Abstract
The success of double cropping in Vitis vinifera L. cultivated in temperate climates relies on bud forcing efficiency, which requires the prompt unlocking of apical dormant buds with sufficient fruitfulness. Chemical dormancy-breaking strategies need to be tested to enhance dormant bud forcing in summer pruning, as hydrogen cyanamide, the most used agent, could damage green organs. This study tested whether foliar applications of cytokinins and auxins could modulate dormancy release, potentially affecting bud forcing dynamics and shoot fruitfulness. The forcing treatments involved trimming primary shoots at the eighth node, removing lateral shoots, and retaining the main leaves and inflorescences. Five treatments were investigated: unforced control, control + 6-Benzyladenine application, forcing (FR), forcing + 6-Benzyladenine application (FBA), and forcing + Naphthaleneacetic acid application (FNAA). Phenological evolution, vegetative and productive parameters, and physiological characteristics have been assessed. Results showed that among the forcing treatments, FBA showed the highest forced/primary shoots ratio (106%), followed by FR (94%) and FNAA (21%). Primary yields were similar across treatments (2.74 kg), but total yield was highest in FBA (4.78 kg, including 2.02 kg from forced grapes), followed by FR (3.62 kg, with 1.09 kg forced). FNAA yielded no forced crop. During forced grapes maturation, photosynthesis rates were higher in forced leaves (11.1 μmol m−2 s−1, as FR and FBA average) than primary leaves (−32%). Forced grapes ripened 47 days later and achieved higher sugar content (21.7 °Brix) and titratable acidity (10.6 g/L) than primary grapes. The findings suggest cytokinins application enhances bud forcing, supporting the feasibility of double cropping, while auxins limited it.
1. Introduction
Double cropping has emerged as a promising viticultural practice to enhance production and differentiate grape quality in temperate climates under the pressure of climate change [1,2,3,4]. Dormant buds, typically programmed to release their dormancy in the spring subsequent to the season in which they formed, can be “forced” to burst within the same growing season through a technique that involves the removal of any active primary meristem on the shoots when berries are at the groat-size stage (BBCH 73) [1]. To be effective, though, forcing must be performed when the dormant buds have already completed bud induction and, as a related consequence, the development of a second, delayed crop is feasible [5,6].
Bud dormancy recognizes three main phases: endodormancy, ecodormancy, and paradormancy [7,8] which, respectively, inhibit growth in relation to the bud maturation state. Endodormancy, driven by internal factors, prevents premature growth during winter. Once surpassed, ecodormancy relies on external environmental conditions, such as temperature and soil moisture, to activate budburst. Paradormancy, or correlative bud inhibition, is regulated by cross regulation of different vegetative organs, involving cytokinins (CK) and auxins (AUX) [9]. The role of paradormancy has been investigated by [10], who highlighted how shoot tips, young main leaves and laterals exert control over other vegetative organs and, namely, the dormant bud. Their study showed that topping the primary shoot and removing the lateral shoots resulted in up to 94% budburst in dormant buds, whereas partial removal led to significantly lower release rates. This emphasizes the importance of fully trimming the primary shoot and eliminating lateral shoots to effectively overcome paradormancy. However, even when these conditions are met, biochemical factors may still limit dormant bud release [5,11].
While extensive research has focused on the optimal timing of forcing and its effects on physiological and productive performance [1,2,12] and on endodormancy release [13], less is known about the factors influencing paradormancy release upon application of the forcing protocol in Vitis vinifera L. grapevines. For instance, Pérez and Noriega [14] identified differences in gene expression influencing hormonal balance in paradormant and endodormant buds, but their findings were limited to bud cuttings exposed to forcing under laboratory conditions, neglecting whole-plant correlative mechanisms. In sub-tropical and tropical climates, dormancy is usually artificially broken with hydrogen cyanamide to achieve uniform budbreak and vegetative growth. Leonel et al. [15] and Lombard et al. [16] found a boost of CK content in buds after hydrogen cyanamide treatments. In woody crops, the roles of AUX and CK have been confirmed to negatively and positively enhance bud outgrowth, respectively [17,18,19]. AUX plays a central role in inhibiting lateral shoots development during active growth [20,21]. The shoot apex topping or bending reduces AUX migration to the below-bud meristem and allows its subsequent development [22]; thus, its exogenous application might effectively restore apical dominance. Conversely, CK is known to promote meristem activity [23,24], bud fruitfulness [25] and the control of lateral shoot branching [26]. CK is also involved in dormancy release anticipation [27,28,29], suggesting its exogenous application could enhance the budburst of dormant buds after trimming and later shoot removal.
This research aims to evaluate the effects of exogenous application of 6-Benzyladenine (BA) and Naphthaleneacetic acid (NAA) on dormant bud forcing at the groat-size stage and their impact on the vine’s vegetative and productive characteristics in both primary and forced canopies.
