Physiological and Metabolomics Analyses Revealed That Overexpression of CBL-Interacting Protein Kinase 23 Accelerate Tuber Sprouting in Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Potato Planting and Tuber Harvesting
2.3. Measurement of Physiological Indicators and Enzyme Activity
2.4. Measurement of Gibberellin and Abscisic Acid Content
2.5. Real-Time Fluorescence Quantitative PCR
2.6. Metabolomics Analysis
2.7. Statistical Analysis
3. Result
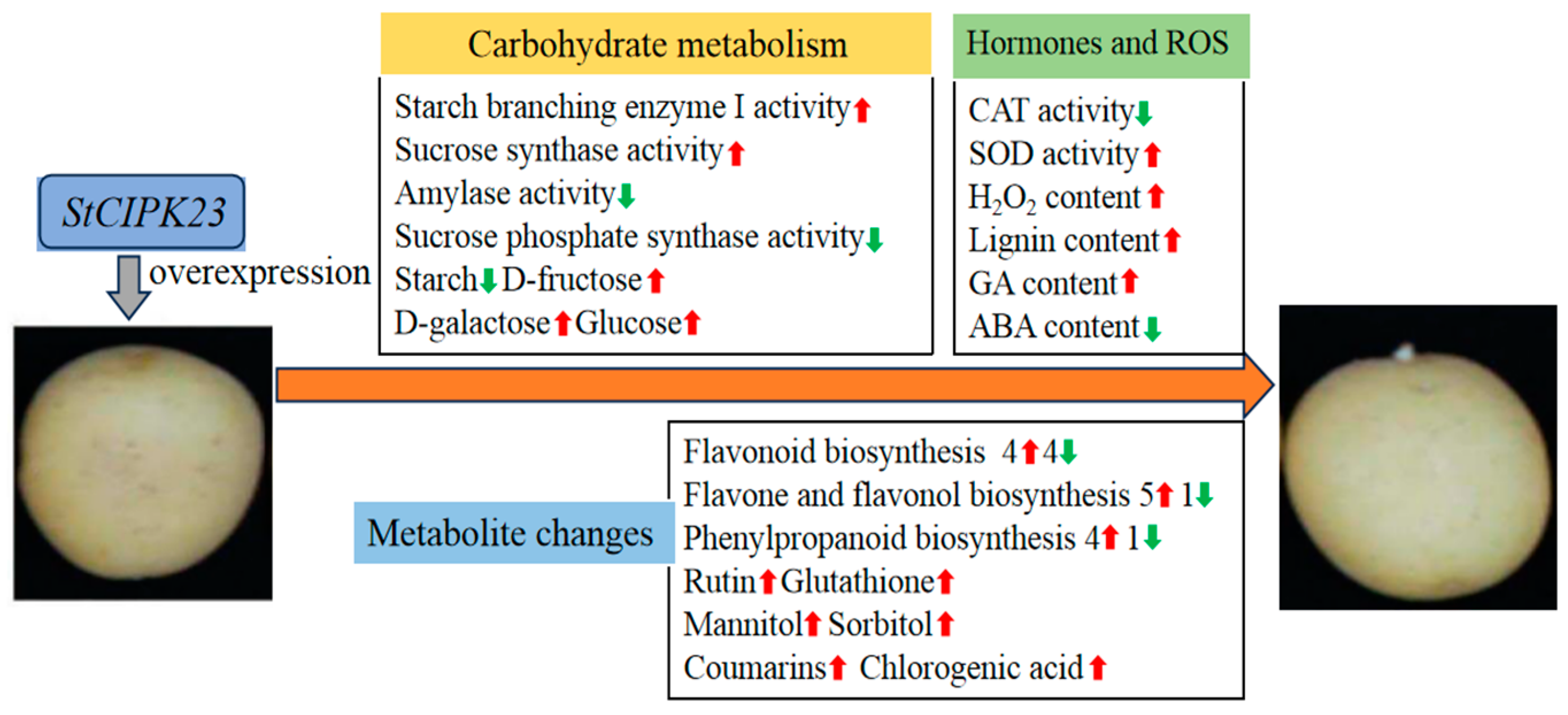
3.1. StCIPK23 Overexpression Accelerates Potato Tuber Sprouting
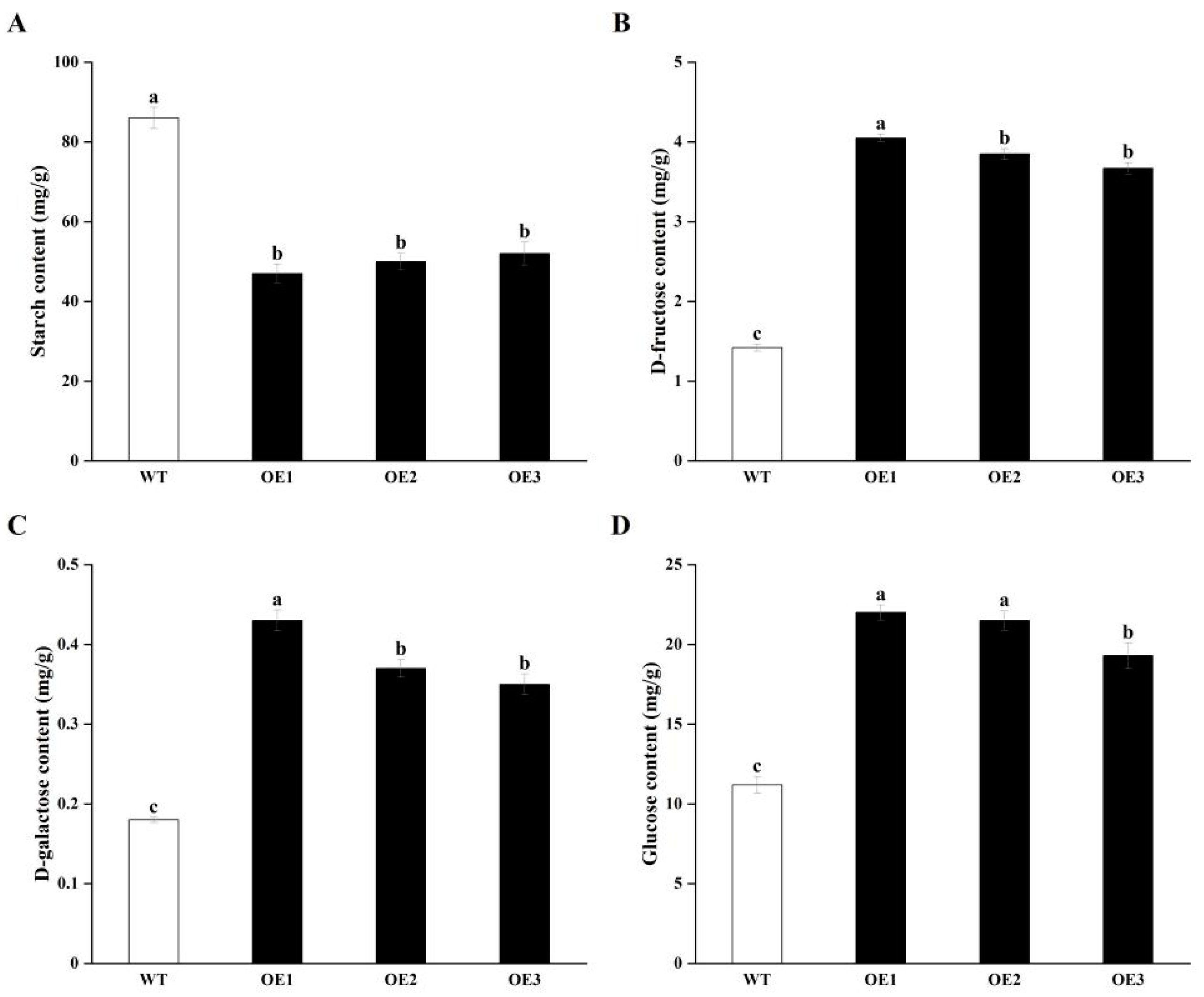
3.2. Overexpressing StCIPK23 Alters Glycometabolism During Tuber Sprouting
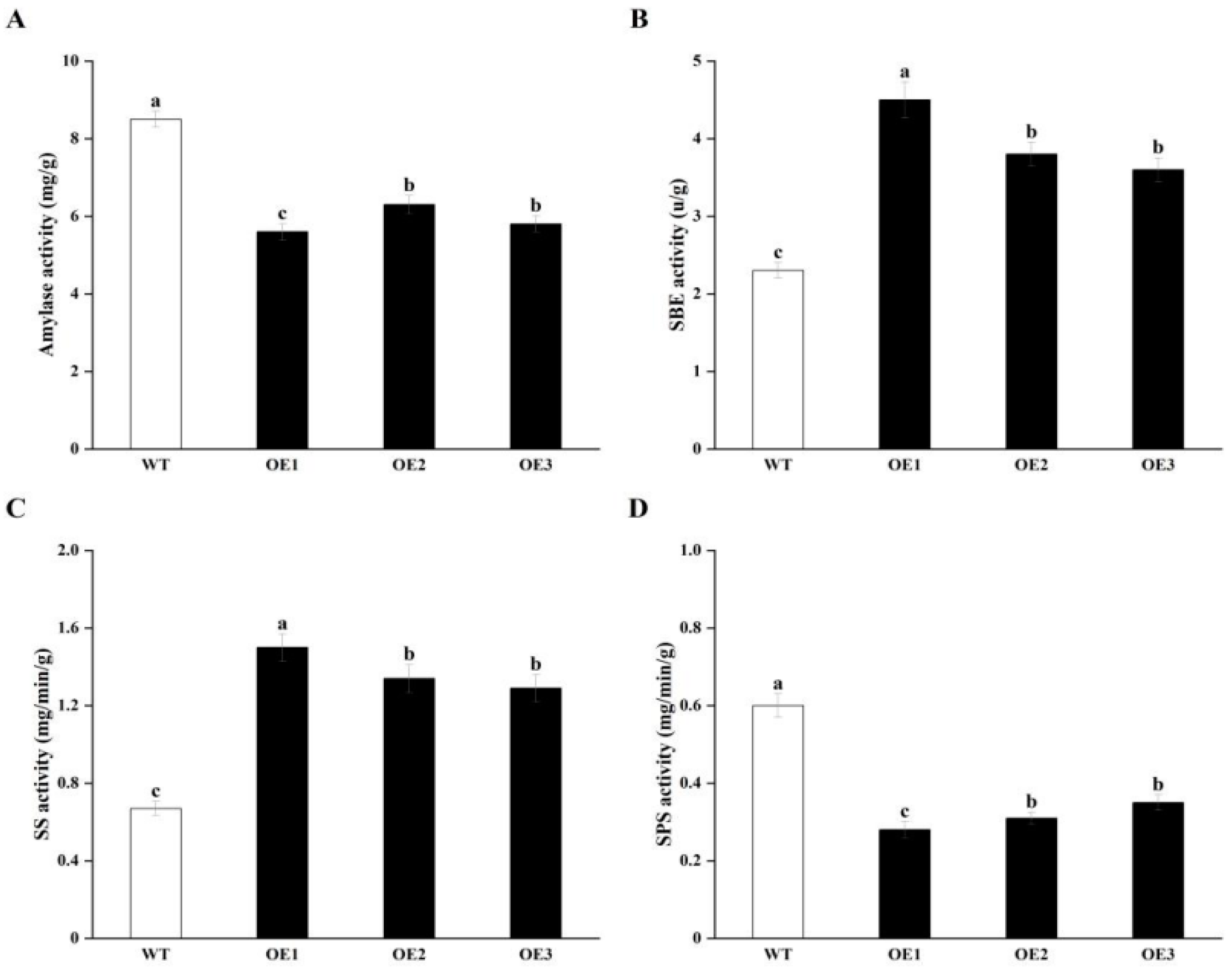
3.3. Overexpressing StCIPK23 Alters Enzyme Activities in Tuber Sprouting
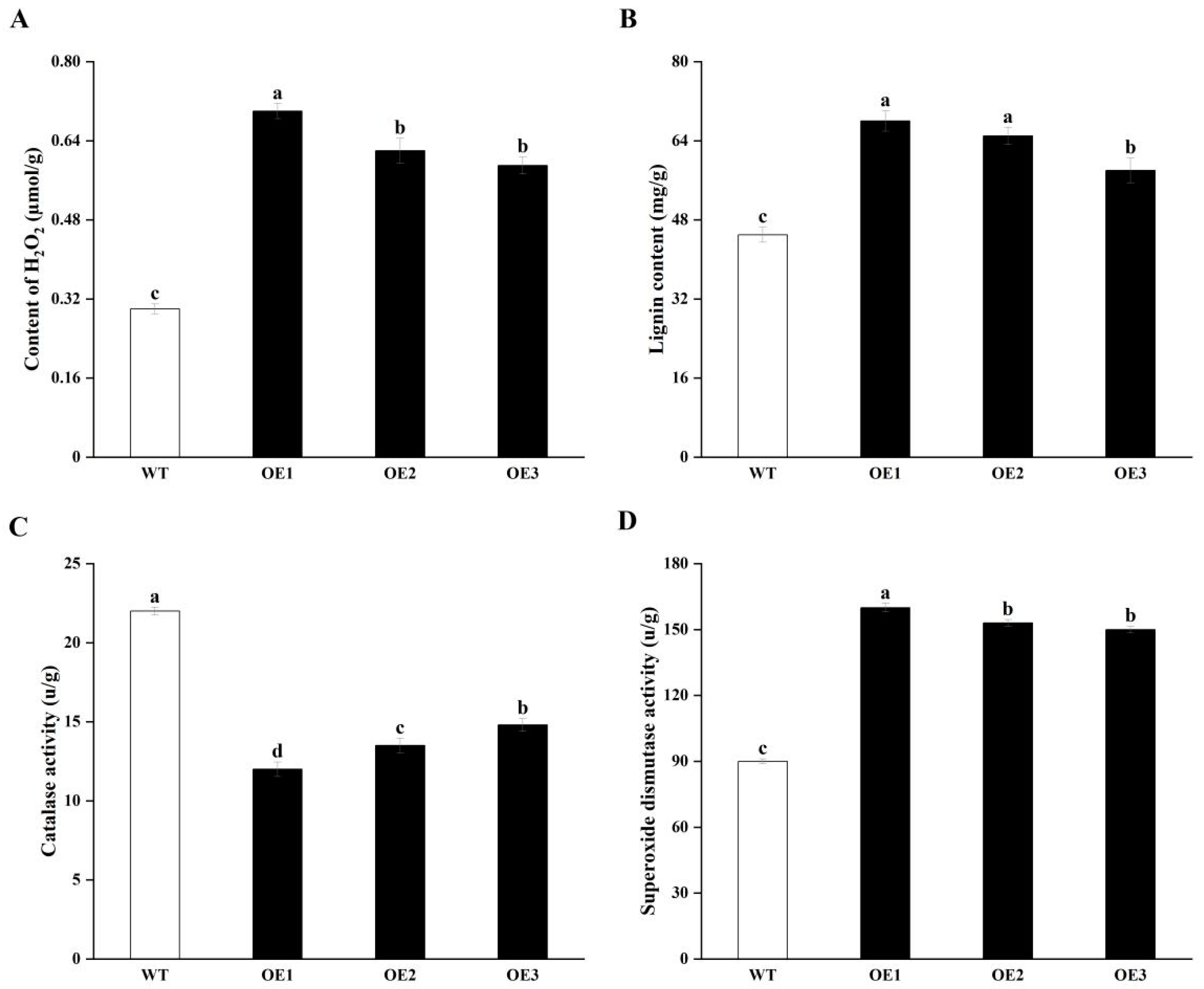
3.4. Overexpressing StCIPK23 Alters the Antioxidant System and Lignin Contents
3.5. Overexpressing StCIPK23 Affects GA and ABA Content
3.6. Overexpressing StCIPK23 Alters Gene Expression Levels During Tuber Sprouting
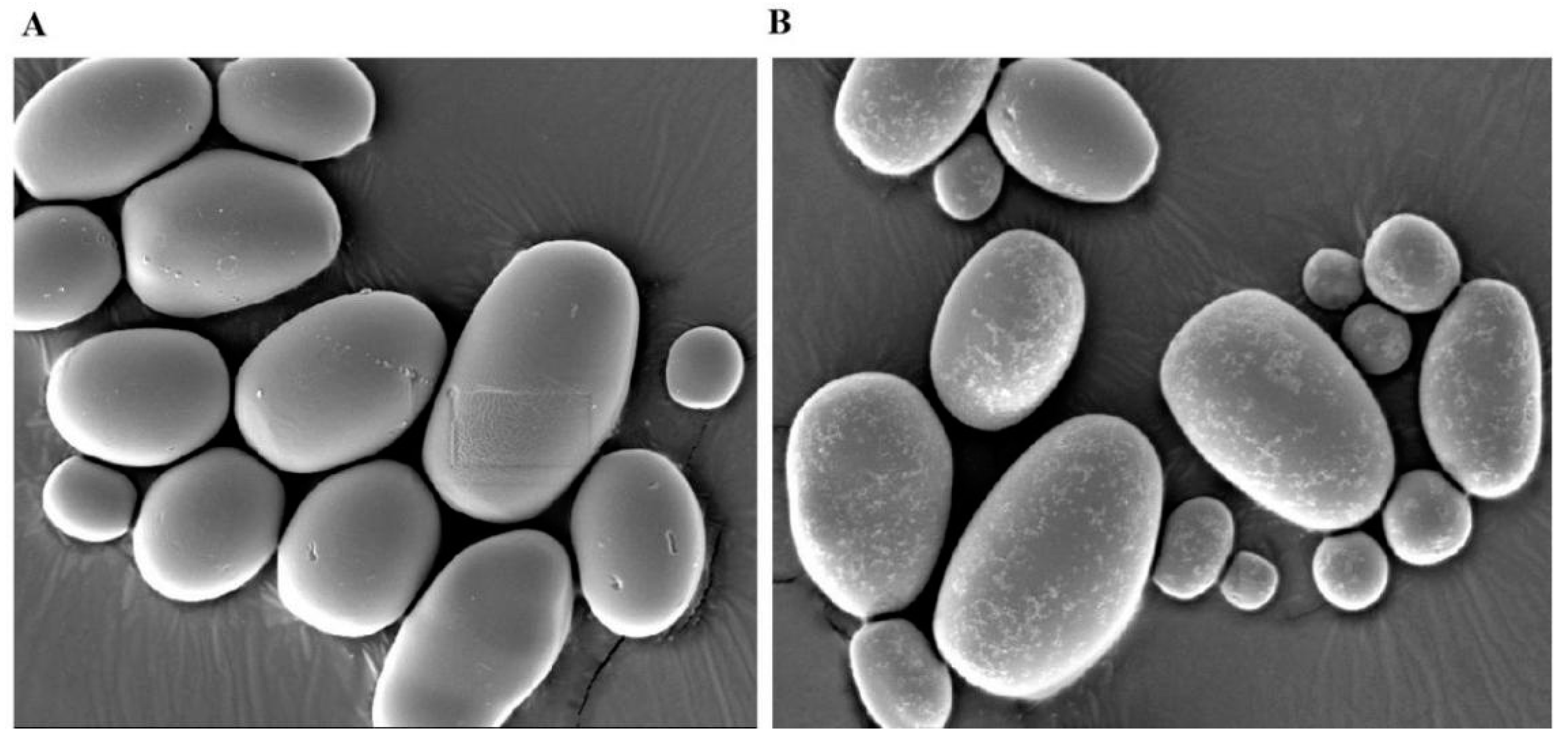
3.7. Overexpressing StCIPK23 Alters Starch Granules During Tuber Sprouting
3.8. Overexpressing StCIPK23 Alters Metabolite Levels During Tuber Sprouting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The potato of the future: Opportunities and challenges in sustainable agrifood systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef]
- Sonnewald, S.; Sonnewald, U. Regulation of potato tuber sprouting. Planta 2014, 239, 27–38. [Google Scholar] [CrossRef]
- Morris, W.L.; Alamar, M.C.; Lopez-Cobollo, R.M.; Castillo Cañete, J.; Bennett, M.; Van der Kaay, J.; Stevens, J.; Kumar Sharma, S.; McLean, K.; Thompson, A.J.; et al. A member of the TERMINAL FLOW-ER 1/CENTRORADIALIS gene family controls sprout growth in potato tubers. J. Exp. Bot. 2019, 70, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Daniels-Lake, B.J.; Prange, R.K. The Canon of Potato Science: 41. Sprouting. Potato Res. 2007, 50, 379–382. [Google Scholar] [CrossRef]
