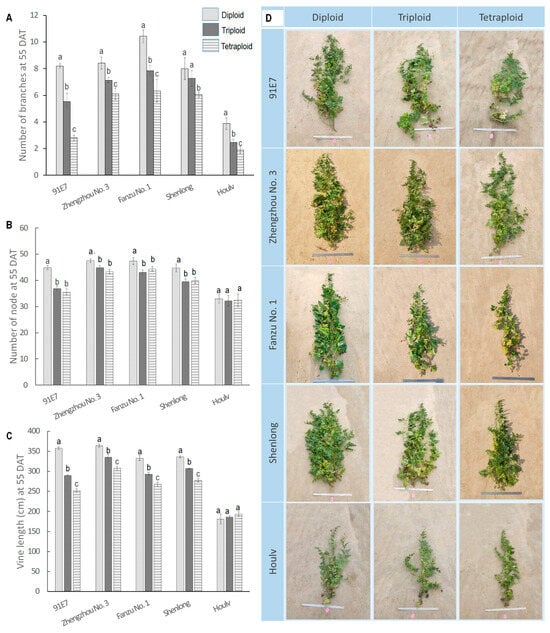
Figure 1.
Branch number, number of nodes, and vine length of five watermelon cultivars with their three ploidy levels. (A) Number of primary branches among different ploidy levels and different genotypes. (B). Number of nodes among different ploidy levels and different genotypes. (C). Vine length among different ploidy levels and different genotypes. (D). Images representing the morphological variation across the diploid, triploid, and tetraploid plants in five watermelon genotypes. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level according to Tukey’s test.
Across genotypes, there was a general trend of decreasing branch numbers from Diploid to Tetraploid. This suggested a potential inverse relationship between ploidy level and branch development. Diploid, triploid, and tetraploid of ‘91E7’ showed the most significant difference in the number of branches, with diploid having 1.44 times more branches than triploid and 2.9 times more branches than tetraploid (8.20, 5.52, and 2.80 branches in diploid, triploid, and tetraploid, respectively). Although the difference between the diploid and triploid of ‘Shenlong’ was not significant, the average number of branches in the diploid was still greater than that of the triploid.
3.1.2. Ploidy Induced Variations in Node Number
The number of nodes in a plant plays a significant role in shaping its architecture. Plants with a higher number of nodes tend to have more longitudinal and lateral growth. In our study, diploid usually had a higher number of nodes compared to triploid and tetraploid (Figure 1B). However, the variation pattern was not similar in all genotypes and was not statistically significant in all varieties. Node number in ‘91E7’ was statistically higher in diploid (44.9 nodes per plant), followed by triploid and tetraploid (36.9 nodes per plant in triploid and 35.55 nodes per plant in tetraploid). In ‘Zhengzhou No. 1’, diploid had 47.44 nodes per plant, which was statistically similar to triploid (44.84 nodes per plant) and tetraploid (43.31 nodes per plant). In ‘Fanzu No. 1’, ‘Shenlong’, and ‘Houlv’, there was also non-significant variation among the ploidy. Node numbers ranged from 43.12 to 47.45 in ‘Fanzu No. 1’, 39.98 to 44.74 in ‘Shenlong’, and 32.15 to 32.94 in ‘Houlv’ (Figure 1).
3.1.3. Ploidy Induced Variations in Vine Length
Vine length is an integral component of plant architecture that significantly impacts the adaptation, growth, and ecological strategies of climbing plants. We observed significant variation in vine length for both genotype and ploidy levels (Figure 1C,D). Diploid ‘Zhengzhou No. 3’ had the longest vine (363.5 cm), which was followed by ‘91E7’, ‘Fanzu No. 1’, and ‘Shenlong’. ‘Houlv’ had the shortest vine length at all ploidy levels (180.94 cm, 185.69 cm, and 193.47 cm in diploid, triploid, and tetraploid, respectively). In general, there is a trend of decreasing vine lengths from diploid to tetraploid, suggesting a potential inverse correlation between ploidy levels and vine development. The maximum reduction in vine length for the ploidy effect was obtained from ‘91E7’, which was 1.23 times longer than triploid and 1.42 times longer than tetraploid. All other genotypes showed similar trends of variation for the ploidy effect except ‘Houlv’. Notably, ‘Houlv’ exhibits a unique response, displaying the shortest diploid vines but an increase in length in the triploid and tetraploid state, though the difference was not significant.
3.1.4. Ploidy-Induced Variations in Stem Thickness and Internodal Length
Stem thickness showed significant variations among different ploidy levels of watermelon (Figure 2A). In the diploid state, ‘Zhengzhou No. 3’ exhibited the thickest stems (4.54 mm), closely followed by ‘91E7, ‘Fanzu No. 1’, and ‘Shenlong’, while ‘Houlv’ had the thinnest stems at 3.988 cm. Moving to triploid, Shenglong had the thickest stem (5.66 mm), followed by ‘91E7’, ‘Zhengzhou No. 3’, ‘Fanzu No. 1’, and ‘Houlv’. In the tetraploid state, Zhenghou No. 3 had the highest value (5.67 mm), which was closely followed by ‘91E7’. In general, stem thickness increased with increased ploidy level; however, the trend was not similar. The variation was significant for diploid, triploid, and tetraploid in ‘Zhengzhou No. 3’ and ‘Houlv’. Conversely, for ‘91E7’, ‘Fanzu No. 1’, and ‘Shenlong’, diploid had significantly thinner stems than triploid and tetraploid, whereas variations were non-significant between the latter two. Internodal length showed a different result, where the diploid had the longest internodal length followed by the triploid, while the tetraploid had the shortest internodal length (Figure 2B). ‘Zhengzhou No. 3’ exhibited the longest internodal lengths in the diploid state (8.23 cm), whereas ‘Houlv’ consistently displayed shorter internodal lengths across all ploidy levels (5.42 cm, 4.46 cm, and 4.19 cm in diploid, triploid, and tetraploid, respectively). Though internodal length gradually decreased with increased ploidy level, the variation was not the same in all the genotypes. ‘Zhengzhou No. 3’ and ‘Fanzu No. 1’ showed statistically significant differences across the ploidy level. However, in ‘91E7’ and ‘Shenlong’, the variation was nonsignificant between diploid and triploid, though tetraploid had a significantly shorter internode than the previous two. In ‘Houlv’, diploid had significantly longer internodal length than triploid and tetraploid, whereas the latter two had non-significant differences between them.
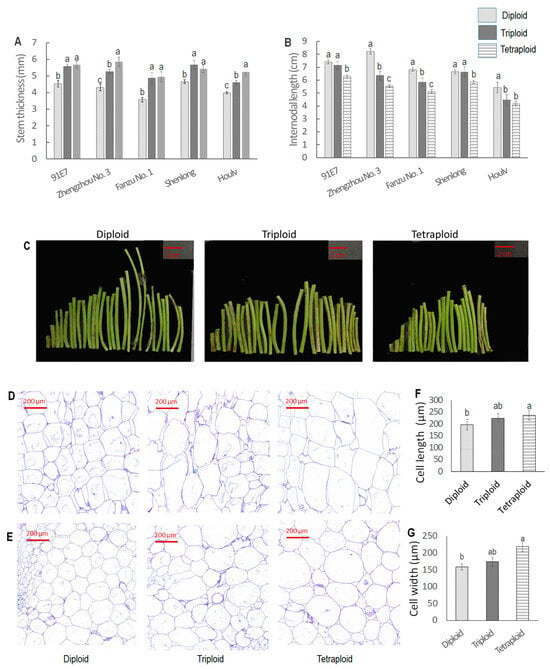
Figure 2.
Variation in stem morphology and anatomy among different ploidy levels of watermelon. (A). Stem thickness of five watermelon genotypes with their three ploidy levels; (B). Internodal length of five watermelons with their three ploidy levels; (C). Picture of intrenodal region of ‘Fanzu No. 1’; (D). Cross-section of stem of Fanzu No. 1; (E). Longitudinal section of stem of Fanzu No. 1; (F,G). Cell length and cell width of the stem. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level according to Tukey’s test.
We also performed a histological observation of stem segments to analyze the ploidy effect on cell size (Figure 2D–G). From the visual images of histology, we could observe a compact cell arrangement with smaller cells in the diploid. Cell size increased with increased ploidy level. Meanwhile, the number of cells per unit area (cell density) was significantly higher in diploid than polyploid.