2. Materials and Methods
2.1. Plant Material and Experimental Layout
The experiment was carried out in 2024 at the experimental farm Gasparini sited at the Università Cattolica del Sacro Cuore (45°02′05.9′′ N 9°43′47.5′′ E), Piacenza, Italy. Within a 7-year-old experimental vineyard, a single row featuring 50 vines of the cv. ‘Ortrugo’ (clone VCR245, grafted to SO4 rootstock) was used. Vine spacing was 1.0 m × 2.2 m (4545 vines/ha). Vines were trained to a spur-pruned cordon tied to a wire 1 m above the ground, with six two-node spurs and a canopy extension of about 1.2–1.5 m above the main wire. Vineyard and pest management were executed following the standard practices of the area. Daily rainfall and maximum, mean and minimum temperatures were recorded by a weather station (Netsens, Firenze, Italy) adjacent to the study site and base 10 °C growing degree days were calculated from April to October [30]. The vineyard was irrigated with a drip irrigation system by applying 100% of the daily vineyard evapotranspiration from 1 June to 31 August. Daily evapotranspiration was estimated using the Hargreaves method [31] and the crop coefficient for grapevine. Water supply ranged between 0.7 and 1.3 mm/day. Irrigation was suspended when rainfall was >5 mm.
The row plot was divided into three randomized blocks each with three vines per treatment. Vines were then assigned to the following five treatments: unforced and unsprayed control (UC); unforced control sprayed with a CK, 6-Benzyladenine (CBA); forcing (FR); Forcing sprayed with 6-Benzyladenine (FBA); and forcing sprayed with an AUX, Naphthaleneacetic acid (FNAA).
Forcing was applied at the groat-size stage (BBCH 73, 6 June, DOY 158) by trimming all growing shoots above node eight and removing all developing summer laterals. The spraying of exogenous CK and AUX was performed on leaves and dormant buds on both sides of the canopies immediately after trimming using a 2 L hand pump. The CBA vines were sprayed on the first eight basal nodes of the canopy and, if present, summer laterals in the same canopy portion were removed. Utilized CK was the commercial formulate Exilis® (SAFAPAC Ltd., Cambridge, UK), 6-Benzyladenine 2%, at a concentration of 50 mg/L. The sprayed AUX was the α-Naphthaleneacetic acid, commercial Germon® (Diachem, Caravaggio, BR, Italy) at a concentration of 100 mg/L.
2.2. Vegetative Development and Physiology Measurements
Before the application of treatments, 30 basal (nodes 1–8), 30 apical (node 9 to tip) primary leaves, and 30 lateral leaves were collected from extra vines and processed through a LI-3050A (LI-COR Biosciences, Lincoln, NE, USA), for average leaf area determination. At forcing application, removed primary and lateral leaves were separated and counted. Removed leaf area was determined by node counting multiplied by the mean leaf area of apical and lateral leaves. The remaining leaf area on the first eight nodes was determined by node counting multiplied by the mean leaf area of basal leaves. Total leaf area per vine was determined by node counting and average leaf area.
Phenological stages were visually determined in both primary and forced canopies according to the BBCH scale [32], once 50% of vines achieved the specific stage (BBCH 05, BBCH 09, BBCH 65, BBCH 73, BBCH 81, BBCH 83, and BBCH 85). At harvest of the forced crop, the surface area of 30 primary, lateral, and forced leaves were determined. After leaf fall, primary, lateral, and forced nodes were counted. Finally, the final total leaf area per vine was estimated based on node counts and mean leaf surface areas of respective leaf categories. On each vine, primary and forced shoots were counted and shoot fruitfulness was determined as clusters/shoots.
Leaf gas exchange parameters as photosynthetic assimilation rate (A), transpiration (E) and stomatal conductance (gs) were measured with a portable single leaf gas analyzer LCi T (ADC Bioscientific Ltd., Hoddesdon, UK) from 11:00 to 12:00 on 20 September 2024 (DOY 264) with clear sky conditions and a photosynthetically active radiation higher than 1500 μmol m−2 s−1 on two primary and two forced leaves (where present) per vine chosen between nodes 5 and 8 on their respective stems. Photosystem II efficiency and leaf chlorophyll content were measured on the same leaves analyzed for gas exchanges. Leaf photosystem II efficiency given as Fv/Fm ratio [33] was measured using the HandyPea chlorophyll fluorescence system (Hansatech Instruments Ltd., Norfolk, UK), after 2 h of dark adaptation using specific leaf clips, whereas leaf chlorophyll content was estimated with a SPAD-502Plus (Konica Minolta, Tokyo, Japan) after inserting a leaf and closing the measuring head without cutting it.
