Abstract
Guizhou Province is one of the regions in China where macadamia is cultivated. The area is characterized by prominent karst landforms, with uneven distribution of precipitation and utilizable water resources, which poses significant challenges to macadamia production. To explore the effects of different drought levels on the anatomical structure and physiological characteristics of macadamia seedlings, and to reveal their adaptation mechanisms and regulatory responses to drought stress, this study established a drought stress experiment on O.C (Own Choice) macadamia seedlings. The seedlings were subjected to stress in a 25% PEG-6000 solution for 0 h (CK), 24 h, 36 h, 48 h, and 72 h, and cellular structural features of stems and leaves were measured, as well as physiological and biochemical indices. The results indicated that macadamia seedlings gradually exhibited dehydration and chlorosis with prolonged drought stress. At 72 h of drought stress, root water potential, leaf water potential, chlorophyll content, relative water content, and root activity decreased by 353%, 98%, 44%, 72%, and 79%, respectively. Leaf thickness, palisade tissue thickness, and spongy tissue thickness were reduced by 19%, 33%, and 29%, respectively. Stomatal density increased by 50%, while stomatal aperture, vessel diameter, and cell wall thickness significantly decreased. Photosynthesis was markedly impaired: Pn, Tr, Gs, WUE, Fv/Fm, qP, and ΦPSII declined by 73%, 25%, 67%, 64%, 0.23, 60%, and 84%, respectively, whereas Ci and qN increased by 107% and 11%, respectively. Cell membranes began to sustain damage after 24 h of drought stress, with electrolyte leakage and MDA content rising by 266% and 672%, respectively, at 72 h. Prolonged drought stress reduced IAA, CTK, and GA levels by 37%, 33%, and 16%, respectively, while ABA content increased by 48%. To counteract drought stress, seedlings activated osmotic adjustment and reactive oxygen species (ROS) scavenging mechanisms. Osmolyte content significantly increased with stress duration, reaching 61%, 73%, 697%, and 107% increments in SS, SP, Pro, and betaine at 72 h. Antioxidant enzyme activities initially rose, peaking at 24 h (SOD, POD, CAT, and APX increased by 132%, 288%, 110%, and 46%, respectively), then gradually declined. By 72 h, SOD and APX activities fell below control levels, while POD and CAT remained elevated. These findings demonstrate that under PEG-6000-simulated drought stress, macadamia seedlings alleviate damage by modifying leaf and stem cellular structures and activating antioxidant and osmotic adjustment mechanisms. This study provides a theoretical basis for understanding the physiological mechanisms of macadamia drought stress response.
1. Introduction
Macadamia (Macadamia integrifolia) is native to Australia and is known as the “King of Nuts” because of its high nutritional content and value [1,2], and is popular among consumers and growers. China began introducing macadamia from Australia in the 1970s, and due to its significant ecological and economic benefits, it has been widely cultivated. After nearly 50 years of development, macadamia is now grown in regions such as Yunnan, Guangxi, Guangdong, and Guizhou Province in China. By the end of 2020, the global macadamia cultivation area reached 6.05 million acres, with China accounting for 4.7 million acres, making it the world’s largest producer of macadamia [3,4,5,6]. However, 51.4% of China’s total land area faces drought, often characterized by uneven precipitation and seasonal dryness [7]. For example, Guizhou Province in southwestern China, with its mountainous terrain and unique karst landscape, experiences extremely uneven distribution of precipitation and available water resources, making it one of the most severe and extreme drought-prone regions [8]. Despite these challenges, macadamia can grow and fruit normally in such environments.
Drought is a natural disaster caused by abnormally low precipitation, with widespread impacts on the environment, economy, and society. Agriculture is particularly sensitive to drought due to its reliance on precipitation and transpiration [9,10]. Drought reduces the available water in plants, and in severe cases, leads to cell dehydration, disruption of membrane structures and proteins, and ultimately cell death. Drought also reduces CO2 diffusion in the mesophyll, lowering photosynthetic efficiency, while high temperatures accompanying drought exacerbate photorespiration, consuming energy and releasing CO2, thereby reducing net photosynthesis [11]. Strong light and impaired carbon fixation cause an imbalance in leaf redox status, generating reactive oxygen species (ROS) and damaging photosynthetic mechanisms [12]. Plants accumulate low-molecular-weight compounds such as sugars, amino acids, and glycine betaine to maintain cellular water content, stabilize proteins and membranes, and scavenge ROS [13,14,15]. Antioxidants like superoxide dismutase and catalase play a critical role in ROS clearance [16,17]. At the hormonal level, plants regulate stomatal closure through ABA synthesis to prevent water loss and enhance drought tolerance by accumulating osmoregulatory substances and antioxidant molecules [18,19]. Studies show that drought induces auxin synthesis, increasing root growth, and exogenous application of auxin enhances drought tolerance [20]. Drought also reduces cytokinin levels to minimize water consumption [21,22], and inhibiting gibberellin synthesis can similarly enhance drought tolerance [23]. These studies reveal the regulatory mechanisms of plant hormones in drought responses.
Macadamia is a tall evergreen tree that often faces challenges such as water scarcity during its growth. Although it shows significant growth in the seasonally arid regions of southeastern Australia, high temperatures (>30 °C) and low rainfall can severely reduce tree growth and nut yield [24,25,26]. Previous studies have demonstrated that macadamia (Macadamia integrifolia) adopts an isohydric strategy, rapidly closing stomata under drought stress to minimize water loss and xylem embolism [27]. Additionally, macadamia has a dense root system that increases the root surface area, enabling greater absorption of water and nutrients [28]. Additionally, its leathery leaves and highly lignified xylem contribute to structural resilience. However, there are few studies on the stem and leaf anatomy of macadamia trees under drought stress, and whether there is a special response mechanism in Australia needs to be further explored. O.C is the most widely cultivated macadamia variety in China, known for its relatively strong drought resistance, stable yield, and quality. Therefore, we selected O.C as the experimental material to conduct a PEG-simulated drought stress experiment, investigating the physiological responses of macadamia to drought stress [29]. The aim is to provide an accurate and scientific evaluation method for macadamia’s drought tolerance and to offer reliable theoretical support for its cultivation and the breeding of drought-resistant varieties.
2. Materials and Methods
2.1. Experimental Materials and Drought Treatment
This study used seeds of O.C macadamia as experimental materials, which were collected from the germplasm resource nursery of the Subtropical Crops Research Institute in Guizhou, China (25.3′23″ N, 104.53′44″ E). After removing the green husk, the seeds were soaked in a 1000-fold dilution of 50% carbendazim wettable powder for 2 h, then drained and immediately sown on a seedbed covered with 20 cm of river sand. When the seedlings reached the 4-leaf stage, they were transplanted into porous plastic pots (19 × 18 cm) containing 3 kg of garden soil, with one seedling per pot. During the cultivation period, each pot was treated with 200 mL of fertilizer diluted 1500 times (N:P:K = 24:10:16) on a weekly basis. After six months of cultivation, seedlings with uniform growth were selected for drought stress treatment.