3.1.5. Ploidy-Induced Variations in Leaf Relate Traits
There was significant variation in leaf size, leaf angle, petiole length, and petiole thickness for both genotype and ploidy level (Figure 3). In the diploid state, ‘Shenglong’ had the largest leaf (146.02 cm2), which was closely followed by ‘91E7’ (125.72 cm2) and ‘Zhengzhou No. 3’ (102.98 cm2) (Figure 3A). ‘Fanzu No. 1’ had the smallest leaf (51.49 cm2), which was followed by ‘Houlv’ (55.23 cm2). The triploid also followed a similar trend for leaf size. In the case of tetraploid, ‘91E7’ had the largest leaf (226.03 cm2) which was followed by Shenglong (204.55 cm2), ‘Zhengzhou No. 3’ (134.35 cm2), ‘Houlv’ (132.77 cm2), and ‘Fanzu No. 1’ (80.98 cm2). In general, leaf size increased with increased ploidy level except in ‘Zhengzhou No. 3’ where the leaf size of diploid was significantly lower than triploid and tetraploid but the latter two had non-significant variation. The maximum changes in leaf size with ploidy level changes were found in ‘Houlv’, where the triploid was 1.84 times larger than the diploid, the tetraploid was 2.40 times larger than the diploid, and the tetraploid was 1.30 times larger than the triploid.
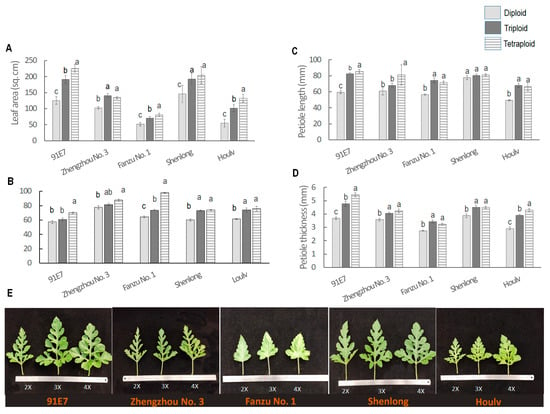
Figure 3.
Variation in leaf morphology among different ploidy levels of watermelon. (A–D) Leaf area, leaf angle, leaf length, and leaf thickness of different ploidy levels of watermelon. (E). Leaf picture of different genotypes with their three ploidy levels. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level according to Tukey’s test.
The leaf angle also showed interesting phenomena. The leaf type was comparatively more erect in diploid than the triploid and tetraploid (Figure 3B). Tetraploid and triploid had larger leaf angles. The maximum leaf angle was found in tetraploid ‘Fanzu No. 1’ (97.82° with the main stem). ‘Fanzu No. 1’ also showed significant variation in leaf angle across the ploidy, where the leaf angles of diploid were 64.15° and 74.76°. In ‘Shenlong’ and ‘Houlv’, the diploid had a significantly smaller leaf angle than the triploid and tetraploid, whereas the latter two had non-significant variation between them. In ‘91E7’, the tetraploid had a significantly larger leaf angle than the diploid and triploid, whereas the latter two had non-significant variation between them.
In the study, both petiole length and thickness were generally greater in tetraploid and triploid watermelons compared to diploids; however, the trend was not similar in all genotypes (Figure 3C,D). In ‘91E7’ and ‘Zhengzhou No. 3’, petiole length significantly increased in triploid and then tetraploid, whereas in ‘Fanzu No. 1’ and ‘Houlv’, petiole length of triploid and tetraploid was significantly higher than diploid, the latter two had non-significant variation. There was statistically non-significant variation among the ploidy in ‘Shenlong’, though the average data followed the trend diploid > Triploid > tetraploid. For petiole thickness, ‘91E7’ and ‘Houlv’ showed significant variation, where tetraploid had the largest thickness followed by triploid and diploid. However, in ‘Zhengzhou No. 3’, ‘Fanzu No. 1’, and ‘Shenlong’, triploid and tetraploid had significantly larger thickness values than the diploid, whereas the first two had statistically non-significant variation.
3.2. Quantification of Endogenous Hormones at Different Growth Stages in Different Tissue Parts
Hormone content was quantified at apical meristem and nodal regions at 15, 25, 35, and 55 DAT. Hormones were measured at the auxiliary bud at 35 DAT. Five major hormones, namely, IAA, Zr, GA3, ABA, and Br, were quantified.
3.2.1. Hormone Quantification at Apical Meristem
For the apical meristem, no hormone showed consistent and significant variation among different ploidy watermelons except Br (Figure 4). IAA content at 15 DAT was higher in diploid, whereas there was no statistical variation between triploid and tetraploid. At 25 DAT and 35 DAT, there was no significant variation in IAA content among the ploidy levels. However, at 55 DAT, Tetraploid had the highest IAA content followed by diploid and triploid.
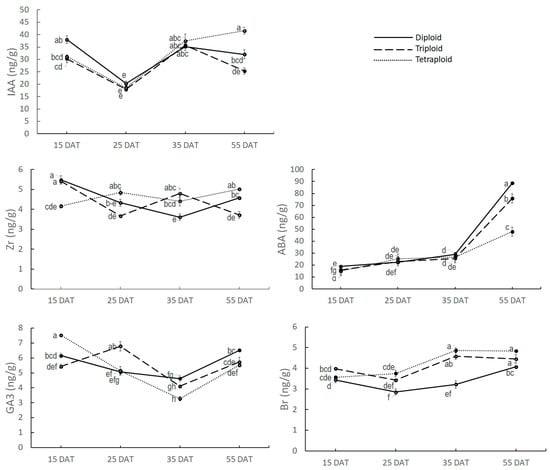
Figure 4.
Five major hormones’ content at apical meristem at different growth stages. Values represent the mean of three replicates ± SE. Values followed by same letters are not significantly different at the 5% probability level according to Tukey’s test.
There was significant variation in Zr content among the ploidy levels, but Zr content was not consistent across the growth phases. Zr content at 15 DAT was highest in diploid (5.48 ng/g), which gradually decreased at 25 DAT (4.33 ng/g and 35 DAT (3.61 ng/g) and finally increased at 55 DAT (4.57 ng/g). For triploid, Zr content was 5.41 ng/g at 15 DAT, then it was decreased to 3.67 ng/g at 25 DAT, then increased to 4.8 ng/g at 35 DAT, and finally decreased again to 3.72 ng/g at 55 DAT. For tetraploid, the initial Zr content at 15 DAT was 4.16 ng/g, which increased to 4.85 ng/g at 25 DAT, then decreased to 4.41 ng/g at 35 DAT, and finally increased again to 5.02 ng/g.
There was variation in GA3 content at AM among the ploidy levels, but the trend was not consistent across the growth stages. GA3 content at AM was 6.16 ng/g in diploid at 15 DAT, which was slightly reduced to 5.05 ng/g at 25 DAT and 4.62 ng/g at 35 DAT, and finally increased to 6.51 ng/g at 55 DAT. In triploid, GA3 content was 5.42 ng/g, which increased at 25 DAT to 6.78 ng/g, then decreased to 4.11 ng/g at 35 DAT, and finally, at 55DAT, it increased again to 5.73 ng/g. GA3 content in tetraploid was 7.53 ng/g at 15 DAT, going down to 5.14 ng/g at 25 DAT and 3.27 ng/g at 35 DAT and finally raised sharply to 5.52 ng/g at 55 DAT.
ABA content was low at 15 DAT in all ploidy levels (19.00 ng/g, 16.01 ng/g, and 15.00 ng/g in diploid, triploid, and tetraploid, respectively) and it increased gradually with the increase in plant age up to 35 DAT, though there was no significant variation among the ploidy levels. However, it showed a sharp increment at 55 DAT. ABA content at 55 DAT was significantly higher in diploid (88.65 ng/g), followed by triploid (75.78 ng/g) and tetraploid (47.95 ng/g), which is very logical because diploid plants gain earlier senescence than that of triploid and tetraploid.
Only Br showed a consistent and significant variation among the ploidy levels throughout the growth phase. Br content ranged from 2.84 ng/g to 4.4.87 ng/g where the lowest reading was found in diploid at 25 DAT and the highest reading was found in tetraploid at 35 DAT. Br was significantly higher at all the growth stages in tetraploid and triploid compared to the diploid. Though the average Br content was higher in tetraploid than diploid, the difference was not statistically significant.