2.3. Yield Components and Fruit Composition
Grapes were sampled every week to monitor ripening trends. The primary crop was harvested on 28 August when grapes achieved a total soluble solids (TSS) of ~20 °Brix and titratable acidity (TA) was ~5 g/L. Forced grapes were harvested on 14 October, when they reached a TSS concentration of 21–22 °Brix and 10–11 g/L TA. The forced date was chosen to optimize fruit maturation and avoid fruit rot and dehydration processes. Single vine yield was determined using a field scale, the total number of clusters per vine recorded and cluster mean weight calculated accordingly. Three representative clusters from each vine were collected. For each cluster, mass and rachis length were measured, and compactness was calculated as the ratio of cluster mass to rachis length (g/cm). All berries for each cluster were separated from the rachis, counted and weighed. The average berry mass (g/berry) was then calculated as the ratio of total berry weight to the number of berries. A random sample of 50 berries was taken from the three clusters, weighed, placed into a zip-seal bag, and stored at −20 °C for subsequent analysis. The remaining berries were crushed to obtain a uniform must. Must TSS concentration was assessed using a digital refractometer (SMART-1, Atago, Bellevue, WA, USA), and pH was measured with a pH meter (pH 60 VioLab, Giorgio Bormac, Carpi, MO, Italy). TA was expressed as g/L of tartaric acid equivalents and determined by titration with 0.1 N NaOH to an endpoint of pH 8.2, using a potentiometric titrator (AT 1000 Series, Hach Company, Loveland, CO, USA).
Malic and tartaric acid concentrations were analyzed using high-performance liquid chromatography (HPLC, Agilent Technologies, Santa Clara, CA, USA). The juice samples were filtered through a 0.22 μm polypropylene syringe filter and diluted before being loaded into autosampler vials. The separation was performed on a Synergy 4u Hydro-RP80 A column (Phenomenex Inc., Torrance, CA, USA), measuring 250 × 4.6 mm. The buffer solution used was 0.2 M KH2PO4, adjusted to pH 2.4 with orthophosphoric acid. A 15 μL sample was injected, and the column was maintained at 30 °C ± 0.1 °C. Detection was carried out using a diode array detector (DAD) monitoring wavelengths from 200 to 700 nm, with quantification at 210 nm UV. Calibration curves were prepared using authentic standards, and concentrations of organic acids were determined by measuring the areas under peaks corresponding to malic and tartaric acid.
Must was diluted 40-fold for Potassium ion (K+) determination. The 50 mL diluted juice was adjusted adding 2 mL of 2.5 M NaCl and followed by the Potassium Ion-Selective Electrode (Crison Instruments S.A., Barcellona, Spain) reading.
2.4. Statistical Analysis
The data were analyzed using a one-way analysis of variance (ANOVA). Differences among significant treatment means were determined using the Student–Newman–Keuls (SNK) post hoc test at a significance level of p ≤ 0.05. Means between primary and forced grapes were separated using the student t-test. Analyses were performed with IBM SPSS Statistics version 29.0 (IBM Corp, Armonk, NY, USA).
3. Results
3.1. Weather Course and Phenology
The spring in 2024 experienced rainy and cool conditions, with the month of May contributing 189 mm of rain over the total of 693 mm recorded from 1 April to 31 October. Afterwards, the season quickly warmed, resulting in a total GDD accumulation of 2310 °C (Figure 1A). Dormant buds began bursting seven days after F application and completed budburst within two weeks from the forcing date. In primary shoots, 339 °C GDD and 61 days elapsed from budburst to full flowering. In the forced canopies, the same transition took 33 days and 781 °C GDD, respectively. Flowering veraison took 49 days and 671 °C GDD in the primary crop cycle vs. 43 days and 781 °C GDD in the forced crop. Veraison to harvest required a similar number of days in primary and forced crops (43 and 47 days, respectively) yet under a dramatically different GDD accumulation (453 °C of primary grapes vs. 781 °C of forced grapes). Primary clusters were harvested on 28 August (DOY 241), which corresponded to the onset of veraison in the forced crop. Finally, forced grapes were harvested on 14 October (DOY 288), 47 days after the primary crop (Figure 1B).
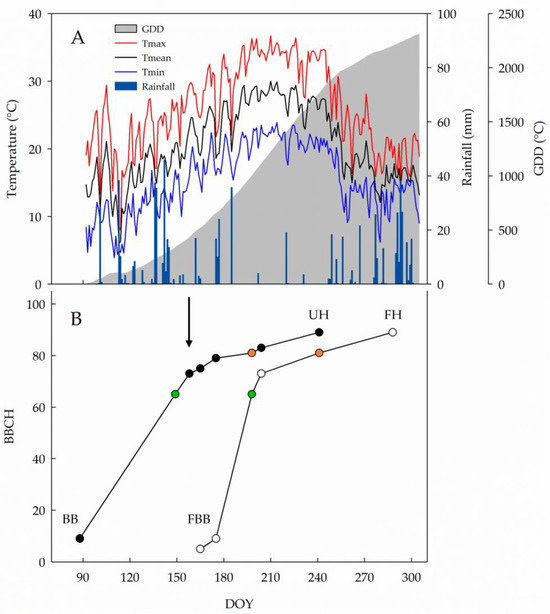
Figure 1.