After removing the seedlings and washing off the soil, a 25% PEG-600 solution was prepared using 1/2 Hoagland nutrient solution as the solvent to simulate drought stress. The macadamia seedlings were subjected to stress in the PEG solution for 0 h, 12 h, 36 h, 48 h, and 72 h, totaling five treatments, with 0 h serving as the control. Each treatment was conducted with three biological replicates, and each replicate involved five seedlings.
2.2. Measurement Indicators and Methods
2.2.1. Observation of Seedling Morphology
Using a digital camera (Canon EOS 90D, Tokyo, Japan), one seedling was fixedly selected in each treatment for taking photographs to record morphological changes in seedlings, while functional leaves in the apical 3rd~5th were selected in each treatment for taking photographs to record morphological changes in leaves.
2.2.2. Observation of Leaf and Stem Structure
The anatomical structures of leaves and stems were analyzed following the method described by Yao et al. with slight modifications [30]. For each seedling, functional leaves from the 3rd to 5th whorls at the top were selected, and the midribs were removed. A 1 cm × 1 cm section from the middle of the leaf was taken as the leaf sample. For stems, a 1~2 cm segment from the middle portion of the stem, after removing the leaves, was collected as the stem sample. Each treatment was repeated three times. The leaf and stem samples were immersed in FAA fixative (75% alcohol/formaldehyde/glacial acetic acid = 90:5:5) for preservation. The samples were then removed in a fume hood, trimmed with a scalpel, dehydrated, embedded in paraffin, and sectioned. Tissue sections were stained with safranin and fast green. An inverted fluorescence microscope (Revolve RVL-100-G, Cerritos, CA, USA) was used to observe and compare the microstructures of leaves and stems under different treatments. Image J (National Institutes of Health, Bethesda, MD, USA) software was employed to measure and analyze the thickness of leaves, palisade tissue, and spongy tissue, as well as the vessel density, vessel diameter, and wall thickness of the xylem in stem segments.
The ultrastructure of stomata was observed using a scanning electron microscope [31], and leaves from the five treatments were immersed in 2.5% glutaraldehyde solution and fixed in a refrigerator at 4 °C for 12 h. Leaves were washed three times with 0.1 mol/L PBS buffer, and dehydrated in graded stages with ethanol solutions (30, 50, 70, 80, 90, 95, and 100%), each time for 20 min. The leaves were washed with isoamyl acetate at 4 °C for 30 min, followed by ethanol solution for 20 min; the samples were replaced with isoamyl acetate at 4 °C for 30 min, and then the dislodged samples were placed in sample cages to be dried with a CO2 critical point dryer (Quorum K850); the dried samples were cut into appropriate sizes with a razor blade and pasted on a sample stage with the lower skin facing upwards, then sprayed with gold coating using an ion sputtering device for 40 s, and placed under a scanning electron microscope (ZEISS EIGMA, Jena, Germany) for observation.
2.2.3. Measurement of Physiological and Biochemical Indicators
The water potential of roots and leaves was determined using a plant water potential tester (LB-PW-II, Hangzhou Lvbo Instrument Co., Ltd., Hangzhou, China). Seedlings were cut from 10 cm above ground and used to determine the water potential of roots and leaves, respectively. Because the petiole of macadamia leaves was too short to be used to determine the water potential, the whole above-ground part of the seedling was inserted into the tester for the determination of the water potential.
The relative water content of the leaves (RWC) was measured according to the method used by Wei et al. [32]. One leaf was collected for each treatment, quickly put into an aluminum box with known weight, and its fresh weight (mf) recorded. Then, the leaves were immersed in distilled water for about 24 h and weighed to obtain sample saturated fresh weight (mt), and then dried to constant weight to obtain dry weight (md), calculated by the following equation:
where mf, mt, and md denote fresh weight, saturated weight, and dry weight, respectively.
Conductivity (C) of macadamia nut leaves was measured according to the method of Barranco et al. with simple adjustments [33]. A quantity of 1 g of fresh leaves was cut, avoiding the leaf veins; contaminants and residues were removed from the leaves with distilled water, the leaves were added to test tubes containing 10 mL of distilled water under vacuum for 10 min and left to stand at 25 °C for 1 h to measure the conductivity (L1), then the tubes were placed in a water bath at 100 °C for 10 min and cooled down to 25 °C for measurement of the final conductivity (L2). The REL was determined using a conductivity meter (DDS-307A, Hangzhou Qiwei Instrument Co., Ltd., Hangzhou, China). The conductivity was calculated as follows, where L0 represents the conductivity of deionized water:
Root activity (RA) was determined using the TTC method. After cleaning the root samples, the amount of TTC reduction was determined using a spectrophotometer and chemical reagents (ethyl acetate, safeties, 1% TTC solution, 0.1 M phosphate buffer at pH 7.5, and 2N sulfuric acid), and the root activity was calculated using the following formula.
TTCr, Wr, and T indicate the amount of TTC reduction [34], root sample weight, and reaction time, respectively.
Total chlorophyll content: total chlorophyll content (Chl) was determined with reference to the method of Kang et al. and calculated according to the following equation [29]:
chlorophyll a = 13.95 A665–6.88 A649
chlorophyll b = 24.96 A649–7.32 A665
Vt denotes constant volume, FW denotes fresh leaf weight.
Photosynthetic parameters: Net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), and transpiration rate (Tr) were measured using an Li-6400XT, and calculation of water use efficiency (WUE) from photosynthetic and transpiration rate was performed. Gas exchange analysis for measuring photosynthesis was conducted using ambient CO2 from the environment [35]. Three to four whorls of leaves at the top of the plants were selected for measurement, and representative growth was chosen for each treatment, including three replications. Photosynthetic parameters were measured from 8:50 a.m. to 11:00 a.m. The LED light intensity was controlled at 800 μmol m−2s−1. A total of 36 h of the treatments in this experiment were in the nighttime, and the measurement of photosynthetic parameters was not performed.
For chlorophyll fluorescence measurements, leaves were darkened using leaf clips for 30 min, and then chlorophyll fluorescence parameters were measured using a pulse amplitude modulated fluorometer (PAM-2500, Walz, Germany). The saturating light pulse was set to approximately 8000 μmol m−2s−1 for 0.8 s, and the photochemical light was set to 269 μmol m−2s−1. Plants were acclimatized to this photochemical light for 5 min. The maximum quantum yield (Fv/Fm), the actual photochemical efficiency (ΦPS II), the photochemical burst coefficient (qP), and the nonphotochemical burst coefficient (qN) of Photosystem II were measured, and all fluorescence parameters were given automatically by the instrument.