From the findings mentioned above, it can be concluded that Br content at AM might have some effect on controlling plant architectural traits.
3.2.2. Hormone Quantification at Nodal Tissue
IAA, Zr, GA3, ABA, and Br contents at nodal regions were also measured (Figure 5). IAA content ranged from 28.05 ng/g at 35 DAT in diploid to 50.42 ng/g at 55 DAT in diploid. However, there was minor variation in IAA content up to 35 DAT across the growth stage and across the ploidy level. At 55 DAT, variation for IAA content was found among the ploidy levels, where diploid had a sharp increase in IAA content (50.42 ng/g), triploid had a moderate level at 37.37 ng/g, and tetraploid had the least amount of IAA content (30.42 ng/g).
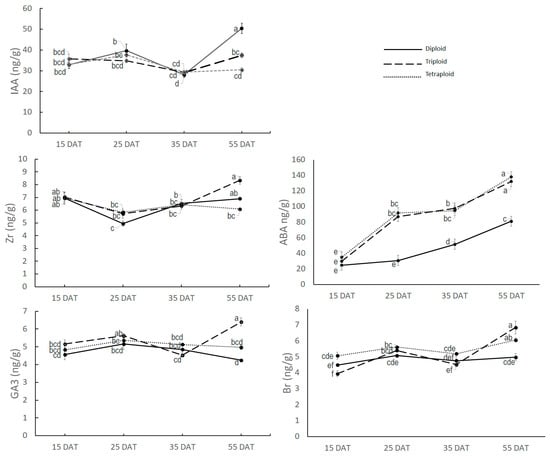
Figure 5.
Five major hormones’ content in nodal region at different growth stages. Values represent the mean of three replicates ± SE. Values followed by same letters are not significantly different at the 5% probability level according to Tukey’s test.
Zr content also showed non-significant variation up to 35 DAT but significant variation at 55 DAT among the ploidy levels. At 55 DAT, the maximum Zr was found in triploid (8.33 ng/g), followed by diploid (6.90 ng/g) and tetraploid (6.08 ng/g).
GA3 and Br also showed a similar pattern where significant variation among the ploidy levels was found only at 55 DAT. GA3 and Br content at 55 DAT was maximum in triploid (6.39 ng/g GA3 and 6.84 ng/g Br), followed by tetraploid (4.96 ng/g GA3 and 6.38 ng/g Br) and diploid (4.25 ng/g GA3 and 5.54 ng/g Br).
The ABA content at the nodal region varied from 24.85 ng/g in diploid at 15 DAT to 140.71 ng/g in tetraploid at 55 DAT. Only ABA showed a significantly higher level of the accumulation rate in the nodal region in tetraploid and triploid. Diploid had comparatively lower levels of accumulation of the ABA hormone in the nodal region. There was no significant variation for ABA content in any growth stage between triploid and tetraploid though the average value was slightly higher in tetraploid than triploid. It can also be noted here that ABA content not only increased with ploidy level increase but also increased with plant age at all ploidy levels.
From the above findings, it is evident that no hormone except ABA varied among the ploidy levels in the active plant growth stage. Therefore, it can be summarized that ABA in the nodal region might have an effect on plant architecture-related traits.
3.2.3. Hormone Quantification at Auxiliary Bud
Hormone quantification was also performed for auxiliary buds (Figure 6). Each auxiliary bud was collected from 35-day-old plants and combined. Zr, IAA, Br, and ABA did not show any significant variation among the ploidy levels. However, GA3 showed high variation among the ploidies. The maximum GA3 content was found in the diploid, which contained 5.27 ng/g GA3, whereas the triploid contained 2.46 ng/g and the tetraploid contained 1.84 ng/g GA3 at the auxiliary bud.
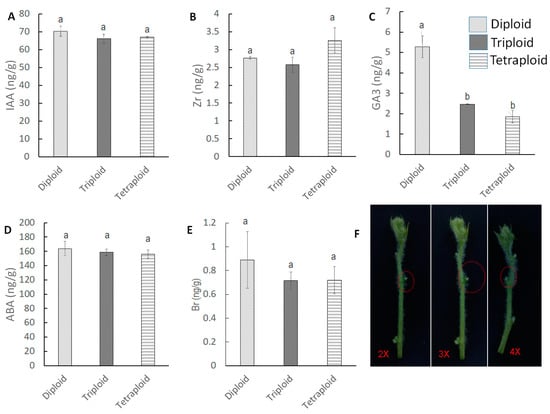
Figure 6.
Five major hormone content at auxiliary bud at 35 DAT. (A–E) IAA, Zr, GA3, ABA and Br content at auxiliary bud. (F) Red circle showing the auxiliary bud growth at diploid, triploid and tetraploid. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level by Tukey’s test.
3.3. Correlation Study between the Morphological and Hormonal Traits
A correlation study was performed with the hormones quantified and the plant architectural traits. Here, hormone concentrations at 35 days and different growth parameters at 35 DAT were compared. First, we compared correlations between the morphological traits (Figure 7A). Vine length was positively correlated with node number (r = 0.852) but negatively correlated with leaf area (r = −0.760). Node number was positively correlated with branch number (r = 0.827) and internodal length (r = 0.737) and negatively correlated with leaf area (r = −0.887) and petiole length (r = −0.732). The number of branches was negatively correlated with leaf area (r = −0.795) and petiole length (r = −0.726). Stem thickness was positively correlated with petiole length (r = 0.779) and petiole thickness (r = 0.729) and negatively correlated with internodal length (r = −0. 740). Leaf area was positively correlated with leaf angle (r = 0.872) and negatively correlated with internodal length (r = −0.794). Internodal length was negatively correlated with leaf angle (r = −0.897) and petiole length (r = −0.907).
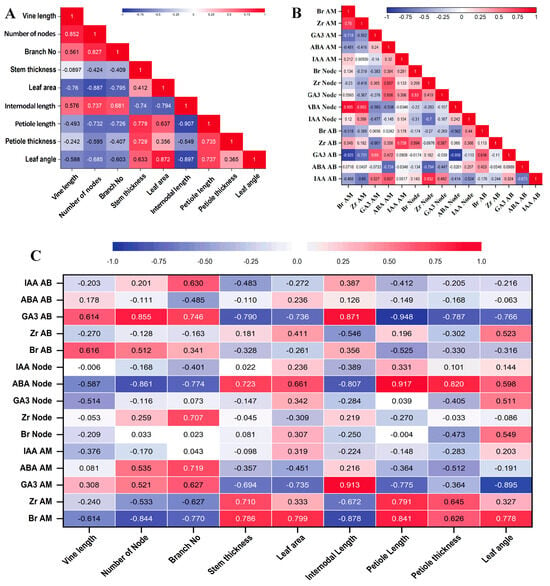
Figure 7.
Heat map showing correlations of different phytohormones with different plant tissues and morphological traits. (A). Correlation among the morphological traits. (B). Correlation among the phytohormones. (C). Correlation between morphological traits and phytohormone content of different tissue parts. The color represents the correlation coefficient (r). Positive correlations are highlighted in red and negative correlations are in blue.
We studied the correlation among the phytohormones (Figure 7B). It was clearly visible that Br at AM was positively correlated with Zr at AM (r = 0.760) and ABA at the node (r = 0.905), but negatively correlated with GA3 at AM (r = −0.718) and GA3 at AB (r = −0.925). Zr at AM was positively correlated with ABA at the node (r = 0.853) and negatively correlated with GA3 at AB (r = −0.731). ABA at AM was negatively correlated with ABA at AB (r = −0.734). IAA at AM was positively correlated with Zr at AB (r = 0.758). Br at the node was positively correlated with GA3 at the node (r = 0.830). Zr at the node was positively correlated with IAA at AB (r = 0.832) and negatively correlated with ABA at AB (r = −0.794). ABA at the node was significantly and negatively correlated with GA3 at AB (r = −0.956).