(A) Seasonal evolution of maximum (Tmax), mean (Tmean) and minimum (Tmin) temperatures (°C), growing degree days (GDD, [30]) and daily rainfall (mm) at the experimental site from 1 April to 31 October 2024. (B) BBCH scale progression recorded in 2024 on unforced (black dots) and forced (white dots) cv. ‘Ortrugo’ canopies. The arrow indicates forcing application date, green and orange dots indicate flowering and veraison phase, respectively. DOY = day of year; BB = budburst; FBB = forced budburst; UH = unforced harvest; FH = forced harvest.
3.2. Vegetative Growth and Physiological Status
In F, FBA and FNAA an average of 1.4 m2 of leaf area was removed at treatments application (Table 1; Figure 2A). The average number of forced shoots per vine was 15 in F, 17 in FBA, and only 3 in FNAA. FBA vines showed the highest forced shoots/primary shoots ratio (106%), while few forced shoots (21%) were counted in FNAA vines (Table 1).

Table 1.
Vegetative growth components and vine balance (LA/Y ratio) recorded in the five treatments representing unforced control (UC) and four different combinations of crop forcing with plant growth regulators application. LA = leaf area; Y = yield; BA = 6-Benzyladenine; NAA = α-Naphthaleneacetic acid.
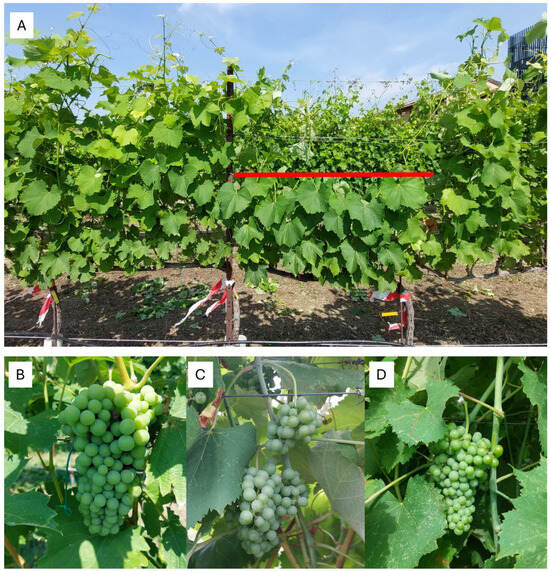
Figure 2.
(A) Grapevine subjected to the forcing treatment (FR) next to untreated grapevines (cv. Ortrugo). Shoots were trimmed to the eight node and lateral shoots have been removed. Red line indicates trimming height. (B) Representative cluster of primary crops in untreated control vines (UC), (C) forced cluster of forced vines (FR) and (D) forced clusters of forced vines sprayed with 6-Benzyladenine (FBA).
In CBA vines, no bud developed from any of the eight nodes subjected to lateral removal and CK spraying. FBA and UC vines showed comparable final LA while FNAA had the lowest final LA.
Regardless of treatment, forced leaves had significantly higher leaf assimilation rates (11.09 μmol m−2 s−1, than primary leaves (7.51 μmol m−2 s−1), which were also mirrored by higher leaf transpiration and stomatal conductance. Conversely, no differences among treatments and between primary and forced leaves have been observed for PS II efficiency or SPAD values (Table 2).

Table 2.
Analysis of single leaf gas exchanges (A and E), intrinsic water use efficiency (WUE), chlorophyll fluorescence (Fv/Fm) and SPAD measurements were conducted in primary (P) and forced (F) leaves on 20 September 2024 in cv. ‘Ortrugo’ grapevines.
3.3. Vine Yield and Fruit Composition
Yield components and cluster morphology of the primary crop were not affected by any of the applied treatments (Table 3). FNAA vines failed to produce forced grapes as dormant buds became unresponsive. By contrast, FR and FBA vines carried 11 and 16 forced clusters which were smaller as compared to the primary clusters leading in turn to a lower cluster compactness (Figure 2B–D). However, fruitfulness of the forced shoots in the FR and FBA treatments was higher than that of primary shoots (Table 3).

Table 3.
Yield components of primary (P) and forced (F) grapes harvested on 27 August and 14 October 2024, respectively, from cv. ‘Ortrugo’ grapevines subjected to forcing treatment, and plant growth regulators applications, compared with unforced control (UC) vines.
Total yield (primary + forced) was the highest in FBA (4.78 kg/vine) followed by FR (3.62 kg/vine) and then by the remaining treatments which relied solely on the primary crop yield. The leaf area-to-yield ratio (LA/Y) was high in UC and CBA (>1.60 m2/kg), whereas in the other treatments it was close to unity (0.99 m2/kg). The primary crop harvest caused the final LA/forced Y to surge to about 2.80 m2/kg in the FR and FBA treatments (Table 1).
Independently by AUX application, Forcing caused a significant decrease in must TSS of the primary crop as compared to the remaining treatments (Table 4). Remarkably, forced grapes had higher TSS (21–22 °Brix) than any other primary grapes (Table 4). Despite this, organic acids were distinctly higher in FR and FBA forced crop, with an almost equal contribution from improved tartaric and malic acid concentrations. Due to the above relative changes, the TSS/TA ratio in forced grapes was almost halved (2.13 and 1.97 for FR and FBA) compared to primary grapes (4.00 across treatments). Berry K+ concentration was also significantly reduced in FR and FBA grapes than in primary grapes, along with a reduced must pH (Table 4).