The hormone contents were determined by HPLC with reference to the method of Zhou et al. [36]. A 1 g frozen sample was ground in an ice bath, 20 mL of 80% pre-cooling methanol was added, sealed, and placed in a 4 °C refrigerator overnight. The extract was filtered and washed twice with 10 mL methanol. After filtration, it was combined with the extract and evaporated at 40 °C until there was no methanol residue. The remaining water phase was transferred to a triangular flask, extracted and decolorized twice with 30 mL petroleum ether. The ether phase was discarded. Then, 0.01 g PVPP (crosslinked polyvinylpyrrolidone) was added to the ultrasonic for 30 min and filtered. Afterwards, it was extracted with 30 mL ethyl acetate 3 times, combined with the lipid phase, and evaporated at 40 °C. The residue was dissolved with methanol (chromatographically pure) and diluted to 2 mL. The test solution was filtered through a 0.45 μm microporous membrane and stored in a refrigerator at 4 °C. Then, the contents of IAA, CTK, GA, and ABA were determined by HPLC.
MDA was determined following the method used by Wu et al. [37]. Using the thiobarbituric acid method, 1 g fresh sample was weighed, 2 mL 10% TCA solution and a small amount of quartz sand were ground in a mortar, 8 mL TCA was added and then fully ground, the mixture was transferred to a centrifuge tube at 4000 r/min for 10 min, and the supernatant was taken as the extraction liquid for use. A quantity of 2 mL was extracted, 2 mL TBA was added and fully mixed in, the mixture was boiled in a water bath for 15 min, then cooled and centrifuged at 4000 r/min for 10 min, after which the supernatant was assessed at 450 nm, 532 nm, and 600 nm wavelengths to determine its light absorption value. The values were then substituted into the following formula for calculation.
V is the volume of extracted liquid and FW is the fresh weight of the sample.
Soluble sugars (SS), soluble proteins (SP), proline (Pro), betaine (BA), and the activities of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) were determined by enzyme-linked immunoabsorbent assay, following the instructions of the kit, which was purchased from Shanghai Tongwei Biotechnology Co., Ltd., Shanghai, China.
2.3. Statistical Analysis of the Data
All data processing and the creation of line charts were conducted in Microsoft Excel 2010. All data were subjected to one-way ANOVA using SPSS v22.0 (IBM Inc., Armonk, NY, USA), and the measurements of anatomical structures were performed using Image J 1.53a.
3. Results
3.1. Effects of Drought Stress on the Morphology of Macadamia Seedlings
In order to understand the effects of drought stress on the morphology of macadamia seedlings, the morphology and leaf phenotype of seedlings from 0 h to 72 h were recorded. The results are shown in Figure 1. The seedling morphology was normal and the leaf blades showed normal green coloration at 0 h. The new leaves at the top lost water and curled, and the old leaves at the bottom showed sporadic brown spots on the surface at 24 h; after 36 h of drought stress, the angle of seedling leaf blade pinch became smaller, the new leaves at the top lost green coloration, the old leaves of the bottom one to two whorls started to dehydrate and lose green coloration, brown spots on the surface of the leaf blades increased, and the brown area was enlarged. The angle of seedling blade pinch continued to become smaller, the top new leaves dried up, the top two to three rounds of leaves lost water and whitened, and the bottom one to two rounds of leaves dried up at 48 h. The angle of seedling leaves continued to become smaller, the overall direction was upward, and the bottom one to three rounds of leaves dried up at 72 h.

Figure 1.
Morphological changes in macadamia seedlings under drought stress.
Drought stress alters the microstructure of plant leaves and stems. As shown in Figure 2 and Table 1, leaf thickness decreases with prolonged drought duration. Compared to 0 h, by 72 h of drought stress, leaf thickness, palisade tissue thickness, and spongy tissue thickness decreased by 19%, 33%, and 29%, respectively. Palisade tissue and spongy tissue are only part of the composition of leaves, and other parts of leaves may not change so significantly after water loss, resulting in a less significant reduction in leaf thickness. In contrast, stomatal density increased with prolonged drought stress, rising by 50% at 72 h compared to 0 h. Stomata remained open during the first 36 h of drought stress but gradually closed thereafter. Stomatal length shortened within the first 24 h of drought stress, decreasing by 21% compared to 0 h, while stomatal width increased by 19%. Subsequently, stomatal length stabilized briefly before increasing again, whereas stomatal width decreased. Vessel diameter and wall thickness increased during the first 36 h of drought stress, rising by 24% and 15%, respectively, compared to 0 h, before showing a declining trend. In the anatomical structure of macadamia leaves and stems, leaf thickness, palisade tissue thickness, and vessel wall thickness responded more slowly to drought stress, showing significant changes until after 36 h compared to 0 h.
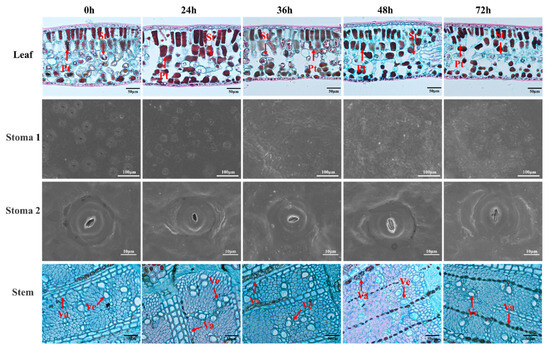
Figure 2.
Microstructure of macadamia leaf and stem under drought stress. Pt and St represent palisade tissue and spongy tissue, respectively, and Va and Ve represent catheter and medullary rays, respectively.

Table 1.
Effects of drought stress on the anatomical structure of macadamia leaves and stems.
3.2. Effects of Drought Stress on Water Potential, Relative Water Content, Root Activity, Conductivity and MDA of Macadamia Seedings
The water content of plants under drought stress decreases and cells are damaged. As shown in Figure 3, root water potential, leaf water potential, relative water content and root activity of macadamia decreased with drought duration and reached the lowest value at 72 h of drought stress, while conductivity and MDA increased with increasing drought stress duration and reached the maximum value at 72 h of drought stress. Compared with 0 h, the root water potential, leaf water potential, relative water content, and root activity decreased by 353%, 98%, 72%, and 79%, respectively, while the relative conductivity and MDA increased by 266% and 672%, respectively, at 72 h. Among them, leaf water potential responded slower, and was significantly reduced at 36 h of drought stress compared with 0 h.
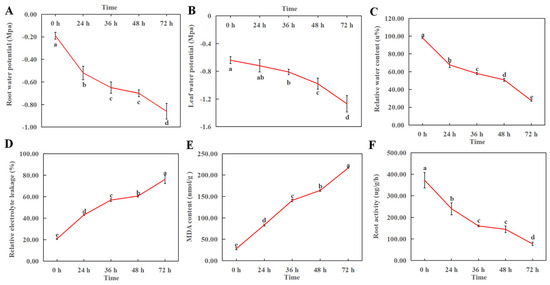
Figure 3.