We compared the hormone levels with morphological traits (Figure 7C), and Br at AM was positively correlated with stem thickness (r = 0.786), leaf area (r = 0.799), petiole length (r = 0.841), and leaf angle (r = 0.778) but negatively correlated with the number of nodes (r = −0.844), branch number (r = −0.770), and internodal length (r = −0.878). Zr at AM was positively correlated with stem thickness (r = 0.710) and petiole length (r = 0.791). GA3 at AM was positively correlated with internodal length (r = 0.913) and negatively correlated with leaf area (r = −0.735), petiole length (r = −0.775), and leaf angle (r = −0.895). ABA at AM was positively correlated with branch number (r = 0.719). Zr at the node was positively correlated with the branch number (r = 0.707). ABA at the node was positively correlated with stem thickness (r = 0.723), petiole length (r = 0.917), and petiole thickness (r = 0.820) and negatively correlated with the number of nodes (r = −0.861), branch number (r = −0.774), and internodal length (r = −0.807). GA3 at AB was positively correlated with the number of nodes (r = 0.855), number of branches (r = 0.746), and internodal length (r = 0.871) and negatively correlated with stem thickness (r = −0.790), leaf area (r = −0.736), leaf angle (r = −0.766), petiole length (r = −0.948), and petiole thickness (r = −0.787).
3.4. Principal Component Analysis of Morphological and Hormonal Traits
In the principal component analysis, all the traits fall into eight principal components, where the first four components explained almost 87% of the variation, while the first two PCs could explain almost 67% of the variations (Figure 8A). It is also noted that all three ploidies were classified separately, with the triploid and tetraploid closer than the diploid (Figure 8B). Among the traits, Br at the node (0.660), ABA at the node (0.555), Br at the apical meristem (0.740), and Zr at the apical meristem (0.644) contributed positively to PC1. ABA at the node (0.801), Br at the apical meristem (0.552), and GA3 at the apical meristem (0.970) contributed positively to PC2 (Figure 8C). The results clearly indicated the importance of Br, Zr, and GA3 at the apical meristem, Br at the node, and ABA at the node for plant architectural traits in polyploid watermelon. As the first two PCs explained almost 67% variation, we plotted PC1 on the X-axis and PC2 on the Y-axis to formulate a score plot. In the score plot, three ploidy levels were placed in three different clusters. Then we created a biplot to see how our experimental traits are affected. In the biplot, high ABA at the node, IAA at the node, and Br at AM and low vine length and number of nodes were associated with triploid plants. Similarly, higher values of Br at the node, ABA at the node, and IAA at the node and leaf area and lower values of vine length, number of node, and branch number were associated with tetraploid. Lower values of ABA at the node, IAA at the node, and Br at AM and higher values of GA3 at auxiliary buds, vine length, number of nodes, and branch numbers were associated with diploid.
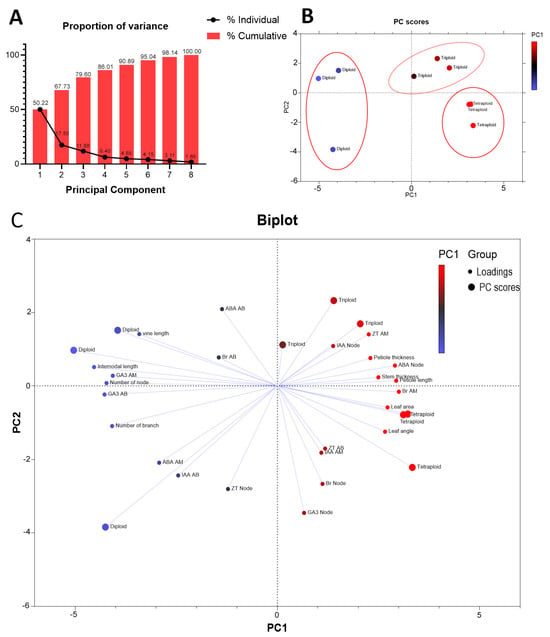
Figure 8.
Principal component analysis (PCA). (A). Proportion of variance among the principal components. (B). Score scatter plot of PC1 versus PC2. (C). Biplot of accessions based on the variance in morphological and biochemical traits.
3.5. Effect of Exogenous Application of GA3, ABA, and Br on Plant Architectural Traits
3.5.1. Effect of Exogenous Application of GA3 on Plant Architectural Traits
GA3 was applied to nodal regions in a way that it does not touch the apical tip of the plant. For branching, GA3 treatment showed significant variation in branch number for all three ploidy levels (diploid, triploid, and tetraploid) (Figure 9A). In all cases, the number of branches increases with the application of the hormone. However, in diploid plants, the increment rate slowed down with the increase in GA3 concentration. For example, it increased 29.09% at 100 µM GA3 and 48.72% at 200 µM GA3 and then decreased to 42.79 at 300 µM GA3. The number of branches gradually increased in triploid and tetraploid plants with increased GA3 concentrations (Figure 9B). The maximum increment in triploid plants was found to be 60.5% at 300 µM GA3, and it was 67.09% in tetraploid plants at 300 µM GA3. The other parameters like vine length, stem thickness, leaf area, and leaf angle were not significantly affected by GA3 treatment (Figure 9C–E).
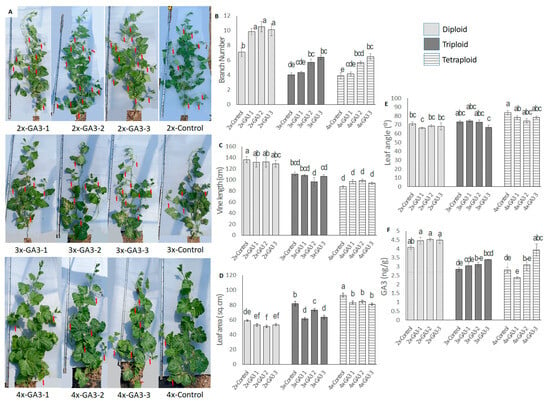
Figure 9.
Effect of exogenous GA3 application in nodal regions of diploid, triploid, and tetraploid watermelon plants. (A). Whole plants under different GA3 treatments on diploid, triploid, and tetraploid watermelon. Red arrows indicate the branches. (B–E) Effect of exogenous GA3 on branch numbers, vine length, leaf angle, and leaf area. (F) Changes in endogenous GA3 concentration after hormone application. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level according to Tukey’s test. Control = freshwater spray, GA3-1 = spraying with 100 µM GA3, GA3-2 = spraying with 200 µM GA3, GA3-3 = spraying with 300 µM GA3.
We also measured the endogenous GA3 content in nodal regions (Figure 9F). Hormone content increased gradually with increased GA3 application. However, the increment was not statistically significant in diploid plants. In diploid plants, the hormone concentration in the control plant was 4.07 ng/g, which increased to 4.52 ng/g after the 200 µM GA3 application. In triploid plants, GA3 content in the control plant was 2.85 ng/g, which increased to 3.59 ng/g after the 300 µM GA3 application, and in tetraploid plants, GA3 content in the control plant was 2.82 ng/g, which increased to 3.94 ng/g after the 300 µM GA3 application.
3.5.2. Effect of Exogenous Application of ABA on Plant Architectural Traits
ABA was applied to different nodal regions in a way that it does not touch the apical tip of the plant. Like exogenous GA3, ABA also showed a significant effect on branching, but unlike GA3, its effect was negative on branching (Figure 10A,B). In all cases, the maximum reduction was found at 150 µM ABA. The maximum reduction after the application of 150 µM ABA was found to be 37.47% in diploid, 23.09% in triploid, and 50% in tetraploid plants. Like GA3, other parameters like vine length, stem thickness, leaf area, and leaf angle were not significantly affected by ABA treatment (Figure 10C–E).
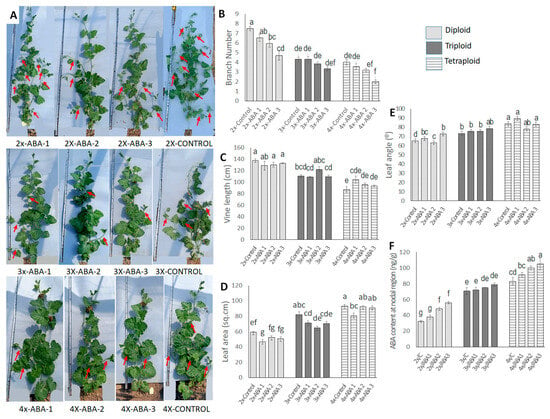
Figure 10.