Table 4.
Final fruit composition of primary (P) and forced (F) grapes harvested on 27 August and 14 October 2024, respectively, from cv. ‘Ortrugo’ grapevines subjected to forcing treatments, phytohormones application and compared with unforced control (UC) vines.
4. Discussion
The responses of forced grapevines to exogenous CK and AUX confirm that at the groat-size stage (BBCH 73), dormant buds inserted at the first eight nodes primarily respond to apical dominance mechanisms. In CBA vines, retaining the shoot tip and the subtending young leaves prevented dormant bud release even in the presence of CK application, suggesting that exogenous CK alone cannot overcome paradormancy, if the shoot apex remains intact. Indeed, paradormancy is exerted by the shoot apex and lateral shoots, where the apex controls the phytomers below [33,34,35,36]. In our study, dormant buds acted as prompt buds and developed once apical dominance and the paradormancy status were removed.
The milder forcing rate observed in FR vines may be attributed to lower endogenous CK levels than FBA vines. Indeed, once trimming removed the main AUX sources (tips and young leaves), in FR some buds stayed dormant. In absence of exogenous CK application, FR vines relied solely in their endogenous CK, evidently insufficient to guarantee a dormancy release comparable to FBA vines. These findings confirm that CK is a pivotal contributor to budbreak during paradormancy, as previous forcing studies reported the endogenous CK content increases steadily after 7 and 14 days from trimming [37]. In our experiment CK was applied at the trimming application, sourcing the endogenous CK pool before the natural accumulation occurred. Indeed, exogenous CK improved bud dormancy release, vegetative growth and yield of forced vines. Forced yield of FBA vines was 53% higher compared to FR, driven by an increased number of clusters and a greater cluster mass. The improved yield of forced grapes in FBA was also linked to the higher shoot fruitfulness than FR. Shoot fruitfulness was higher in both forced shoots than primary ones. Forcing delayed inflorescence development of both forced crops to a warmer and drier period (DOY 198) than primary grapes (DOY 149). Indeed, the cool and wet conditions in 2024 springtime could have impaired the primary inflorescences development in the grapevine [38], explaining the reduced number of inflorescences of primary grapes.
FBA vines experienced a further improvement in the shoot fruitfulness of forced grapes than FR vines. This may be linked with dormant bud fruitfulness, suggesting the involvement of CK. In our experiment, the floral induction was likely ongoing since treatments were applied after the fruit set (BBCH 73). Floral initiation and differentiation are reputed to span from early formation of the bud up to veraison [39]. In FBA vines, the application could have increased endogenous CK, promoting floral induction and differentiation in forced buds. In this regard, Crane et al., [25] reported CK upregulates genes linked to the formation of inflorescences and also reported higher levels of CK in the most fruitful buds. In addition, a higher ratio of zeatin-riboside (an endogenous CK) on gibberellins could increase the number of inflorescences after pinching and chlormequat applications in cv. Summer Black (V. labrusca x V. vinifera) buds [40]. CK also enhances the development of inflorescences [40,41,42] and the flowering process [43]. Double cropping in temperate climates involves a shorter period between dormant bud formation and bud forcing compared to a standard single-crop system, which result in incomplete floral differentiation and smaller clusters than primary grapes, as confirmed by our results and reported by previous studies [1,2,11,43,44,45,46]. Our study highlights the application of CK as a strategy to increase forced clusters mass, compensating the smaller forced inflorescence.
NAA application restored a status of an apical dominance condition in FNAA vines that was sufficient to inhibit the development of a significant fraction of buds, compared to FR vines. In fact, the rate of forced budburst in FNAA was 21% and shoots were unfruitful. From a practical perspective, our work demonstrates that the balance between CK and AUX is a crucial factor to ensure the success of bud forcing. The pending question is how to practically manage such a balance, without exogenous CK applications. If AUX control is easily removed by trimming primary shoots and removing lateral shoots [22], changing the endogenous CK level represents a more complicated task. In unforced canopies, endogenous CK has been reported to gradually decrease during the season [37]. Additionally, rootstock type has been shown to affect CK content in xylem exudates and the budburst rate of cv. Thompson seedless [47]. Thus, endogenous CK can be affected both in time and based on plant material. Similarly, adjusting the node height when trimming the main cane may impact the maturation state and the hormonal balance of the buds at the time of forcing [5]. All these elements should be considered to tune forcing application and maximize forcing success rate. Previous studies showed different outcomes in forcing success rate and final forced to primary shoots ratio, depending on cultivar and time of forcing [1,2,44]. Bud hormonal balance may be one of the factors of such variability.