Effects of drought stress on root water potential (A), leaf water potential (B), leaf relative water content (C), root activity (D), leaf relative electrical conductivity (E) and MDA (F) of macadamia. The short vertical line in the folded line indicates the error bar. Different lowercase letters indicate significant differences at each point in time (p < 0.05).
3.3. Effects of Drought Stress on the Photosynthetic Characteristics of Macadamia Seedling Leaves
Drought stress adversely affects the photosynthetic efficiency of plants. As shown in Table 2, the chlorophyll content and photosynthetic efficiency of macadamia decreased with prolonged drought stress, reaching their lowest values at 72 h of drought stress. In contrast, Ci and qN increased with the duration of drought stress, peaking at 72 h. Compared to 0 h, by 72 h of drought stress, chlorophyll content, Pn, Tr, Gs, WUE, Fv/Fm, qP, and ΦPSII decreased by 44%, 73%, 25%, 67%, 64%, 0.23, 60%, and 84%, respectively, while Ci and qN increased by 107% and 11%, respectively. Among these parameters, Tr, WUE, Fv/Fm, qP, and qN responded more slowly to drought stress, showing significant decreases until after 48 h compared to 0 h.

Table 2.
Chlorophyll and photosynthetic parameters of macadamia seedling leaves under drought stress.
3.4. Effect of Drought Stress on Hormones in Macadamia Seedlings
Hormones play a crucial regulatory role in plant growth and development, and drought stress can significantly alter plant hormone levels. As shown in Table 3, the contents of IAA, CTK, and GA in macadamia seedlings decreased with prolonged drought stress, reaching their lowest values at 72 h, while ABA content increased with the duration of drought stress, peaking at 72 h. Compared to 0 h, the IAA, CTK, and GA content at 72 h decreased by 37%, 33%, and 16%, respectively, whereas the ABA content increased by 48%. Among these, GA responded more slowly to drought stress, showing a significant decrease after 36 h compared to 0 h.

Table 3.
Effects of drought stress on macadamia hormones content.
3.5. Effects of Drought Stress on Osmoregulatory Substances in Macadamia Seedlings
Drought stress disrupts the osmotic balance in plant leaves, leading to an increase in the content of osmotic adjustment substances. As shown in Table 4, the levels of osmotic adjustment substances in macadamia seedlings rose with prolonged drought stress, reaching their maximum values at 72 h. Compared to 0 h, after 72 h of drought stress, soluble sugar content, soluble protein, proline, and BA content increased by 61%, 73%, 696%, and 107%, respectively.

Table 4.
Effect of drought stress on osmoregulatory substances content.
3.6. Effects of Drought Stress on Antioxidant Activity in Macadamia Seedlings
Drought stress can lead to the production of ROS in plants, resulting in oxidative damage to membrane lipids and proteins. Plants mitigate this by increasing both enzymatic and non-enzymatic antioxidants to scavenge ROS. As shown in Table 5, the antioxidant enzyme activities in macadamia respond relatively quickly to drought stress, with all activities peaking at 24 h of drought stress. The activities of the four enzymes initially increased and then decreased with prolonged drought duration, albeit to varying extents. At 24 h of drought stress, the activity of SOD reached 147 U/g, an increase of 132% compared to 0 h. By 36 h, the activity began to decline, and by 48 h, it was significantly lower than at 0 h. At 72 h, the activity dropped to its lowest value of 26 U/g, a decrease of 59% compared to 0 h. The activity of APX increased after 24 h, 36 h, and 48 h of drought stress, with the highest and most stable activities observed at 24 h and 36 h, at 2 U/g and 2 U/g, respectively, representing increases of 46% and 48% compared to 0 h. However, by 72 h, APX activity significantly decreased to below the level at 0 h, a reduction of 9%. The activities of POD and CAT followed a similar trend, peaking at 24 h of drought stress at 112,988 U/g and 602 U/g, respectively, which were increases of 288% and 110% compared to 0 h. Between 36 h and 72 h, the activities of POD and CAT gradually declined, but by 72 h, they remained significantly higher than at 0 h. The total flavonoid content showed a decreasing trend with prolonged drought duration. Compared to 0 h, the total flavonoid content decreased by 29%, 29 %, 32%, and 27% after 24 h, 36 h, 48 h, and 72 h of drought stress, respectively.

Table 5.
Effect of drought stress on antioxidant substances content.
4. Discussion
4.1. Effects of Drought Stress on Anatomy and Physiology
Polyethylene glycol (PEG) is a high-molecular weight, water-soluble polymer that, when dissolved in water, induces osmotic stress in plants and is widely used to induce drought stress responses in plants [38]. In this study, the leaves of macadamia seedlings gradually lost their green coloration with drought duration, and leaf dehydration and browning could be clearly seen from the time of 36 h of stress. From the anatomical and ultrastructural structures, it was observed that the thickness of spongy tissue decreased with increasing drought time, leaf thickness and palisade tissue thickness responded slower and started to decrease until 36 h. The diameter of the stem’s vessel and the thickness of the tube wall increased until 36 h and decreased from 48 h. The width of the stomata increased and then decreased until they closed, and the density of the stomata increased with increasing drought time. Root water potential, leaf water potential, leaf relative water content, and root activity decreased with increasing drought time, and conversely, conductivity increased. In previous studies, drought stress is the first line of defense affecting growth, and plants can improve water transport and reduce water loss by closing stomata, increasing stomatal density, thickening palisade tissues, and thinning spongy tissues [39], which in turn improve water transport [40,41], while at the same time, xylem vessel diameter decreases, vessel density increases, and wall thickness increases to enhance xylem resistance to drought-induced hydraulic failures [42]. In response to deleterious heat and drought stress, leaves prevent plant water loss under drought conditions by reducing water potential; stomatal closure helps plants to maintain leaf water potential but also reduces carbon dioxide uptake, which affects photosynthesis, leading to lipid peroxidation caused by conversion of excess electron fluxes to O2- and ROS that are not scavenged in a timely manner, damage to DNA and cellular proteins as well as leakage of intracellular electrolytes, ultimately destroying the structure and function of the cell membrane [43]. In our study, root activity of macadamia seedlings decreased with increasing drought duration, and conductivity and MDA continued to increase, which is consistent with the performance of potato [44], olive [45] and hemp oak [46].