Effect of exogenous ABA application to nodal regions in diploid, triploid, and tetraploid watermelon plants. (A). The pictorial view of whole plants under different ABA treatments on diploid, triploid, and tetraploid watermelon. Red arrows indicate the branches. (B–E) Effect of exogenous ABA on number of branches, vine length, leaf angle, and leaf area. (F) Changes in endogenous ABA concentration after hormone application. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level according to Tukey’s test. Control = freshwater spray, ABA 1 = spraying with 50 µM ABA, ABA 2 = spraying with 100 µM ABA, ABA 3 = spraying with 150 µM ABA.
ABA content was also measured in nodal regions after the application of ABA. Endogenous ABA content increased gradually with increased ABA application (Figure 10F). In diploid plants, it increased to 56.33 ng/g when 150 µM ABA was applied, which was 67.30% higher than the control plants. In triploid plants, endogenous ABA increased with the application of a higher ABA concentration but the increment was not statistically significant. In tetraploid plants, ABA content increased with the increased concentration of the applied hormone and the maximum ABA content was found when ABA was applied at 150 µM ABA concentration, which was 36.40% higher than the control plants.
3.5.3. Effect of Exogenous Application of Br on Plant Architectural Traits
Exogenous Br showed interesting changes in diploid watermelon plants. We applied Br at the apical meristem of the diploid plants (Figure 11A). Exogenous Br showed a negative effect on the vine length of diploid watermelon plants and the rate of reduction increased with increased Br concentrations (Figure 11A,D). The average vine length of 35-day-old control plants was 127.08 cm, and it was 107.58 cm at 100 µM Br, which shows a 15.17% length reduction. Internodal length also decreased with 1 µM and 10 µM Br concentrations but then increased again at the 100 µM Br concentration (Figure 11F). The internodal length of the control plant was 36.87 mm, which was reduced to 33.27 mm at 1 µM Br and 30.87 mm at 10 µM Br but increased to 37.42 mm at 100 µM Br. Exogenous Br had a positive effect on stem thickness, leaf area, and leaf angle (Figure 11B,C). Stem thickness showed an 8.30% increment at 1 µM Br and a 30.56% increment at 10 µM Br compared to the control plants. However, it decreased by 3.38% when the Br concentration was increased to 100 µM (Figure 11E). Leaf area increased at 1 µM and 10 µM concentrations. Leaf area increased by 32.97% at 1 µM Br and 50.57% at 10 µM Br compared to the control plants. However, at higher concentrations like 100 µM, it showed only a 17.60% increment compared to the control plants (Figure 11G). Leaf angle showed a 28.10% increment at 1 µM Br, a 37.56% increment at 10 µM Br, and an 80.31% increment at 100 µM Br compared to the control group plants (Figure 11H).
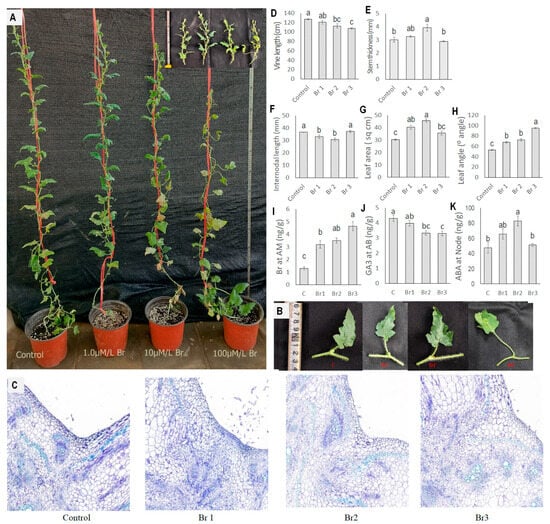
Figure 11.
Effect of exogenous Br application in nodal region on diploid watermelon. (A). The pictorial view of whole plants under different treatments. (B) Effect of exogenous Br on leaf angle and leaf size changes under different treatments. (C) Cross-section in nodal region showing increased cell size with increased Br doses. (D–H) Morphological changes under Br application. (I–K) Endogenous hormonal changes after Br application. Values represent the mean of three replicates ± SE. Values followed by same letters in the column are not significantly different at the 5% probability level according to Tukey’s test. Control = freshwater spray, Br 1 = spraying with 1 µM Br, Br 2 = spraying with 10 µM Br, Br 3 = spraying with 100 µM Br.
We also measured the hormone content in the apical meristem and nodal regions (Figure 11I–K). Br content increased with increased concentrations of the applied hormone. We observed the endogenous Br content in control plants to be 1.3 ng/g, which increased 3.57 times when 100 µM Br was applied. It also changed the concentration of ABA and GA3 in the nodal and auxiliary bud regions.
4. Discussion
The comprehensive study of various watermelon genotypes across different ploidy levels reveals distinct variations in plant architectural traits, highlighting the profound impact of polyploidy on plant morphology and growth patterns. Diploid watermelon plants exhibited a higher number of branches and nodes compared to their triploid and tetraploid counterparts. They also showed longer internodal length and vine length (Figure 1 and Figure 2). In contrast to diploids, triploid and tetraploid watermelon plants exhibited a more compact growth form, characterized by shorter plants with less branching. However, these higher ploidy levels were associated with larger leaves, increased leaf angle, and greater petiole length and thickness (Figure 3). Similar results were reported by Corneillie et al. [32] for the leaf size, stem diameter, and lateral branches of inflorescence in Arabidopsis thaliana. However, they reported longer inflorescence in tetraploid than diploid. Increased biomass of polyploid was also observed in Miscanthus spp. and Salix spp. [33,34]. The result can be easily explained by the increased gene copies of polyploid resulting in increased cell and organ size. However, an increase in gene number does not always result in an increase in biomass production. Smaller plant sizes in tetraploid than diploid were also reported in Triticum monococcum and apples [35,36,37]. Slower growth with short but thick stems and large leaves was reported in autotetraploid britch, poplus, and eucalyptus tree species [3,38,39]. Ren et al. [40], Corneillie et al. [33], and Rosellini et al. [41] described this shorter but thicker stem as being due to shorter but wider cells in tetraploid compared to its diploid counterpart.
The comprehensive analysis of hormone content in watermelon plants across different ploidy levels revealed intricate patterns of hormonal variation, contributing to our understanding of plant physiology and development. In the AM, IAA, GA3, Zr, and ABA showed fluctuations across different growth stages and ploidy levels, but not in a consistent manner (Figure 4). The significant increase in ABA at 55 DAT in diploids might correlate with early senescence, aligning with the findings of Van Norman et al. [42] regarding ABA’s role in plant development and stress responses. Br showed consistent variation at AM, being significantly higher in tetraploid and triploid plants compared to diploids. Though Ma et al. [36] found the lower Br concentration at the shoot tip to be responsible for the dwarfism of tetraploid apples, they concluded that Br acts in a dose-dependent manner. Both Br mutant plants and higher doses of Br can be detrimental to plant growth. The nodal region displayed a significantly higher accumulation rate of ABA in tetraploid and triploid plants (Figure 5), aligning with the role of ABA in stress adaptation and growth regulation [43]. The increase in ABA content with plant age across all ploidy levels further highlights its role in plant maturation and stress responses. A notable variation in GA3 content was observed in the auxiliary buds, with diploid plants showing the highest levels (Figure 6). This finding is particularly interesting as it suggests a potential link between the GA3 concentration and the development of auxiliary buds, potentially influencing branching patterns, as discussed by Yamaguchi [44].