- Di, X.; Wang, Q.; Zhang, F.; Feng, H.; Wang, X.; Cai, C. Advances in the modulation of potato tuber dormancy and sprouting. Int. J. Mol. Sci. 2024, 25, 5078. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Zhang, C.; Zhang, N.; Wen, Y.; Wang, D. Control of potato tuber dormancy and sprouting by expression of sense and antisense genes of pyrophosphatase in potato. Acta Physiol. Plant. 2016, 38, 69. [Google Scholar] [CrossRef]
- Deng, M.; Peng, J.; Zhang, J.; Ran, S.; Cai, C.; Yu, L.; Ni, S.; Huang, X.; Li, L.; Wang, X. The cysteine-rich peptide Snakin-2 negatively regulates tubers sprouting through modulating lignin biosynthesis and H2O2 accumulation in potato. Int. J. Mol. Sci. 2021, 22, 2287. [Google Scholar] [CrossRef]
- Liu, S.F.; Cai, C.C.; Li, L.P.; Wen, H.; Liu, J.; Li, L.Q.; Wang, Q.; Wang, X.Y. StSN2 interacts with the brassinosteroid signaling suppressor StBIN2 to maintain tuber dormancy. Hortic. Res. 2023, 10, uhad228. [Google Scholar] [CrossRef]
- Ding, X.; Liu, B.; Sun, X.; Sun, X.; Zheng, C. New functions of CIPK gene family are continue to emerging. Mol. Biol. Rep. 2022, 49, 6647–6658. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Lu, J.; Wang, L.; Min, F.; Guo, M.; Wei, Q.; Wang, W.; Dong, X.; Mao, Y.; et al. Potato calcineurin B-like protein CBL4, interacting with calcineurin B-like protein-interacting protein kinase CIPK2, positively regulates plant resistance to stem canker caused by Rhizoctonia solani. Front. Microbiol. 2023, 13, 1032900. [Google Scholar] [CrossRef]
- Ma, R.; Liu, W.; Li, S.; Zhu, X.; Yang, J.; Zhang, N.; Si, H. Genome-Wide Identification, characterization and expression analysis of the CIPK gene family in potato (Solanum tuberosum L.) and the role of StCIPK10 in response to drought and osmotic stress. Int. J. Mol. Sci. 2021, 22, 13535. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, G.; Zhang, J.; Wang, G. Analysis of gene expression in early seed germination of rice: Landscape and genetic regulation. BMC Plant Biol. 2022, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Wang, W.; Feng, L.; Yin, H.; Xiong, F.; Ren, M. Target of rapamycin regulates potassium uptake in Arabidopsis and potato. Plant Physiol. Biochem. 2020, 155, 357–366. [Google Scholar] [CrossRef]