4.2. Effects of Drought Stress on Photosynthetic Characteristics
Chlorophyll, as the main photosynthetic pigment in plant leaves, reflects the photosynthetic capacity of leaves and overall plant vigor [47]. In this study, the chlorophyll content in the leaves of macadamia seedlings decreased with increasing drought duration: from 0 h to 72 h chlorophyll content decreased by 44.00%, and the photosynthetic capacity of the leaves was also affected with the continuation of the drought, with a significant decrease in Pn, Tr, Gs, and WUE, and a significant increase in Ci, which may be a direct result of the closure of stomata combined with a lack of water to meet their photosynthetic needs [43]. The increase in intercellular carbon dioxide concentration (Ci) was attributed to drought compromising reaction sites such as photosystem II and the antioxidant enzyme system, limiting photosynthesis and carbon dioxide diffusion [48]. Interestingly, Tr and WUE did not change significantly between 0 and 24 h, and were significantly lower than the hourly values at 0 h until 48 h, which may be related to stomatal closure and changes in physiological activities within the leaf.
In general, light energy absorbed by chlorophyll is mainly consumed through electron transfer, chlorophyll fluorescence, and heat dissipation, and there is an inversely proportional relationship between these three pathways. Photosynthesis and heat dissipation can be evaluated and analyzed by qP and qN. The larger the value of qP, the higher the electron transfer activity in PSII, and qN reflects the light energy absorbed by chlorophyll and dissipated in the form of heat; if it is not dissipated in a timely manner, the excessive light energy in the reaction center of PSII will destroy the photosynthetic tissues. ΦPSII represents the actual PSII light energy conversion efficiency, indicating the proportion of light energy entering the PSII reaction center that is used for photochemical reactions [49,50,51]. In this study, seedlings showed a gradual decrease in Fv/Fm, indicating that drought stress reduces the electron transport capacity and primary photochemical activity of PSII, as well as the detrimental effect of excessive excitation energy accumulation on photosynthesis [52]. qP was reduced, which reflects the reduced photosynthetic efficiency and electron transport activity of seedlings [53]; the decrease in qP may be caused by an increase in the proportion of closed PSII reaction centers [54]. ΦPSII is reduced, indicating a blockage in the photosynthetic electron transfer rate of leaves. qN is significantly increased, indicating that excess excitation energy is dissipated in the form of heat, thus protecting PSII [55]. Because stomatal closure weakens carbon fixation and leads to excess energy, it enhances photodamage to the photosystem [56]. We also noted that the values of Fv/Fm, qP and qN were relatively stable between 0 h and 24 h of exposure to drought stress, possibly due to the ability of macadamia nuts to produce certain substances on their own, thus counteracting the adverse effects caused by a certain degree of drought stress.
4.3. Effect of Drought Stress on Hormone Levels
Endogenous hormones, as key regulators and signaling molecules in plant growth and development, exert significant physiological effects even at extremely low concentrations. Under drought stress conditions, plants dynamically modulate the levels of endogenous hormones to coordinate their growth and development processes, thereby minimizing the adverse effects of drought [57]. IAA influences various plant processes, including cell differentiation, root morphology, root gravitropism, seed dormancy, and floral organ development [58]. CTK and IAA regulate many aspects of plant development, often acting as an antagonistic hormone pair: IAA promotes root growth and inhibits shoot growth, while CTK has the opposite effect [59]. In this study, IAA content decreased under drought stress, likely due to the disruption of normal physiological processes affecting auxin synthesis. The reduction in CTK levels slowed shoot growth, thereby reducing water loss. GAs primarily function to promote stem elongation. In this study, GA content in macadamia decreased under drought stress. Nir et al. suggested that reduced GA levels can slow plant growth, mitigating the impact of water scarcity on normal physiological activities and effectively enhancing drought resistance [60]. ABA is a critical hormone that regulates short-term responses (stomatal closure) and long-term adaptations (root structure remodeling) to drought stress [61]. In this study, ABA content in macadamia seedlings increased with prolonged drought stress. Bhusal et al. proposed that drought stress significantly elevates ABA levels in plants, with more severe drought leading to a more pronounced increase in ABA concentration [62]. Under drought conditions, ABA reduces stomatal conductance and significantly lowers the net photosynthetic rate, thereby effectively alleviating the damage caused by drought stress [62].
4.4. Effects of Drought Stress on Osmoregulatory Substances
Osmoregulation is one of the main strategies of plants to cope with drought and salt stress [63], and osmotic potential has been repeatedly shown to decrease during drought stress in order to maintain the expansion of the leaf pulp under the reduced water potential [64]. SS, SP, Pro and BA are important osmoregulators in plants that play an important role in plant stress [65], which are not only involved in maintaining water content and osmoregulation in plants facing drought stress, but also in membrane protection and scavenging of free radicals [66,67]. In the present study, with the prolongation of drought duration, the leaves of macadamia seedlings showed a significant increase in MDA, SS, SP, Pro and BA contents, which all increased gradually; SS content remained in equilibrium between 0 h and 24 h, and started to increase gradually after 36 h, and the rest of the substances had a tendency to increase at each time period. Among these substances, MDA and Pro content changed the most, and from 0 h to 72 h, they increased in the leaves by almost the same multiples, 671.88% and 696.38%, respectively, whereas SS, SP, and BA increased by 60.64%, 72.55%, and 106.58%, respectively. From the range of change, Pro may be the main osmotic regulator of macadamia in response to drought stress, and similar results have been obtained in other crops [68,69,70]. Pro has multiple functions in plants: maintaining the relative water content of the plant, stabilizing the membrane and protein structure as well as reducing photodamage in the bursa membranes through ROS scavenging [71], enhancing antioxidant defense mechanisms in synergy with SOD, POD, and CAT [72,73], and there is a high correlation between plant stress tolerance and Pro accumulation, with drought-tolerant varieties accumulating more Pro than sensitive varieties [69].