Forced yield of FR vines aligned with previous field studies [1,4,44]. Both FR and FBA treatments produced a higher total yield (primary + forced) than UC, CBA and FNAA vines. Interestingly, the application of CK improved yield without negatively altering fruit composition (Table 4), since yield and LA increased proportionally in FBA vines, leading to vine balances similar to FR. Despite the extent of the trimming, both forced treatments gradually restored vine balance to optimal ranges for berry ripening [48], avoiding suboptimal TSS and TA concentrations that can result from impaired vine balance [49]. Our study suggests that forcing can be enhanced without relying on extreme forcing conditions, such as the complete removal of primary leaves and grapes [12,43] and may also optimize the higher water and nutrient inputs required to force vine regrowth and produce two crops on the same vine [50].
In both FR and FBA, postponing the forced crop ripening to a cooler period led to higher TA levels [51], especially for malate [2,3]. In fact, vines of cv. ‘Ortrugo’ are particularly prone to losing acidity during warm seasons [52]. When other summer pruning strategies failed to maintain acidity [53], in our study, the forced crop preserved acidity well above the minimum threshold of 6.5 g/L for sparkling wine production. Overall, the ripening of forced grapes was less dependent from bud forcing rate but closely tied to environmental factors and vine vegetative growth, including relatively warm late-season temperatures, a more efficient (i.e., younger) photosynthetic apparatus, and the increased LA/Y ratio of the forced crop.
5. Conclusions
The results provide new insights into the same-season bud forcing in Vitis vinifera grapes, highlighting the role of paradormancy. The removal of shoot tip combined with the application of BA lead to a significant increase in forced budburst and forced yield without affecting fruit composition compared to FR treatment. Conversely, NAA inhibited the development of a complete forced canopy—only few shoots developed, leaving most buds dormant and unproductive. Identifying the optimal endogenous CK in relation to the forcing rate could enhance double cropping performances without the need for exogenous CK applications. These findings provide a practical approach to mitigating the challenges of hot and early ripening seasons, improving the adaptability of grape cultivation to a warming climate. The preliminary nature of our study considered the 2024 vegetative season. The promising results lay the foundations for further experimentation of CK to test the feasibility of Vitis vinifera double cropping over multiple vintages. Future research should focus on testing exogenous CK application on different grape cultivars and how endogenous CK could ease bud dormancy release and facilitate the double cropping technique in temperate climates.
Author Contributions
Conceptualization, F.D.Z. and S.P.; Data curation, F.D.Z., T.F. and S.P.; Funding acquisition, T.F. and S.P.; Investigation, H.T. and G.C.; Methodology, F.D.Z., T.F. and S.P.; Visualization, F.D.Z., T.F. and S.P.; Writing—original draft, F.D.Z., T.F. and S.P.; Writing—review and editing, H.T. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This study was supported by the Doctoral School on the Agro-Food System (Agrisystem) of the Università Cattolica del Sacro Cuore (Italy).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors want to thank Maria Giulia Parisi for the technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CK | Cytokinins |
| AUX | Auxins |
| UC | Unforced Control |
| CBA | Control + 6-Benzyladenine |
| FR | Forced |
| FBA | Forced + 6-Benzyladenine |
| FNAA | Forced + Naphthaleneacetic acid |
| TSS | Total Soluble Solids |
| TA | Titratable Acidity |
References
- Poni, S.; Gatti, M.; Tombesi, S.; Squeri, C.; Sabbatini, P.; Lavado Rodas, N.; Frioni, T. Double Cropping in Vitis vinifera L. Pinot Noir: Myth or Reality? Agronomy 2020, 10, 799. [Google Scholar] [CrossRef]
- Poni, S.; Del Zozzo, F.; Santelli, S.; Gatti, M.; Magnanini, E.; Sabbatini, P.; Frioni, T. Double cropping in Vitis vinifera L. cv. Pinot Noir: Agronomical and physiological validation. Aust. J. Grape Wine Res. 2021, 27, 508–518. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Sanz, F.; Yeves, A.; Gil-Muñoz, R.; Martínez, V.; Intrigliolo, D.S.; Buesa, I. Forcing bud growth by double-pruning as a technique to improve grape composition of Vitis vinifera L. cv. Tempranillo in a semi-arid Mediterranean climate. Sci. Hortic. 2019, 256, 108614. [Google Scholar] [CrossRef]