- Huang, F.Y.; Lu, Y.F.; Li, Z.; Zhang, L.; Xie, M.Q.; Ren, B.; Lu, L.M.; Li, L.Q.; Yang, C.Q. Over-expression of CBL-interacting protein kinases 23 improves tolerance to low-nitrogen stress in potato plants. Horticulturae 2024, 10, 526. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Liu, X.W.; Nie, B.H.; Song, B.T.; Du, P.; Liu, S.X.; Li, L.; Zhao, Z.Q. Nitrogen management can inhibit or induce the sprouting of potato tubers: Consequences of regulation tuberization. Postharvest Biol. Technol. 2022, 183, 111722. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Zhou, D.; Cai, W.; Rehman, M.; Feng, Y.; Kong, Y.; Liu, X.; Fahad, S.; Deng, G. Impact of dormancy periods on some physiological and biochemical indices of potato tubers. PeerJ 2023, 11, e15923. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Sabba, R.; Bussan, A.J. Tuber water and pressure potentials decrease and sucrose contents increase in response to moderate drought and heat stress. Am. J. Potato Res. 2009, 86, 519–532. [Google Scholar] [CrossRef]
- Xi, Z.X.; Duan, H.M.; Zhang, N.; Majeed, Y.; Jin, H.; Li, W.; Chen, Z.; Chen, S.; Tang, J.H.; Zhang, Y.; et al. Genome-wide identification of GATA family genes in potato and characterization of StGATA12 in response to salinity and osmotic stress. Int. J. Mol. Sci. 2024, 25, 12423. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Y.C.; Bi, Y.; Chen, S.J.; Li, Y.C.; Yuan, L.; Wang, Y.; Wang, D. Postharvest treatment with β-aminobutyric acid induces resistance against dry rot caused by Fusarium sulphureum in potato tuber. Agric. Sci. China 2010, 9, 1372–1380. [Google Scholar] [CrossRef]
- Li, L.Q.; Lyu, C.C.; Chen, J.; Lu, Y.; Yang, S.; Ni, S.; Zheng, S.; Yu, L.; Wang, X.; Wang, Q.; et al. Snakin-2 interacts with cytosolic glyceraldehyde-3-phosphate dehydrogenase 1 to inhibit sprout growth in potato tubers. Hortic. Res. 2022, 9, uhab060. [Google Scholar] [CrossRef]
- Ross, H.A.; Davies, H.V. Amylase activity in potato tubers. Potato Res. 1987, 30, 675–678. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yuki, K.; Park, S.Y.; Ohya, T. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989, 30, 833–839. [Google Scholar] [CrossRef]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Peivastegan, B.; Hadizadeh, I.; Nykyri, J.; Nielsen, K.L.; Somervuo, P.; Sipari, N.; Tran, C.; Pirhonen, M. Effect of wet storage conditions on potato tuber transcriptome, phytohormones and growth. BMC Plant Biol. 2019, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Alisdair, R.F.; Lothar, W. Molecular and biochemical triggers of potato tuber development. Plant Physiol. 2001, 127, 1459–1465. [Google Scholar]