Though hormones play a regulatory role in developing plant architecture, the regulations are not so linear; rather, there is a complex crosstalk between the hormones [45]. The correlation study examining the relationship between hormone content and plant architectural traits in watermelons highlights the complex interactions between hormonal regulation and plant morphology (Figure 7). In our study, Br at AM showed a significant positive correlation with Zr at AM and a significant negative correlation with GA3 at AM. Yuldashev et al. [46] also showed that the exogenous application of Br can increase the CK level in wheat seedlings. Chone et al. [47] and Zu et al. [48] showed the synergistic effect of Br and CK on somatic embryogenesis in leaf explants of Coffea arabica and ovule initiation of arabidopsis. However, the negative correlation observed between Br and GA is somewhat perplexing, given that most researchers have reported positive regulation of Br and GA [49,50]. Notably, Xia et al. [51] described the downregulation of GA in response to a higher Br dose. This phenomenon can be explained by the findings of a previous study conducted by Ma et al. [36], who mentioned that the action of Br is dose-dependent. The positive correlation of GA with Br and the negative correlation with ABA align with the well-established roles of GA and Br as growth-promoting hormones and ABA as a stress-related hormone [52,53]. Besides the significant positive correlations between Br at AM and ABA at the node, Zr at AM and ABA at the node, IAA at AM and Zr at AB, and Zr at the node and IAA at AB and the significant negative correlations between Br at AM and GA3 at AB, Zr at AM and GA3 at AB, ABA at AM and ABA at AB, Zr at the node and ABA at AB, and ABA at the node and GA3 at AB, they might also be associated with their signal transduction mechanism. Interestingly, hormonal interactions with morphological traits revealed that Br at AM, ABA at the node, and GA3 at AB interact with most of the plant’s architectural traits. Among them, Br at AM positively interacted with stem thickness, leaf area, petiole length, and leaf angle and negatively correlated with the number of nodes, branch number, and internodal length. ABA at the node positively correlated with stem thickness, leaf area, petiole length, petiole thickness, and leaf angle, whereas it showed negative correlations with node number, branch number, and internodal length. GA3 in the auxiliary branch positively affected the node and branch numbers and internodal length, while negatively impacting stem and leaf dimensions. A subsequent biplot analysis revealed associations of specific traits with each ploidy level: high ABA at the node, IAA at the node, and Br at AM gave the plants a compact stature with less branching, which was characteristics of triploid and tetraploid, whereas lower values of ABA at the node, IAA at the node, and Br at AM but higher values of GA3 at the auxiliary bud gave plants a thin stature with profuse branching, which was associated with diploid (Figure 8). These correlations reflect the complex and significant role of hormones in shaping the architectural traits of watermelon plants, indicating a deep interconnection between physiological processes and plant structure. For further confirmation of the result, we treated Fanzu No. 1’ with different doses of Gibberellin, ABA, and Br. We sprayed GA3 and ABA at different nodal regions and Br on the apical meristem regions. GA3 increased the number of branches in both diploid and polyploid watermelon plants, where the exogenous application of ABA to the nodal region reduced the branch number in all three ploidy levels. Gibberellin showed a different effect on shoot growth. Its negative regulatory role on auxiliary shoot outgrowth was reported in pea, turf grass, and populous [54,55,56,57]. On the other hand, its positive role in auxiliary shoot-out growth was reported in arabidopsis, citrus, snapdragon, sweet cherry, and Jatropha [58,59,60,61,62]. The suppressing role of ABA in auxiliary bud development was also mentioned by (González et al., 2017) [63]. Yao and Finlayson (2015) suggested that exogenous ABA can affect the expression of the IAA biosynthesis enzyme Tryptophan Aminotransferase Of Arabidopsis1 (TAA1), the auxin transporter PIN-FORMED1, and the cell cycle genes CYCLIN A2;1 and Proliferating Cell Nuclear Antigen1 (PCNA1) in buds, thus suppressing auxiliary bud outgrowth [64]. The most interesting factor was shown by the exogenous application of Br at the apical meristem. It increased the leaf angle and leaf area but reduced the vine length and internodal length, which resembles the traits of polyploid. The positive role of Br on leaf size has also been reported by many researchers [65,66]. The effect is mainly related to cell expansion [67,68]. Zhang and Kang (2014) showed that exogenous Br application can upregulate the expression of cell growth-related genes like NtCYCD3; 1, NtARGOS, NtGRF5, NtGRF8, and NtXTH [69]. The role of Br in regulating leaf angle has been reported by many researchers, but most of these are concerning monocotyledonous plants [70,71,72].
5. Conclusions
In conclusion, the impact of genome duplication on plant morphology is evident, with diploid plants exhibiting expansive growth forms characterized by higher branching and longer vine lengths. In contrast, triploid and tetraploid plants display a compact stature with distinct features such as larger leaves and thicker stems. Hormonal analysis underscores the importance of Br, ABA, and GA3 in shaping the plant architecture at different ploidy levels, revealing a complex interplay in growth and development. These findings contribute valuable insights for breeding programs and agricultural practices, guiding the optimization of growth conditions for diverse watermelon varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10060569/s1, Figure S1: Picture showing chromosome number in diploid, triploid and tetraploid. Supplementary File S1: ANOVA table for morphological traits. Supplementary File S2: ANOVA table for hormone content at apical meristem (AM); hormone content at nodal tissue; hormone content at AB. Supplementary File S3: ANOVA table for different parameters after ABA spray; GA3 spray; Br spray. Supplementary File S4: Correlation co-efficient for morphological traits; Correlation co-efficient among hormones; Correlation between hormones and traits.
Author Contributions
Conceptualization, W.L.; methodology, E.M., H.Z. and M.O.K.; software, E.M.; validation, E.M., X.L. and N.H.; formal analysis, E.M.; investigation, E.M. and M.O.K.; resources, X.L. and H.Z.; data curation, E.M.; writing—original draft preparation, E.M.; writing—review and editing, H.Z. and M.Z.S.; visualization, E.M.; supervision, W.L. and N.H.; project administration, W.L; funding acquisition, W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (SQ2023YFE0201239), the Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2021-ZFRI), the China Agriculture Research System of MOF and MARA (CARS-25-03), the National Key Research and Development Program of China (2023YFF1000100), the Basic Research Funds of Chinese Academy of Agricultural Sciences (1610192023201), the Major Science and Technology Projects of Henan Province (221100110400), and the Funding of Joint Research on Agricultural Variety Improvement of Henan Province (2022010503).
Data Availability Statement
Data supporting the findings of this work are available in Supplementary Files.
Acknowledgments
This research work was a part of the PhD study of Eftekhar Mahmud at Zhengzhou Fruit Research Institute, Zhengzhou, Henan, China. We acknowledge all of the members of Polyploid Watermelon Breeding Group and the support staffs of ZFRI for their cordial support in performing the research work.
Conflicts of Interest