4.5. Effects of Drought Stress on Antioxidant Activity
MDA is one of the products of membrane lipid peroxidation, and its content can represent the degree of membrane lipid peroxidation and indirectly reflect the antioxidant capacity of plant tissues [74]. When the balance between reactive oxygen species production and scavenging is disturbed in plant cells, an antioxidant defense system composed of enzymes and non-enzymes is continuously activated to maintain redox homeostasis [75]: SOD is the first line of defense against reactive oxygen species toxicity [76], and APX plays an important role in the protection of plant cells against reactive oxygen species damage [77]. CAT directly decomposes hydrogen peroxide into water and oxygen [78], APX catalyzes the conversion of H2O2 to H2O [79], and flavonoids remove reactive oxygen species (ROS), inhibit ROS-producing enzymes, promote antioxidant enzyme activity, and repair damage caused by UV radiation [80]. In this study, MDA content increased continuously with the extension of stress time, indicating that under drought stress, the cell membrane structure and function of macadamia seedlings were impaired, and the cells were producing ROS in a continuous manner, with altered membrane permeability, thus affecting a series of physiological and biochemical responses. The activities of SOD and APX increased and then decreased with the duration of drought; SOD activity continued to increase from 0 h to 36 h, then decreased from 48 h to 72 h, as compared with 0 h, while APX activity increased between 0 h and 48 h and decreased at 72 h. The activities of POD and CAT varied consistently, and both enzyme activities were higher than 0 h at all times of drought stress, and gradually decreased with the extension of drought time. The highest activities of all four enzymes were detected at 24 h. The content of total flavonoids decreased with drought stress duration. Similar changes were observed in olive, Conocarpus erectus, Acacia modesta, and Salix tetrasperma; drought led to a significant increase in lipid peroxidation in olive, causing an increase in SOD, POD, CAT and APX activities, effectively scavenging ROS and H2O2 from the cells, thus reducing electron loss in the leaves [81,82,83]. At the same time, the antioxidant enzyme activities were not consistently born elevated, but experienced elevation and then decreased, which is consistent with the results of Qi et al., who reported that antioxidant enzyme activities increased and then decreased with drought time in their study [84]. The sustained decrease in flavonoid content with drought duration that we detected, in contrast to the results of drought stress-induced flavonoid biosynthesis in leaves of Bupleurum chinense [85] and tea [86], may be due to the high PEG concentration, which caused the extreme drought to reduce the metabolic capacity of the plant, as verified in the study of Scutellaria baicalensis [87].
5. Conclusions
Under drought stress, the seedlings of macadamia gradually showed dewatering and degreening. On the one hand, leaf thickness, palisade tissue thickness, sponge tissue thickness, stomatal pore diameter and duct diameter decreased, leaf water content and root activity decreased significantly, and photosynthesis was inhibited. The contents of MDA, relative conductivity, soluble sugar, soluble protein, proline, betaine and ABA increased continuously under drought stress, while the contents of IAA, CTK and GA decreased continuously. The activity of SOD, POD, CAT and APX increased first and then decreased. Under PEG-6000 simulated drought stress, macadamia seedlings alleviated the damage caused by drought stress by changing the cell structure of leaves and stems and activating antioxidant and osmotic regulatory mechanisms.
Author Contributions
All authors contributed to the study’s conception. Material preparation was performed by H.C. and G.G. Z.K. analyzed the data, constructed the figures, and wrote the draft. W.W., H.Z. and X.T. conceived and designed the experiment, provided financial support, and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Subsidies for the Cultivation of Forest Tree Seeds and Seedlings (2025-GZZM-KBM-01); Forestry Science and Technology Innovation Platform Operation Subsidy Funds (2020132540); Subsidies for the Cultivation of Forest Tree Seeds and Seedlings [2023] No. 4; Ministry of Agriculture Opening Project Fund of Key Laboratory of Tropical Fruit Biology, Ministry of Agriculture & Rural Affairs.
Data Availability Statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
All authors have acknowledged the content of the article and agreed to this publication.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nagao, M.A.; Hirae, H.H.; Stephenson, R.A. Macadamia: Cultivation and physiology. Crit. Rev. Plant Sci. 1992, 10, 441–470. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Shuai, X.; Dai, T.; Chen, M.; Liang, R.; Du, L.; Chen, J.; Liu, C. Comparative study of chemical compositions and antioxidant capacities of oils obtained from 15 macadamia (Macadamia integrifolia) cultivars in China. Foods 2021, 10, 1031. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, S.; Tan, Q.; Wang, W.; Zhao, L.; Zhang, C.; Bao, Y.; Liu, Q.; Wang, W.; Zou, M.; et al. Chromosomal-level genome of macadamia (Macadamia integrifolia). Trop. Plants 2022, 1, 3. [Google Scholar] [CrossRef]
- Tu, X.H.; Wu, B.; Xie, Y.; Xu, L.; Wu, Z.; Lv, X.; Wei, F.; Du, L.; Chen, H. A comprehensive study of raw and roasted macadamia nuts: Lipid profile, physicochemical, nutritional, and sensory properties. Food Sci. Nutr. 2021, 9, 1688–1697. [Google Scholar] [CrossRef]
- Kang, Z.M.; He, F.P.; Geng, J.J.; Zeng, H.; Tu, X.; Wang, W. Evaluation of quality characters of Macadamia suitable for planting in Guizhou. Non-Wood For. Res. 2020, 38, 117–124. [Google Scholar]
- Yang, M.; Mou, Y.; Meng, Y.; Liu, S.; Peng, C.; Zhou, X. Modeling the effects of precipitation and temperature patterns on agricultural drought in China from 1949 to 2015. Sci. Total Environ. 2020, 711, 135139. [Google Scholar] [CrossRef]
- Wang, C.L.; Zhong, S.B.; Yao, G.N.; Huang, Q.Y. BME spatiotemporal estimation of annual precipitation and detection of drought hazard clusters using space–time scan statistics in the Yun-Gui-Guang region, Mainland China. J. Appl. Meteorol. Climatol. 2017, 56, 2301–2316. [Google Scholar] [CrossRef]
- Kiem, A.S.; Johnson, F.; Westra, S.; Dijk, A.; Rouillard, A.; Sivakumar, B.; Mehrotra, R. Natural hazards in Australia: Droughts. Clim. Chang. 2016, 139, 37–54. [Google Scholar] [CrossRef]