- Aksenova, N.P.; Sergeeva, L.I.; Konstantinova, T.N.; Golyanovskaya, S.A.; Kolachevskaya, O.O.; Romanov, G.A. Regulation of potato tuber dormancy and sprouting. Russ. J. Plant Physiol. 2013, 60, 301–312. [Google Scholar] [CrossRef]
- Wiltshire, J.J.J.; Cobb, A.H. A review of the physiology of potato tuber dormancy. Ann. Appl. Biol. 1996, 129, 553–569. [Google Scholar] [CrossRef]
- Faivre-Rampant, O.; Cardle, L.; Marshall, D.; Viola, R.; Taylor, M.A. Changes in gene expression during meristem activation processes in Solanum tuberosum with a focus on the regulation of an auxin response factor gene. J. Exp. Bot. 2004, 55, 613–622. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, R.; Niu, S.; Si, H.; Yang, Q.; Rajora, O.P.; Li, X.Q. Heat-stress-induced sprouting and differential gene expression in growing potato tubers: Comparative transcriptomics with that induced by postharvest sprouting. Hortic. Res. 2021, 8, 226. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Cortelezzi, J.I.; Capiati, D.A. The protein phosphatase 2A catalytic subunit StPP2Ac2b is involved in the control of potato tuber sprouting and source–sink balance in tubers and sprouts. J. Exp. Bot. 2022, 73, 6784–6799. [Google Scholar] [CrossRef] [PubMed]
- Hajirezaei, M.; Börnke, F.; Peisker, M.; Takahata, Y.; Lerchl, J.; Kirakosyan, A.; Sonnewald, U. Decreased sucrose content triggers starch breakdown and respiration in store-d potato tubers (Solanum tuberosum). J. Exp. Bot. 2003, 54, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Farré, E.M.; Bachmann, A.; Willmitzer, L.; Trethewey, R.N. Acceleration of potato tuber sprouting by the expression of a bacterial pyrophosphatase. Nat. Biotechnol. 2001, 19, 268–272. [Google Scholar] [CrossRef]
- Hu, Q.; Tang, C.; Zhou, X.; Yang, X.; Luo, Z.; Wang, L.; Yang, M.; Li, D.; Li, L. Potatoes dormancy release and sprouting commencement: A review on current and future prospects. Food Front. 2023, 4, 1001–1018. [Google Scholar] [CrossRef]