The authors declare that there are no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kihara, H. Triploid Watermelons. Proc. Am. Soc. Hortic. Sci. 1951, 58, 217–230. [Google Scholar]
- Smith, W.K.; Vogelmann, T.C.; DeLucia, E.H.; Bell, D.T.; Shepherd, K.A. Leaf Form and Photosynthesis. Bioscience 1997, 47, 785–793. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Qiu, B.; Ma, Z.; Lu, T.; Kang, X.; Yang, J. Induction and Characterization of Tetraploid Through Zygotic Chromosome Doubling in Eucalyptus Urophylla. Front. Plant Sci. 2022, 13, 870698. [Google Scholar] [CrossRef]
- Lynch, J.P. Harnessing Root Architecture to Address Global Challenges. Plant J. 2022, 109, 415–431. [Google Scholar] [CrossRef]
- Valladares, F.; Laanisto, L.; Niinemets, Ü.; Zavala, M.A. Shedding Light on Shade: Ecological Perspectives of Understorey Plant Life. Plant Ecol. Divers. 2016, 9, 237–251. [Google Scholar] [CrossRef]
- Mencuccini, M. Dwarf Trees, Super-Sized Shrubs and Scaling: Why Is Plant Stature so Important? Plant Cell Environ. 2015, 38, 1–3. [Google Scholar] [CrossRef]
- Jaillais, Y.; Chory, J. Unraveling the Paradoxes of Plant Hormone Signaling Integration. Nat. Struct. Mol. Biol. 2010, 17, 642–645. [Google Scholar] [CrossRef]
- Eichmann, R.; Richards, L.; Schäfer, P. Hormones as Go-Betweens in Plant Microbiome Assembly. Plant J. 2021, 105, 518–541. [Google Scholar] [CrossRef]
- Ortiz-García, P.; González Ortega-Villaizán, A.; Onejeme, F.C.; Müller, M.; Pollmann, S. Do Opposites Attract? Auxin-Abscisic Acid Crosstalk: New Perspectives. Int. J. Mol. Sci. 2023, 24, 3090. [Google Scholar] [CrossRef]
- Comai, L. The Advantages and Disadvantages of Being Polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Tsukaya, H. Controlling Size in Multicellular Organs: Focus on the Leaf. PLoS Biol. 2008, 6, e174. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, J.C.; Ramirez-Parra, E. Whole Genome Duplications in Plants: An Overview from Arabidopsis. J. Exp. Bot. 2015, 66, 6991–7003. [Google Scholar] [CrossRef] [PubMed]
- Tavan, M.; Mirjalili, M.H.; Karimzadeh, G. In Vitro Polyploidy Induction: Changes in Morphological, Anatomical and Phytochemical Characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult. 2015, 122, 573–583. [Google Scholar] [CrossRef]
- Vergara, F.; Kikuchi, J.; Breuer, C. Artificial Autopolyploidization Modifies the Tricarboxylic Acid Cycle and GABA Shunt in Arabidopsis Thaliana Col-0. Sci. Rep. 2016, 6, 26515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, X.; Hao, B.; Ge, S.; Luo, J. Duplication and DNA Segmental Loss in the Rice Genome: Implications for Diploidization. New Phytol. 2005, 165, 937–946. [Google Scholar] [CrossRef]
- Lavania, U.C. Genomic and Ploidy Manipulation for Enhanced Production of Phyto-Pharmaceuticals. Plant Genet. Resour. 2005, 3, 170–177. [Google Scholar] [CrossRef]
- He, N.; Umer, M.J.; Yuan, P.; Wang, W.; Zhu, H.; Lu, X.; Xing, Y.; Gong, C.; Batool, R.; Sun, X.; et al. Physiological, Biochemical, and Metabolic Changes in Diploid and Triploid Watermelon Leaves during Flooding. Front. Plant Sci. 2023, 14, 1108795. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, S.; Lu, X.; He, N.; Gao, L.; Dou, J.; Bie, Z.; Liu, W. Genome Duplication Improves the Resistance of Watermelon Root to Salt Stress. Plant Physiol. Biochem. 2018, 133, 11–21. [Google Scholar] [CrossRef]
- Tossi, V.E.; Martínez Tosar, L.J.; Laino, L.E.; Iannicelli, J.; Regalado, J.J.; Escandón, A.S.; Baroli, I.; Causin, H.F.; Pitta-Álvarez, S.I. Impact of Polyploidy on Plant Tolerance to Abiotic and Biotic Stresses. Front. Plant Sci. 2022, 13, 869423. [Google Scholar] [CrossRef]
- Ramsey, J.; Schemske, D.W.; Ramsey, J.; Schemske, D.W. Pathways, Mechanisms, and Rates of Polyploid Formation in Flowering Plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Davis, A.R.; Webber, C.L.; Liu, W.; Perkins-Veazie, P.; Levi, A.; King, S. Watermelon Quality Traits as Affected by Ploidy. HortScience 2013, 48, 1113–1118. [Google Scholar] [CrossRef]
- Xu, M.; Gao, M.; Amanullah, S.; Guo, Y.; Bao, X.; Duan, Y.; Liu, X.; Liu, J.; Gao, Y.; Luan, F. Fine Genetic Mapping Confers a Major Gene Controlling Leaf Shape Variation in Watermelon. Euphytica 2023, 219, 92. [Google Scholar] [CrossRef]
- Mandizvo, T.; Odindo, A.O.; Mashilo, J.; Sibiya, J.; Beck-Pay, S.L. Phenotypic Variability of Root System Architecture Traits for Drought Tolerance among Accessions of Citron Watermelon (Citrullus lanatus Var. Citroides (L.H. Bailey). Plants 2022, 11, 2522. [Google Scholar] [CrossRef]
- Gebremeskel, H.; Dou, J.; Li, B.; Zhao, S.; Muhammad, U.; Lu, X.; He, N.; Liu, W. Molecular Mapping and Candidate Gene Analysis for GA3 Responsive Short Internode in Watermelon (Citrullus lanatus). Int. J. Mol. Sci. 2020, 21, 290. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Fan, M.; He, Y.; Guo, P. A Mutation in the Intron Splice Acceptor Site of a GA3ox Gene Confers Dwarf Architecture in Watermelon (Citrullus lanatus L.). Sci. Rep. 2020, 10, 14915. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, A.; He, W.; Li, Q.; Zhao, B.; Zhao, H.; Ke, X.; Guo, Y.; Sun, P.; Yang, T.; et al. GRAS Family Member LATERAL SUPPRESSOR Regulates the Initiation and Morphogenesis of Watermelon Lateral Organs. Plant Physiol. 2023, 193, 2592–2604. [Google Scholar] [CrossRef]
- Khan, M.N.-E.-A.; Hassan, J.; Biswas, M.S.; Khan, H.I.; Sultana, H.; Suborna, M.N.; Rajib, M.M.R.; Akter, J.; Gomasta, J.; Anik, A.A.M. Morphological and Anatomical Characterization of Colchicine-Induced Polyploids in Watermelon. Hortic. Environ. Biotechnol. 2023, 64, 461–474. [Google Scholar] [CrossRef]
- Zhang, N.; Bao, Y.; Xie, Z.; Huang, X.; Sun, Y.; Feng, G.; Zeng, H.; Ren, J.; Li, Y.; Xiong, J.; et al. Efficient Characterization of Tetraploid Watermelon. Plants 2019, 8, 419. [Google Scholar] [CrossRef]
- Jaskani, M.J.; Kwon, S.W.; Kim, D.H. Comparative Study on Vegetative, Reproductive and Qualitative Traits of Seven Diploid and Tetraploid Watermelon Lines. Euphytica 2005, 145, 259–268. [Google Scholar] [CrossRef]
- Feng, Z.; Bi, Z.; Fu, D.; Feng, L.; Min, D.; Bi, C.; Huang, H. A Comparative Study of Morphology, Photosynthetic Physiology, and Proteome between Diploid and Tetraploid Watermelon (Citrullus lanatus L.). Bioengineering 2022, 9, 746. [Google Scholar] [CrossRef]
- Mo, Z.; Feng, G.; Su, W.; Liu, Z.; Peng, F. Transcriptomic Analysis Provides Insights into Grafting Union Development in Pecan (Carya illinoinensis). Genes 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Corneillie, S.; De Storme, N.; Van Acker, R.; Fangel, J.U.; De Bruyne, M.; De Rycke, R.; Geelen, D.; Willats, W.G.T.; Vanholme, B.; Boerjan, W. Polyploidy Affects Plant Growth and Alters Cell Wall Composition. Plant Physiol. 2019, 179, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Dudits, D.; Török, K.; Cseri, A.; Paul, K.; Nagy, A.V.; Nagy, B.; Sass, L.; Ferenc, G.; Vankova, R.; Dobrev, P.; et al. Response of Organ Structure and Physiology to Autotetraploidization in Early Development of Energy Willow Salix Viminalis. Plant Physiol. 2016, 170, 1504–1523. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, K.; Jezowski, S.; Kaczmarek, Z. In Vitro Induction of Polyploidy by Colchicine Treatment of Shoots and Preliminary Characterisation of Induced Polyploids in Two Miscanthus Species. Ind. Crops Prod. 2010, 32, 88–96. [Google Scholar] [CrossRef]
- Kuspira, J.; Bhambmani, R.N.; Shimada, T. Genetic and Cytogenetic Analyses of the A Genome of Triticum Monococcum. I. Cytology, Breeding Behaviour, Fertility, and Morphology of Induced Autotetraploids. Can. J. Genet. Cytol. 1985, 27, 51–63. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, H.; Zhang, L.; Zhang, F.; Ou, C.; Wang, F.; Zhang, Z. Involvement of Auxin and Brassinosteroid in Dwarfism of Autotetraploid Apple (Malus × Domestica). Sci. Rep. 2016, 6, 26719. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J. Plant Cytogenetics; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1439884196. [Google Scholar]