- Wilhite, D.A.; Sivakumar MV, K.; Pulwarty, R. Managing drought risk in a changing climate: The role of national drought policy. Weather. Clim. Extrem. 2014, 3, 4–13. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Ghaffar, A.; Zeidi, M.A.; Siddique, K.H.M.; Farooq, M. Plant photosynthesis under heat stress: Effects and management. Environ. Exp. Bot. 2023, 206, 105178. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Kuromori, T.; Urano, K.; Shinozaki, K.Y.; Shinozaki, K. Drought stress responses and resistance in plants: From cellular responses to long-distance intercellular communication. Front. Plant Sci. 2020, 11, 556972. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional genomics in plant abiotic stress responses and tolerance: From gene discovery to complex regulatory networks and their application in breeding. Proc. Jpn. Acad. Ser. B 2022, 98, 470–492. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.; Motyka, V.; Strnad, M.; Schmülling, T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA 2001, 98, 10487–10492. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Stephenson, R.A.; Gallagher, E.C.; Doogan, V.J.; Mayer, D.G. Nitrogen and environmental factors influencing macadamia quality. Aust. J. Exp. Agric. 2000, 40, 1145–1150. [Google Scholar] [CrossRef]
- Emmanuel, J.Z.; Yoseph, N.A.; Kadmiel, M. Climatic suitability predictions for the cultivation of macadamia in Malawi using climate change scenarios. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bringhenti, T.; Joubert, E.; Abdulai, I.; Hoffmann, M.P.; Moriondo, M.; Taylor, P.J.; Roetter, R.P. Effects of environmental drivers and irrigation on yields of macadamia orchards along an altitudinal gradient in South Africa. Sci. Hortic. 2023, 321, 112326. [Google Scholar] [CrossRef]
- Theunis, G.S.; Nicolette, J.T.; Stephanie, J.E.M. The seasonal regulation of gas exchange and water relations of field grown macadamia. Sci. Hortic. 2020, 267, 109346. [Google Scholar] [CrossRef]
- Mlungisi, S.; Michele, T.; Alistair, C. The Macadamia bloom—What are the hydrological implications? Sci. Hortic. 2022, 292, 110628. [Google Scholar] [CrossRef]
- Kang, Z.M.; Zhang, W.; Guo, G.Z.; Pan, X.J.; Huang, D.; Wang, R.; Shen, X.J. Morphological and physiological responses of 14 macadamia rootstocks to drought stress and a comprehensive evaluation of drought resistance. Environ. Exp. Bot. 2024, 219, 105630. [Google Scholar] [CrossRef]
- Yao, X.C.; Meng, L.F.; Zhao, W.L.; Mao, G.L. Changes in the morphology traits, anatomical structure of the leaves and transcriptome in Lycium barbarum L. under salt stress. Front. Plant Sci. 2023, 14, 1090366. [Google Scholar] [CrossRef]
- Matthaeus, W.J.; Schmidt, J.; White, J.D.; Zechmann, B. Novel perspectives on stomatal impressions: Rapid and non-invasive surface characterization of plant leaves by scanning electron microscopy. PLoS ONE 2020, 15, e0238589. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Hou, K.; Zhang, H.; Wang, X.; Wu, W. Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L. under drought stress. Ind. Crops Prod. 2020, 151, 112465. [Google Scholar] [CrossRef]
- Barranco, D.; Ruiz, N.; Gomes, M. Frost tolerance of eight olive cultivars. HortScience 2005, 40, 558–560. [Google Scholar] [CrossRef]
- Xue, W.; Liu, W.; Ma, R.; Zhang, S.H.; Yu, X.; Li, T.; Luan, X.Y.; Cui, X.W.; Liu, J.; Zhang, C.W. The toxic mechanism of tetracycline on root tips in hulless barley (Hordeum vulgare L. var. nudum). J. Hazard. Mater. 2023, 460, 132453. [Google Scholar] [CrossRef]
- Zhang, Q.; Xing, D.; Wu, Y.; Zhao, K.; Wang, J.; Mao, R. Effects of Low-Phosphorus Stress on Use of Leaf Intracellular Water and Nutrients, Photosynthesis, and Growth of Brassica napus L. Horticulturae 2024, 10, 821. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Wang, X.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Effects of Different Drought Degrees on Physiological Characteristics and Endogenous Hormones of Soybean. Plants 2022, 11, 2282. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Kuang, J.; Ge, Q.; Zhang, Y.; Wang, Z. Overexpression of SmLEA enhances salt and drought tolerance in Escherichia coli and Salvia miltiorrhiza. Protoplasma 2014, 251, 1191–1199. [Google Scholar] [CrossRef]
- Skriver, K.; Mundy, J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 1990, 2, 503. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Karimi, M.; Venditti, A.; Zahra, N.; Siddique, K.H.M.; Farooq, M. Plant Adaptation to Drought Stress: The Role of Anatomical and Morphological Characteristics in Maintaining the Water Status. J. Soil Sci. Plant Nutr. 2024, 25, 409–427. [Google Scholar] [CrossRef]
- Zhu, J.C.; Cai, D.F.; Wang, J.P.; Cao, J.H.; Wen, Y.C.; He, J.P.; Zhao, L.; Wang, D.G.; Zhang, S.F. Physiological and anatomical changes in two rapeseed (Brassica napus L.) genotypes under drought stress conditions. Oil Crop Sci. 2021, 6, 97–104. [Google Scholar] [CrossRef]
- Haffani, S.; Mezni, M.; Nasri, M.B.; Chaibi, W. Comparative leaf water relations and anatomical responses of three vetch species (Vicia narbonensis L., V. sativa L. and V. villosa Roth.) to cope with water stress. Crop Pasture Sci. 2017, 68, 691–702. [Google Scholar] [CrossRef]
- Zimmermann, J.; Link, R.M.; Hauck, M.; Leuschner, C.; Schuldt, B. 60-year record of stem xylem anatomy and related hydraulic modification under increased summer drought in ring-and diffuse-porous temperate broad-leaved tree species. Trees 2021, 35, 919–937. [Google Scholar] [CrossRef]
- Weber AP, M.; Bar-Even, A. Update: Improving the efficiency of photosynthetic carbon reactions. Plant Physiol. 2019, 179, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Xu, X.F.; Sun, Y.M.; Zhang, J.L.; Li, C.Z. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Trabelsi, L.; Gargouri, K.; Hassena, A.B.; Trabelsi, L.; Gargouri, K.; Hassena, A.B.; Mbadra, C.; Ghrab, M.; Ncube, B.; Staden, V.J. Impact of drought and salinity on olive water status and physiological performance in an arid climate. Agric. Water Manag. 2019, 213, 749–759. [Google Scholar] [CrossRef]
- Liu, D.; Guo, H.L.; Yan, L.P.; Cao, L.; Zhai, S.S.; Xu, Y. Physiological, Photosynthetic and Stomatal Ultrastructural Responses of Quercus acutissima Seedlings to Drought Stress and Rewatering. Forests 2023, 15, 71. [Google Scholar] [CrossRef]
- Arunyanark, A.; Jogloy, S.; Akkasaeng, C.; Vorasoot, N.; Kesmala, R.C.; Nageswara, R.; Wright, G.C.; Patanothai, A. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop Sci. 2008, 194, 113–125. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.E.; Zhao, Y.Q.; Ding, C.B.; Liao, J.Q.; Hu, C.; Zhou, L.J.; Zhang, Z.W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef]
- Peterson, R.B.; Sivak, M.N.; Walker, D.A. Relationship between steady-state fluorescence yield and photosynthetic efficiency in spinach leaf tissue. Plant Physiol. 1988, 88, 158–163. [Google Scholar] [CrossRef]
- Guo, C.F.; Sun, Y.; Tang, Y.H.; Zhang, M.G. Effect of water stress on chlorophyll fluorescence in leaves of tea plant (Camellia sinensis). Chin. J. Eco-Agric. 2009, 17, 560–564. [Google Scholar] [CrossRef]