- Yang, B.; Chen, M.; Zhan, C.; Liu, K.; Cheng, Y.; Xie, T.; Zhu, P.; He, Y.; Zeng, P.; Tang, H.; et al. Identification of OsPK5 involved in rice glycolytic metabolism and GA/ABA balance for improving seed germination via genome-wide association study. J. Exp. Bot. 2022, 73, 3446–3461. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, L.; Guo, W.; Wu, W. Transcription factor TERF1 promotes seed germination under osmotic conditions by activating gibberellin acid signaling. Plant Sci. 2022, 322, 111350. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, J.; Liu, J.; Xie, C.; Song, B. Amylase analysis in potato starch degradation during cold storage and sprouting. Potato Res. 2014, 57, 47–58. [Google Scholar] [CrossRef]
- Hou, J.; Liu, T.F.; Reid, S.; Zhang, H.L.; Peng, X.J.; Sun, K.L.; Du, J.; Sonnewald, U.; Song, B.T. Silencing of α-amylase StAmy23 in potato tuber leads to delayed sprouting. Plant Physiol. Biochem. 2019, 139, 411–418. [Google Scholar] [CrossRef]
- Yin, M.; Hu, D.; Yu, X.; Wang, Y.; Song, S.; Wang, C.; Hu, Q.; Wen, Y. Polyacrylamide regulated phytohormone balance and starch degradation to promote seed-potato sprouting and emergence. Plants 2024, 13, 2796. [Google Scholar] [CrossRef]
- Geigenberger, P.; Reimholz, R.; Deiting, U.; Sonnewald, U.; Stitt, M. Decreased expression of sucrose phosphate synthase strongly inhibits the water stress-induced synthesis of sucrosein growing potato tubers. Plant J. 1999, 19, 119–129. [Google Scholar] [CrossRef]
- Dai, H.F.; Fu, M.R.; Yang, X.Y.; Chen, Q.M. Ethylene inhibited sprouting of potato tubers by influencing the carbohydrate metabolism pathway. J. Food Sci. Technol. 2016, 53, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Tan, F.; Zhao, H.; Wang, D.; Si, H.; Chen, Q. Changes in ROS production and antioxidant capacity during tuber sprouting in potato. Food Chem. 2017, 237, 205–213. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, N.; Wang, K.; Luo, Y.; Wei, H.; Si, H. StSnRK1.1 protein kinase positively regulates tuber dormancy release of potato. Sci. Hortic. 2024, 337, 113505. [Google Scholar] [CrossRef]