- Lavado Rodas, N.; Hernández, D.U.; Cardona, D.M.; Ramírez, L.A.M.; Losada, M.H.P.; Sánchez, M.E.V. Forcing vine regrowth under different irrigation strategies: Effect on polyphenolic composition and chromatic characteristics of cv. Tempranillo wines grown in a semiarid climate. Front. Plant Sci. 2023, 14, 1128174. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, K.; Que, Y.; Li, Y. Grapevine double cropping: A magic technology. Front. Plant Sci. 2023, 14, 1173985. [Google Scholar] [CrossRef]
- Li-Mallet, A.; Rabot, A.; Geny, L. Factors controlling inflorescence primordia formation of grapevine: Their role in latent bud fruitfulness? A review. Botany 2016, 94, 147–163. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endo-, Para-, and Ecodormancy: Physiological Terminology and Classification for Dormancy Research. Hortscience 1987, 22, 371–377. [Google Scholar] [CrossRef]
- Or, E. Grape Bud Dormancy Release—The Molecular Aspect. In Grapevine Molecular Physiology & Biotechnology; Springer: Dordrecht, The Netherlands, 2009; pp. 1–29. [Google Scholar] [CrossRef]
- Lavee, S.; May, P. Dormancy of grapevine buds—Facts and speculation. Aust. J. Grape Wine Res. 1997, 3, 31–46. [Google Scholar] [CrossRef]
- Bugnon, F.; Bessis, R. Biologie de la Vigne: Acquisitions Récentes et Problèmes Actuels; Masson: Paris, France, 1968. [Google Scholar]
- Martinez De Toda, F.; Garcia, J.; Balda, P. Preliminary results on forcing vine regrowth to delay ripening to a cooler period. VITIS-J. Grapevine Res. 2019, 58, 17–22. [Google Scholar] [CrossRef]
- Gu, S.; Jacobs, S.D.; McCarthy, B.S.; Gohil, H.L. Forcing vine regrowth and shifting fruit ripening in a warm region to enhance fruit quality in ‘Cabernet Sauvignon’ grapevine (Vitis vinifera L.). J. Hortic. Sci. Biotechnol. 2012, 87, 287–292. [Google Scholar] [CrossRef]
- Noriega, X.; Pérez, F.J. Cell cycle genes are activated earlier than respiratory genes during release of grapevine buds from endodormancy. Plant Signal. Behav. 2017, 12, e1321189. [Google Scholar] [CrossRef]
- Pérez, F.J.; Noriega, X. Sprouting of paradormant and endodormant grapevine buds under conditions of forced growth: Similarities and differences. Planta 2018, 248, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Leonel, S.; Tecchio, M.; Cóser, G. Dormancy Breaking of the Fig Tree with Hydrogen Cyanamide and Garlic Extrate. Br. J. Appl. Sci. Technol. 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Lombard, P.J.; Cook, N.C.; Bellstedt, D.U. Endogenous cytokinin levels of table grape vines during spring budburst as influenced by hydrogen cyanamide application and pruning. Sci. Hortic. 2006, 109, 92–96. [Google Scholar] [CrossRef]
- Jackson, J.E. The Biology of Apples and Pears; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar] [CrossRef]
- Baldini, E. Arboricoltura Generale; CLUEB: Bologna, Italy, 1986. [Google Scholar]
- Abo-Hamed, S.; Collin, H.A.; Hardwick, K. Biochemical and physiological aspects of leaf development in cocoa (Theobroma cacao). New Phytol. 1981, 89, 191–200. [Google Scholar] [CrossRef]
- Thimann, K.V.; Skoog, F.; William, G. On the inhibition of bud development and other functions of growth substance in vicia faba. Proc. R. Soc. London. Ser. B Contain. Pap. Biol. Character 1934, 114, 317–339. [Google Scholar] [CrossRef]
- Shimizu-Sato, S.; Tanaka, M.; Mori, H. Auxin–cytokinin interactions in the control of shoot branching. Plant Mol. Biol. 2009, 69, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.G. Apical dominance. Bot. Rev. 1991, 57, 318–358. [Google Scholar] [CrossRef]
- Kurakawa, T.; Ueda, N.; Maekawa, M.; Kobayashi, K.; Kojima, M.; Nagato, Y.; Sakakibara, H.; Kyozuka, J. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 2007, 445, 652–655. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar]
- Crane, O.; Halaly, T.; Pang, X.; Lavee, S.; Perl, A.; Vankova, R.; Or, E. Cytokinin-induced VvTFL1A expression may be involved in the control of grapevine fruitfulness. Planta 2012, 235, 181–192. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Liu, J.; Sherif, S.M. Hormonal Orchestration of Bud Dormancy Cycle in Deciduous Woody Perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Senning, M.; Hedden, P.; Sonnewald, U.; Sonnewald, S. Reactivation of meristem activity and sprout growth in potato tubers require both cytokinin and gibberellin. Plant Physiol. 2011, 155, 776–796. [Google Scholar] [CrossRef]
- Faust, M.; Erez, A.; Rowland, L.J.; Wang, S.Y.; Norman, H.A. Bud dormancy in perennial fruit trees: Physiological basis for dormancy induction, maintenance, and release. Hortscience 1997, 32, 623–629. [Google Scholar] [CrossRef]
- Winkler, A.J. General Viticulture, 4th ed.; University of California Press: Berkeley, CA, USA, 1974; ISBN 9780520025912. [Google Scholar]