- Zhang, X.; Chen, K.; Wang, W.; Liu, G.; Yang, C.; Jiang, J. Differences in Leaf Morphology and Related Gene Expression between Diploid and Tetraploid Birch (Betula pendula). Int. J. Mol. Sci. 2022, 23, 12966. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, Y.; Han, Q.; Kang, X. Molecular Mechanism of Slow Vegetative Growth in Populus Tetraploid. Genes 2020, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, S.; Xu, T.; Kang, X. Morphological, Transcriptome, and Hormone Analysis of Dwarfism in Tetraploids of Populus alba × P. glandulosa. Int. J. Mol. Sci. 2022, 23, 9762. [Google Scholar] [CrossRef]
- Rosellini, D.; Ferradini, N.; Allegrucci, S.; Capomaccio, S.; Zago, E.D.; Leonetti, P.; Balech, B.; Aversano, R.; Carputo, D.; Reale, L.; et al. Sexual Polyploidization in Medicago Sativa L.: Impact on the Phenotype, Gene Transcription, and Genome Methylation. G3 Genes Genomes Genet. 2016, 6, 925–938. [Google Scholar] [CrossRef]
- Van Norman, J.M.; Zhang, J.; Cazzonelli, C.I.; Pogson, B.J.; Harrison, P.J.; Bugg, T.D.H.; Chan, K.X.; Thompson, A.J.; Benfey, P.N. Periodic Root Branching in Arabidopsis Requires Synthesis of an Uncharacterized Carotenoid Derivative. Proc. Natl. Acad. Sci. USA 2014, 111, E1300–E1309. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic Acid: Emergence of a Core Signaling Network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin Metabolism and Its Regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Müller, M. Hormonal Cross-Talk in Plant Development and Stress Responses. Front. Plant Sci. 2013, 4, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Yuldashev, R.; Avalbaev, A.; Bezrukova, M.; Vysotskaya, L.; Khripach, V.; Shakirova, F. Cytokinin Oxidase Is Involved in the Regulation of Cytokinin Content by 24-Epibrassinolide in Wheat Seedlings. Plant Physiol. Biochem. 2012, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chone, R.M.S.; Rocha, D.I.; Monte-Bello, C.C.; Pinheiro, H.P.; Dornelas, M.C.; Haddad, C.R.B.; Almeida, J.A.S. Brassinosteroid Increases the Cytokinin Efficiency to Induce Direct Somatic Embryogenesis in Leaf Explants of Coffea arabica L. (Rubiaceae). Plant Cell Tissue Organ Cult. 2018, 135, 63–71. [Google Scholar] [CrossRef]
- Zu, S.-H.; Jiang, Y.-T.; Chang, J.-H.; Zhang, Y.-J.; Xue, H.-W.; Lin, W.-H. Interaction of Brassinosteroid and Cytokinin Promotes Ovule Initiation and Increases Seed Number per Silique in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.J.; Quittenden, L.J. Interactions between Brassinosteroids and Gibberellins: Synthesis or Signaling? Plant Cell 2016, 28, 829–832. [Google Scholar] [CrossRef]
- Unterholzner, S.J.; Rozhon, W.; Papacek, M.; Ciomas, J.; Lange, T.; Kugler, K.G.; Mayer, K.F.; Sieberer, T.; Poppenberger, B. Brassinosteroids Are Master Regulators of Gibberellin Biosynthesis in Arabidopsis. Plant Cell 2015, 27, 2261–2272. [Google Scholar] [CrossRef]
- Xia, X.; Liu, Y.; Zhang, L.; Qi, Z.; Zhou, Y.; Yu, J. Overexpression of Brassinosteroid Synthesis Gene DWARF Promotes Resistance to Botrytis Cinerea by Inhibiting Gibberellin Synthesis in Solanum lycopersicum L. Plant Stress 2023, 9, 100170. [Google Scholar] [CrossRef]
- Bai, M.Y.; Shang, J.X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.P.; Wang, Z.Y. Brassinosteroid, Gibberellin and Phytochrome Impinge on a Common Transcription Module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Gibberellins and Abscisic Acid Signal Crosstalk: Living and Developing under Unfavorable Conditions. Plant Cell Rep. 2013, 32, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Agharkar, M.; Lomba, P.; Altpeter, F.; Zhang, H.; Kenworthy, K.; Lange, T. Stable Expression of AtGA2ox1 in a Low-Input Turfgrass (Paspalum notatum Flugge) Reduces Bioactive Gibberellin Levels and Improves Turf Quality under Field Conditions. Plant Biotechnol. J. 2007, 5, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper Gibberellin Localization in Vascular Tissue Is Required to Control Auxin-Dependent Leaf Development and Bud Outgrowth in Hybrid Aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.K.; Case, D.B.; Jacobs, W.P. Auxin-Gibberellin Interaction in Apical Dominance. Plant Physiol. 1967, 42, 1329–1333. [Google Scholar] [CrossRef]
- Zawaski, C.; Busov, V.B. Roles of Gibberellin Catabolism and Signaling in Growth and Physiological Response to Drought and Short-Day Photoperiods in Populus Trees. PLoS ONE 2014, 9, e86217. [Google Scholar] [CrossRef] [PubMed]
- Choubane, D.; Rabot, A.; Mortreau, E.; Legourrierec, J.; Péron, T.; Foucher, F.; Ahcène, Y.; Pelleschi-Travier, S.; Leduc, N.; Hamama, L.; et al. Photocontrol of Bud Burst Involves Gibberellin Biosynthesis in Rosa sp. J. Plant Physiol. 2012, 169, 1271–1280. [Google Scholar] [CrossRef] [PubMed]
- Elfving, D.C.; Visser, D.B.; Henry, J.L. Gibberellins Stimulate Lateral Branch Development in Young Sweet Cherry Trees in the Orchard. Int. J. Fruit Sci. 2011, 11, 41–54. [Google Scholar] [CrossRef]
- Koorneef, M.; Elgersma, A.; Hanhart, C.J.; van Loenen-Martinet, E.P.; Van Rijn, L.; Zeevaart, J.A.D. A Gibberellin Insensitive Mutant of Arabidopsis Thaliana. Physiol. Plant. 1985, 65, 33–39. [Google Scholar] [CrossRef]
- Marth, P.C.; Audia, W.V.; Mitchell, J.W. Effects of Gibberellic Acid on Growth and Development of Plants of Various Genera and Species. Bot. Gaz. 1956, 118, 106–111. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.; Chen, M.-S.; Pan, B.-Z.; Ye, K.; Xu, Z.-F. Gibberellin Promotes Shoot Branching in the Perennial Woody Plant Jatropha Curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- González-Grandío, E.; Pajoro, A.; Franco-Zorrilla, J.M.; Tarancón, C.; Immink, R.G.H.; Cubas, P. Abscisic Acid Signaling Is Controlled by a BRANCHED1/HD-ZIP I Cascade in Arabidopsis Axillary Buds. Proc. Natl. Acad. Sci. USA 2017, 114, E245–E254. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Finlayson, S.A. Abscisic Acid Is a General Negative Regulator of Arabidopsis Axillary Bud Growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, D.M.; Joazeiro, C.A.P.; Li, J.; Hunter, T.; Chory, J. Brassinosteroid-Insensitive-1 Is a Ubiquitously Expressed Leucine-Rich Repeat Receptor Serine/Threonine Kinase. Plant Physiol. 2000, 123, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Khan, R.; Wu, X.; Zhou, L.; Xu, N.; Du, S.; Ma, X. Exogenous Application of Brassinosteroids Regulates Tobacco Leaf Size and Expansion via Modulation of Endogenous Hormones Content and Gene Expression. Physiol. Mol. Biol. Plants 2021, 27, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fan, X.-Y.; Cao, D.-M.; Tang, W.; He, K.; Zhu, J.-Y.; He, J.-X.; Bai, M.-Y.; Zhu, S.; Oh, E.; et al. Integration of Brassinosteroid Signal Transduction with the Transcription Network for Plant Growth Regulation in Arabidopsis. Dev. Cell 2010, 19, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Németh, K.; Koncz-Kálmán, Z.; Mathur, J.; Kauschmann, A.; Altmann, T.; Rédei, G.P.; Nagy, F.; Schell, J.; Koncz, C. Brassinosteroids Rescue the Deficiency of CYP90, a Cytochrome P450, Controlling Cell Elongation and de-Etiolation in Arabidopsis. Cell 1996, 85, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Kang, M.B. Brassinosteroid-Mediated Regulation of Agronomic Traits in Rice. Plant Cell Rep. 2014, 33, 683–696. [Google Scholar] [CrossRef]
- Ji, X.; Gao, Q.; Zhuang, Z.; Chang, F.; Peng, Y. WGCNA Analysis of the Effect of Exogenous BR on Leaf Angle of Maize Mutant Lpa1. Sci. Rep. 2024, 14, 5238. [Google Scholar] [CrossRef]
- Perez, M.B.M. Genetic and Brassinosteroid Control Underlying Leaf Angle Variation across the Canopy in Sorghum. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2018. [Google Scholar]
- Wang, Z.-Y.; Bai, M.-Y.; Oh, E.; Zhu, J.-Y. Brassinosteroid Signaling Network and Regulation of Photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).