- Li, L.Q.; Chen, J.; Lu, Y.F.; Ren, B.; Huang, X.L.; Yu, L.P.; Zeng, F.C.; Wang, Q.; Wang, X.Y.; Lu, L.M. Physiological and proteomic analyses of γ-aminobutyric acid (GABA)-treated tubers reveals that StPOD42 promotes sprouting in potato. J. Plant Physiol. 2022, 278, 153826. [Google Scholar] [CrossRef]
- Drerup, M.M.; Schlücking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The Calcineurin B-Like calcium sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef]
- Beltrán, E.G.; Personat, J.M.; Torre, F.D.L.; Pozo, O.D. A universal stress protein involved in oxidative stress Is a phosphorylation target for protein kinase CIPK6. Plant Physiol. 2016, 173, 836–852. [Google Scholar] [CrossRef]
- Wróbel, S.; Kęsy, J.; Treder, K. Effect of growth regulators and ethanol on termination of dormancy in potato tubers. Am. J. Potato Res. 2017, 94, 544–555. [Google Scholar] [CrossRef]
- Ma, Q.J.; Sun, M.H.; Lu, J.; Liu, Y.J.; You, C.X.; Hao, Y.J. An apple CIPK protein kinase targets a novel residue of AREB transcription factor for ABA-dependent phosphorylation. Plant Cell Environ. 2017, 40, 2207–2219. [Google Scholar] [CrossRef]
- Kumar, S.; Bandyopadhyay, N.; Saxena, S.; Hajare, S.N.; More, V.; Tripathi, J.; Dahia, Y.; Gautam, S. Differential gene expression in irradiated potato tubers contributed to sprout inhibition and quality retention during a commercial scale storage. Sci. Rep. 2024, 14, 13484. [Google Scholar] [CrossRef]
- Liang, B.; Cao, J.; Wang, R.; Fan, C.; Wang, W.; Hu, X.; He, R.; Tai, F. ZmCIPK32 positively regulates germination of stressed seeds via gibberellin signal. Plant Physiol. Biochem. 2023, 199, 107716. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.G.; Bea, S.H.; Jun, B.K.; Kim, S.T.; Kwon, S.Y.; Kim, S.H. Overexpression of ADP-glucose pyrophosphorylase (IbAGPaseS) affects expression of carbohydrate regulated genes in sweet potato [Ipomoea batatas (L.) Lam. Cv. Yulmi]. Genes Genom. 2015, 37, 595–605. [Google Scholar] [CrossRef]
- Abel, G.J.W.; Springer, F.; Willmitzer, L.; Kossmann, J. Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.). Plant J. 1996, 10, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dees, D.; Dechesne, A.; Huang, X.F.; Visser, R.G.F.; Trindade, L.M. Starch phosphorylation plays an important role in starch biosynthesis. Carbohydr. Polym. 2017, 157, 1628–1637. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Li, M.; Zhang, X.J.; Chen, J.X.; Ge, X.; Li, S.Q.; Tian, J.C.; Tian, S.L. Unraveling the mechanism of potato (Solanum tuberosum L.) tuber sprouting using transcriptome and metabolome analyses. Front. Plant Sci. 2024, 14, 1300067. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.Y.; Feng, Y.H.; Yang, Y.; Feng, C.; Li, J.H.; Zaman, Q.; Kong, Y.X.; Fahad, S.; Deng, G. Integrated physiological, transcriptomic and metabolomic analyses reveal potential mechanisms of potato tuber dormancy release. Physiol. Plant. 2025, 177, e70081. [Google Scholar] [CrossRef]
- Suchova, L.S.; Machá, K.I.; Eder, J.; Bibik, N.D.; Korableva, N.P. Changes in the levels of free IAA and cytokinins in potato tubers during dormancy and sprouting. Biol. Plant. 1993, 35, 387–391. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Zong, Y.Y.; Liang, W.; Kong, R.; Gong, D.; Han, Y.; Li, Y.C.; Bi, Y.; Prusky, D. Sorbitol immersion accelerates the deposition of suberin polyphenolic and lignin at wounds of potato tubers by activating phenylpropanoid metabolism. Sci. Hortic. 2022, 297, 110971. [Google Scholar] [CrossRef]
- Yan, J.; Bi, H.H.; Liu, Y.Z.; Zhang, M.; Zhou, Z.Y.; Tan, J.W. Phenolic compounds from Merremia umbellata subsp. orientalis and their allelopathic effects on Arabidopsis seed germination. Molecules 2010, 15, 8241–8250. [Google Scholar]
- Dong, Q.J.; Xu, X.Y.; Fan, C.X.; Xiao, J.P. Transcriptome and metabolome analyses reveal chlorogenic acid accumulation in pigmented potatoes at different altitudes. Genomics 2024, 116, 110883. [Google Scholar] [CrossRef]




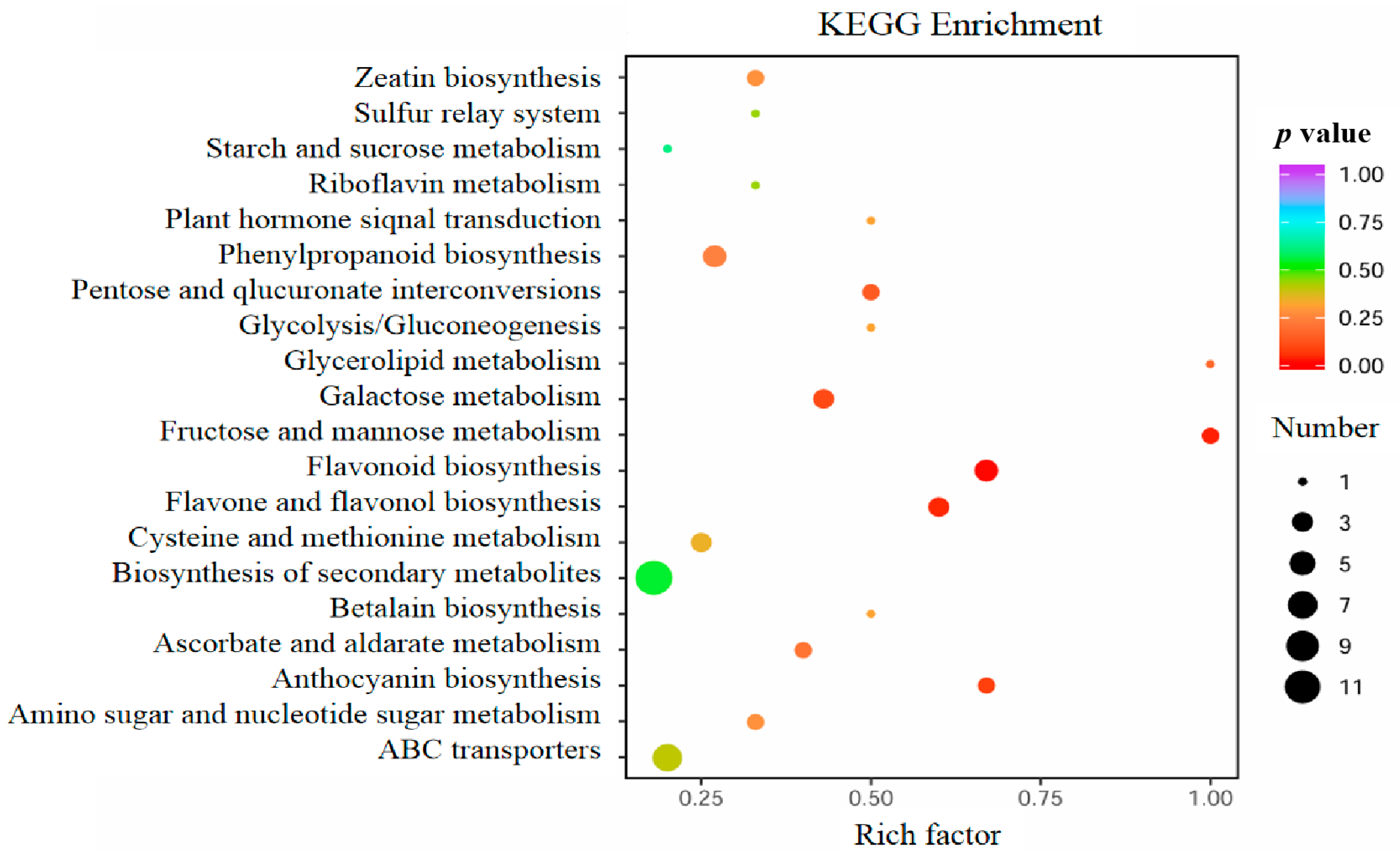
| Index | Compounds | Class | Log2FC | p Value |
|---|---|---|---|---|
| mws0921 | p-Coumaryl alcohol | Phenolic acids | 10.60 | 2.6594 × 10−5 |
| pme1086 | Glutathione reduced form | Amino acids and derivatives | 6.06 | 1.4914 × 10−4 |
| Lmmp002334 | Quercetin-glucoside-glucoside-rhamnoside | Flavonols | 5.91 | 5.2036 × 10−5 |
| mws1077 | Scopolin | Coumarins | 4.68 | 6.1833 × 10−7 |
| Hmdp002169 | 6-Hydroxy-7-methoxy-coumarin | Coumarins | 3.97 | 1.7789 × 10−12 |
| pmb0550 | Cyanidin 3-O-glucoside (Kuromanin) | Anthocyanins | 3.46 | 9.6507 × 10−7 |