- Hargreaves, G.H.; Samani, Z.A. Reference Crop Evapotranspiration from Temperature. Applied Engineering in Agriculture. Am. Soc. Agric. Biol. Eng. 1985, 1, 96–99. [Google Scholar]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Baker, N.R.; Oxborough, K. Chlorophyll fluorescence as a probe of photosynthetic productivity. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 65–82. [Google Scholar]
- Blažková, J.; Krekule, J.; Macháčková, I.; Procházka, S. Auxin and Cytokinins in the Control of Apical Dominance in Pea — A Differential Response Due to Bud Position. J. Plant Physiol. 1999, 154, 691–696. [Google Scholar] [CrossRef]
- Brown, C.L.; McAlpine, R.G.; Kormanik, P.P. Apical Dominance and Form in Woody Plants: A Reappraisal. Am. J. Bot. 1967, 54, 153–162. [Google Scholar] [CrossRef]
- Crabbé, J.J. Correlative Effects Modifying the Course of Bud Dormancy in Woody Plants. Z. Pflanzenphysiol. 1984, 113, 465–469. [Google Scholar] [CrossRef]
- Pou, A.; Balda, P.; Albacete, A.; Martínez De Toda, F. Forcing vine regrowth to delay ripening and its association to changes in the hormonal balance. VITIS-J. Grapevine Res. 2019, 58, 95–101. [Google Scholar] [CrossRef]
- Srinivasan, C.; Mullins, M.G. Reproductive Anatomy of the Grape-vine (Vitis vinifera L.): Origin and Development of the Anlage and its Derivatives. Ann. Bot. 1976, 40, 1079–1084. [Google Scholar] [CrossRef]
- Carmona, M.J.; Chaïb, J.; Martínez-Zapater, J.M.; Thomas, M.R. A molecular genetic perspective of reproductive development in grapevine. J. Exp. Bot. 2008, 59, 2579–2596. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, B.; Lin, L.; Cheng, G.; Zhou, S.; Xie, S.; Shi, X.; Cao, M.; Zhang, Y.; Bai, X. Evolutionary, interaction and expression analysis of floral meristem identity genes in inflorescence induction of the second crop in two-crop-a-year grape culture system. J. Genet. 2018, 97, 439–451. [Google Scholar] [CrossRef]
- Srinivasan, C.; Mullins, M.G. Control of Flowering in the Grapevine (Vitis vinifera L.): Formation of Inflorescences in Vitro by Isolated Tendrils. Plant Physiol. 1978, 61, 127–130. [Google Scholar] [CrossRef]
- Srinivasan, C.; Mullins, M.G. Flowering in Vitis: Effects of genotype on cytokinin-induced conversion of tendrils into inflorescences. VITIS-J. Grapevine Res. 1980, 19, 293. [Google Scholar] [CrossRef]
- Carmo Vasconcelos, M.; Greven, M.; Winefield, C.S.; Trought, M.C.T.; Raw, V. The Flowering Process of Vitis vinifera: A Review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar] [CrossRef]
- Martínez de Toda, F. Grapevine double cropping: A reality, not a myth. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Oliver-Manera, J.; García-Tejera, O.; Mata, M.; Girona, J. Cumulative response of Tempranillo vines to the crop forcing technique and pre-forcing and post-veraison water stress in terms of yield and grape and wine quality. Irrig. Sci. 2023, 41, 571–587. [Google Scholar] [CrossRef]
- Oliver-Manera, J.; García-Tejera, O.; Mata, M.; Girona, J. Long-Term Study of the Crop Forcing Technique on cv. Tempranillo (Vitis vinifera L.) Vines and Suggested Irrigation Strategies to Improve Water Use Efficiency of Forced Vines. Agronomy 2024, 14, 130. [Google Scholar] [CrossRef]
- Nikolaou, N.; Koukourikou, M.; Karagiannidis, N. Effects of various rootstocks on xylem exudates cytokinin content, nutrient uptake and growth patterns of grapevine Vitis vinifera L. cv. Thompson seedless. Agronomie 2000, 20, 363. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf Area/Crop Weight Ratios of Grapevines: Influence on Fruit Composition and Wine Quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar] [CrossRef]
- Del Zozzo, F.; Canavera, G.; Pagani, S.; Gatti, M.; Poni, S.; Frioni, T. Post-Spring Frost Canopy Recovery, Vine Balance, and Fruit Composition in cv. Barbera Grapevines. Aust. J. Grape Wine Res. 2022, 2022, 6596021. [Google Scholar] [CrossRef]
- Poni, S.; Frioni, T.; Gatti, M. Summer pruning in Mediterranean vineyards: Is climate change affecting its perception, modalities, and effects? Front. Plant Sci. 2023, 14, 1227628. [Google Scholar] [CrossRef]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef]
- Frioni, T.; Bertoloni, G.; Squeri, C.; Garavani, A.; Ronney, L.; Poni, S.; Gatti, M. Biodiversity of Local Vitis vinifera L. Germplasm: A Powerful Tool Toward Adaptation to Global Warming and Desired Grape Composition. Front. Plant Sci. 2020, 11, 608. [Google Scholar] [CrossRef]
- Gatti, M.; Garavani, A.; Cantatore, A.; Parisi, M.G.; Bobeica, N.; Merli, M.C.; Vercesi, A.; Poni, S. Interactions of summer pruning techniques and vine performance in the white Vitis vinifera cv. Ortrugo. Aust. J. Grape Wine Res. 2015, 21, 80–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).