- Gilmore, A.M.; Yamamoto, H.Y. Zeaxanthin formation and energy-dependent fluorescence quenching in pea chloroplasts under artificially mediated linear and cyclic electron transport. Plant Physiol. 1991, 96, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. An integrative approach to photoinhibition and photoprotection of photosynthesis. Environ. Exp. Bot. 2018, 154, 1–3. [Google Scholar] [CrossRef]
- Epron, D.; Dreyer, E. Effects of severe dehydration on leaf photosynthesis in Quercus petraea (Matt.) Liebl.: Photosystem II efficiency, photochemical and nonphotochemical fluorescence quenching and electrolyte leakage. Tree Physiol. 1992, 10, 273–284. [Google Scholar] [CrossRef]
- Havaux, M.; Strasser, R.J.; Greppin, H. A theoretical and experimental analysis of the q P and q N coefficients of chlorophyll fluorescence quenching and their relation to photochemical and nonphotochemical events. Photosynth. Res. 1991, 27, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Magdaong NC, M.; Blankenship, R.E. Photoprotective, excited-state quenching mechanisms in diverse photosynthetic organisms. J. Biol. Chem. 2018, 293, 5018–5025. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Mizoi, J.; Shinozaki, K.; Shinozaki, K.Y. Complex plant responses to drought and heat stress under climate change. Plant J. 2024, 117, 1873–1892. [Google Scholar] [CrossRef]
- Barcia, R.A.; Pena, L.B.; Zawoznik, M.S.; Benavides, M.P.; Gallego, S.M. Osmotic adjustment and maintenance of the redox balance in root tissue may be key points to overcome a mild water deficit during the early growth of wheat. Plant Growth Regul. 2014, 74, 107–117. [Google Scholar] [CrossRef]
- Lintunen, A.; Paljakka, T.; Jyske, T.; Peltoniemi, M.; Sterck, F.; Arx, G.V.; Cochard, H.; Copini, P.; Maria, C.C.; Sylvain, D. Osmolality and non-structural carbohydrate composition in the secondary phloem of trees across a latitudinal gradient in Europe. Front. Plant Sci. 2016, 7, 726. [Google Scholar] [CrossRef]
- Hein, J.A.; Sherrard, M.E.; Manfredi, K.P.; Abebe, T. The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol. 2016, 16, 248. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Ashraf, U.; Hussain, S.; Shahzad, B.; Khan, I.; Wang, L. Effect of progressive drought stress on growth, leaf gas exchange, and antioxidant production in two maize cultivars. Environ. Sci. Pollut. Res. 2016, 23, 17132–17141. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Ahanger, M.A.; Su, Y.Y.; Lei, Y.F.; Nabil, S.A.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L.X. Improved drought tolerance by AMF inoculation in maize (Zea mays) involves physiological and biochemical implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Zegaoui, Z.; Planchais, S.; Cabassa, C.; Djebbar, R.; Belbachir, O.A.; Carol, P. Variation in relative water content, proline accumulation and stress gene expression in two cowpea landraces under drought. J. Plant Physiol. 2017, 218, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Kishor PB, K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhris, M.; Abdallah, F.B. Changes in gas exchange, proline accumulation and antioxidative enzyme activities in three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2009, 67, 345–352. [Google Scholar] [CrossRef]
- Slabbert, M.M.; Krüger, G.H.J. Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 2014, 95, 123–128. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Emam, Y.; Pessarakli, M. Changes in endogenous hormonal status in corn (Zea mays) hybrids under drought stress. J. Plant Nutr. 2013, 36, 1695–1707. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Kurepa, J.; Smalle, J.A. Auxin/Cytokinin Antagonistic Control of the Shoot/Root Growth Ratio and Its Relevance for Adaptation to Drought and Nutrient Deficiency Stresses. Int. J. Mol. Sci. 2022, 23, 1933. [Google Scholar] [CrossRef]
- Nir, I.; Moshelion, M.; Weiss, D. The Arabidopsis gibberellin methyl transferase 1 suppresses gibberellin activity, reduces whole-plant transpiration and promotes drought tolerance in transgenic tomato. Plant Cell Environ. 2014, 37, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Furutera, A.; Seki, K.; Toyoda, Y.; Tanaka, K.; Sugimoto, Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 2008, 46, 786–793. [Google Scholar] [CrossRef]
- Miller, G.A.D.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Chang-Quan, W.; Rui-Chang, L. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol. Plant. 2008, 30, 841–847. [Google Scholar] [CrossRef]
- Sekmen, A.H.; Ozgur, R.; Uzilday, B.; Turkan, I. Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot. 2014, 99, 141–149. [Google Scholar] [CrossRef]
- Gokce, A.; Sekmen Cetinel, A.H.; Turkan, I. Involvement of GLR-mediated nitric oxide effects on ROS metabolism in Arabidopsis plants under salt stress. J. Plant Res. 2024, 137, 485–503. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic stress tolerance in plants: Myriad roles of ascorbate peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Wang, N.; Liu, W.J.; Yu, L.; Guo, Z.W.; Chen, Z.J.; Jiang, S.H.; Xu, H.F.; Fang, H.C.; Wang, Y.C.; Zhang, Z.Y. HEAT SHOCK FACTOR A8a modulates flavonoid synthesis and drought tolerance. Plant Physiol. 2020, 184, 1273–1290. [Google Scholar] [CrossRef]
- Rasheed, F.; Gondal, A.; Kudus, K.A.; Zafar, Z.; Nawaz, M.F.; Khan, W.R.; Abdullah, M.; Ibrahim, F.H.; Depardieu, C.; Pazi, A.M.M. Effects of soil water deficit on three tree species of the arid environment: Variations in growth, physiology, and antioxidant enzyme activities. Sustainability 2021, 13, 3336. [Google Scholar] [CrossRef]
- Denaxa, N.K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- Dreyer, A.; Dietz, K.J. Reactive oxygen species and the redox-regulatory network in cold stress acclimation. Antioxidants 2018, 7, 169. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, L.; Ghani, M.I.; Peng, Q.; Fan, R.D.; Hu, X.J.; Chen, X.Y.L. Effects of drought stress induced by hypertonic polyethylene glycol (PEG-6000) on Passiflora edulis sims physiological properties. Plants 2023, 12, 2296. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, L.; Yang, X.; Zhang, T.; Lan, Y.M.; Zhao, Y.; Han, M.; Yang, L.M. Drought stress induces biosynthesis of flavonoids in leaves and saikosaponins in roots of Bupleurum chinense DC. Phytochemistry 2020, 177, 112434. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.L.; Wang, Y.; Xie, H.; Qiu, C.; Zhang, S.N.; Xiao, J.; Li, H.Y.; Chen, L.; Xinghui Li, X.H.; Ding, Z.T. Drought stress triggers proteomic changes involving lignin, flavonoids and fatty acids in tea plants. Sci. Rep. 2020, 10, 15504. [Google Scholar] [CrossRef]
- Li, P.; Ren, G.X.; Wu, F.; Chen, J.X.; Jiang, D.; Liu, C.S. Root-specific flavones and critical enzyme genes involved in their synthesis changes due to drought stress on Scutellaria baicalensis. Front. Ecol. Evol. 2023, 11, 1113823. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).