| pme1261 | Pantothenol | Saccharides and Alcohols | 3.25 | 1.8434 × 10−2 |
| Hmcp001757 | Quercetin-O-rhamnoside-O-Hexoside-O-rhamnoside | Flavonols | 3.17 | 4.4403 × 10−5 |
| Lmhp008763 | LysoPE 16:1 (2n isomer) | LPE | 3.14 | 7.7189 × 10−4 |
| mws1639 | Isofraxidin | Coumarins | 2.68 | 9.9598 × 10−9 |
| mws0036 | Hesperidin | Dihydroflavone | −1.98 | 9.1199 × 10−9 |
| mws0055 | Tangeretin | Flavonols | −2.01 | 5.7735 × 10−1 |
| mws0043 | Nobiletin | Flavonoid | −2.01 | 5.2729 × 10−2 |
| pme0001 | Hesperetin 7-O-neohesperidoside (Neohesperidin) | Dihydroflavone | −2.02 | 1.1439 × 10−7 |
| mws0052 | Baicalin | Flavonoid | −2.17 | 2.8922 × 10−10 |
| pme0460 | Epicatechin | Flavanols | −2.40 | 2.0138 × 10−7 |
| pmp001252 | p-Coumaroyltyramine | Phenolamine | −3.54 | 3.5747 × 10−1 |
| pme3544 | Limonin | Others | −3.59 | 1.6466 × 10−4 |
| mws2118 | Phlorizin | Chalcones | −3.93 | 1.6868 × 10−14 |
| pmb3002 | Chrysoeriol-7-O-rutinoside | Flavonoid | −11.25 | 4.4212 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Wang, F.; Zhang, X.; Lu, Y.; Ren, B.; Yang, S.; Lu, L.; Li, L. Physiological and Metabolomics Analyses Revealed That Overexpression of CBL-Interacting Protein Kinase 23 Accelerate Tuber Sprouting in Potato. Horticulturae 2025, 11, 342. https://doi.org/10.3390/horticulturae11040342
Zhou F, Wang F, Zhang X, Lu Y, Ren B, Yang S, Lu L, Li L. Physiological and Metabolomics Analyses Revealed That Overexpression of CBL-Interacting Protein Kinase 23 Accelerate Tuber Sprouting in Potato. Horticulturae. 2025; 11(4):342. https://doi.org/10.3390/horticulturae11040342
Chicago/Turabian StyleZhou, Fang, Fengjuan Wang, Xing Zhang, Yifei Lu, Bi Ren, Shimin Yang, Liming Lu, and Liqin Li. 2025. "Physiological and Metabolomics Analyses Revealed That Overexpression of CBL-Interacting Protein Kinase 23 Accelerate Tuber Sprouting in Potato" Horticulturae 11, no. 4: 342. https://doi.org/10.3390/horticulturae11040342
APA StyleZhou, F., Wang, F., Zhang, X., Lu, Y., Ren, B., Yang, S., Lu, L., & Li, L. (2025). Physiological and Metabolomics Analyses Revealed That Overexpression of CBL-Interacting Protein Kinase 23 Accelerate Tuber Sprouting in Potato. Horticulturae, 11(4), 342. https://doi.org/10.3390/horticulturae11040342






