Ascorbic Acid Mitigates Aluminum Stress Through Improved Antioxidant Mechanism in Highbush Blueberry (Vaccinium corymbosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Plant Growth Analysis
2.3. Determination of Aluminum and Calcium Concentrations
2.4. Chlorophyll Fluorescence and Gas-Exchange Measurements
2.5. Determination of Photosynthetic Pigments
2.6. Lipid Peroxidation Assay
2.7. Antioxidant Determination
2.8. Ascorbate and Dehydroascorbate Determination
2.9. Superoxide Dismutase (SOD) Activity
2.10. Analysis of Root Exudates
2.11. Experimental Design and Statistical Analyses
3. Results
3.1. Relative Growth Rate
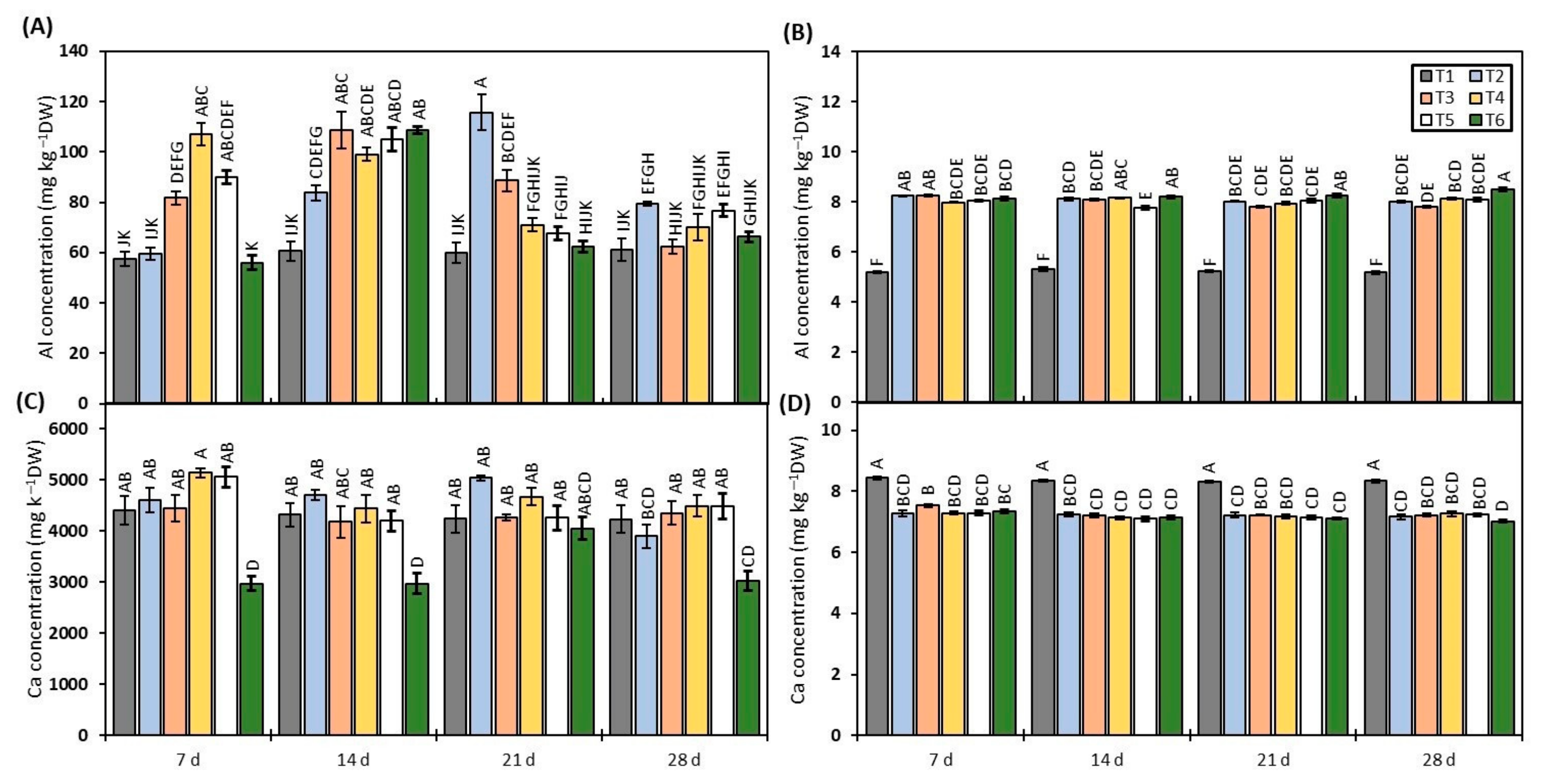
3.2. Determination of Al and Ca Content
3.3. Gas Exchange Parameters
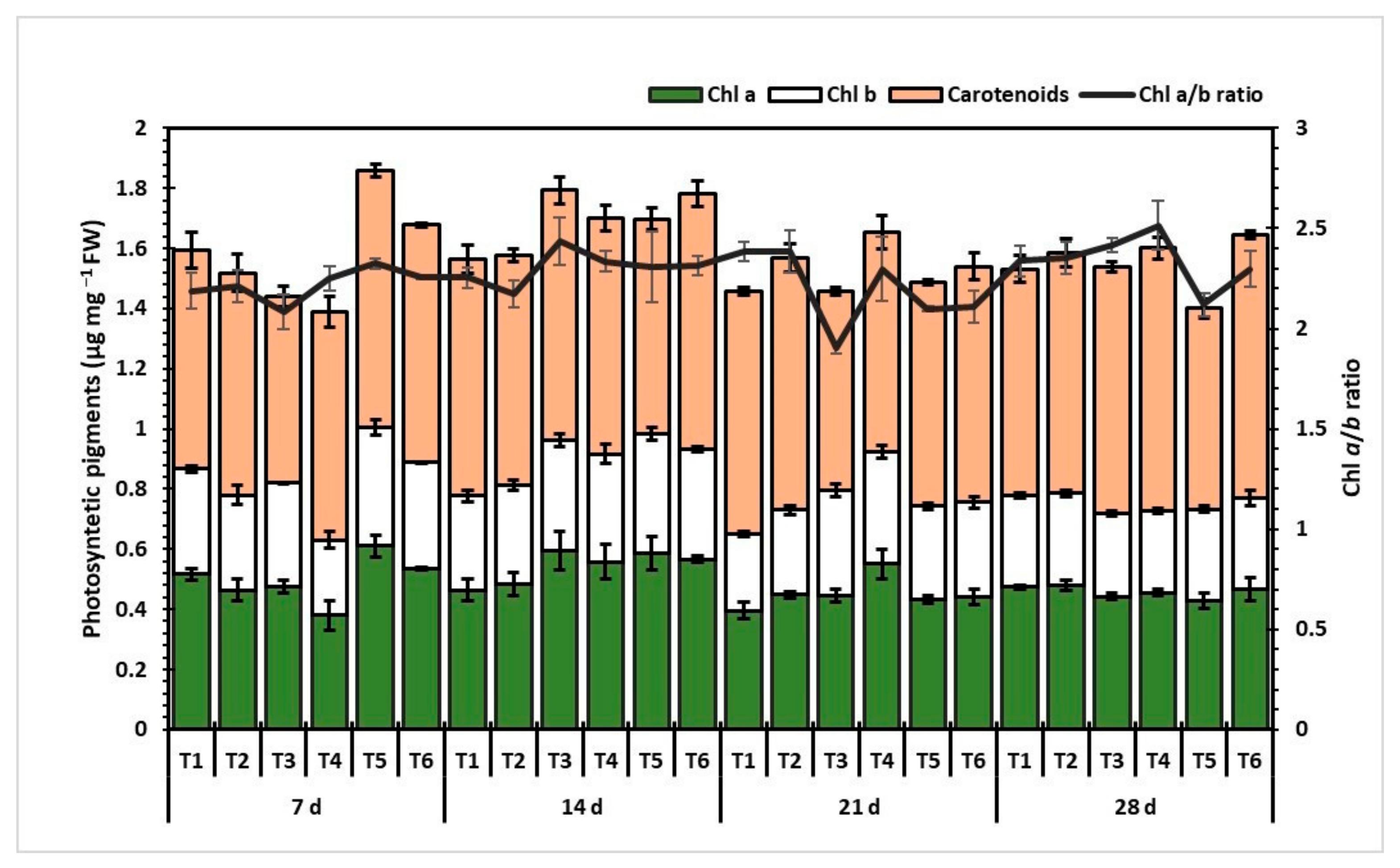
3.4. Photosynthetic Pigments
3.5. Antioxidants and Lipid Peroxidation Assay
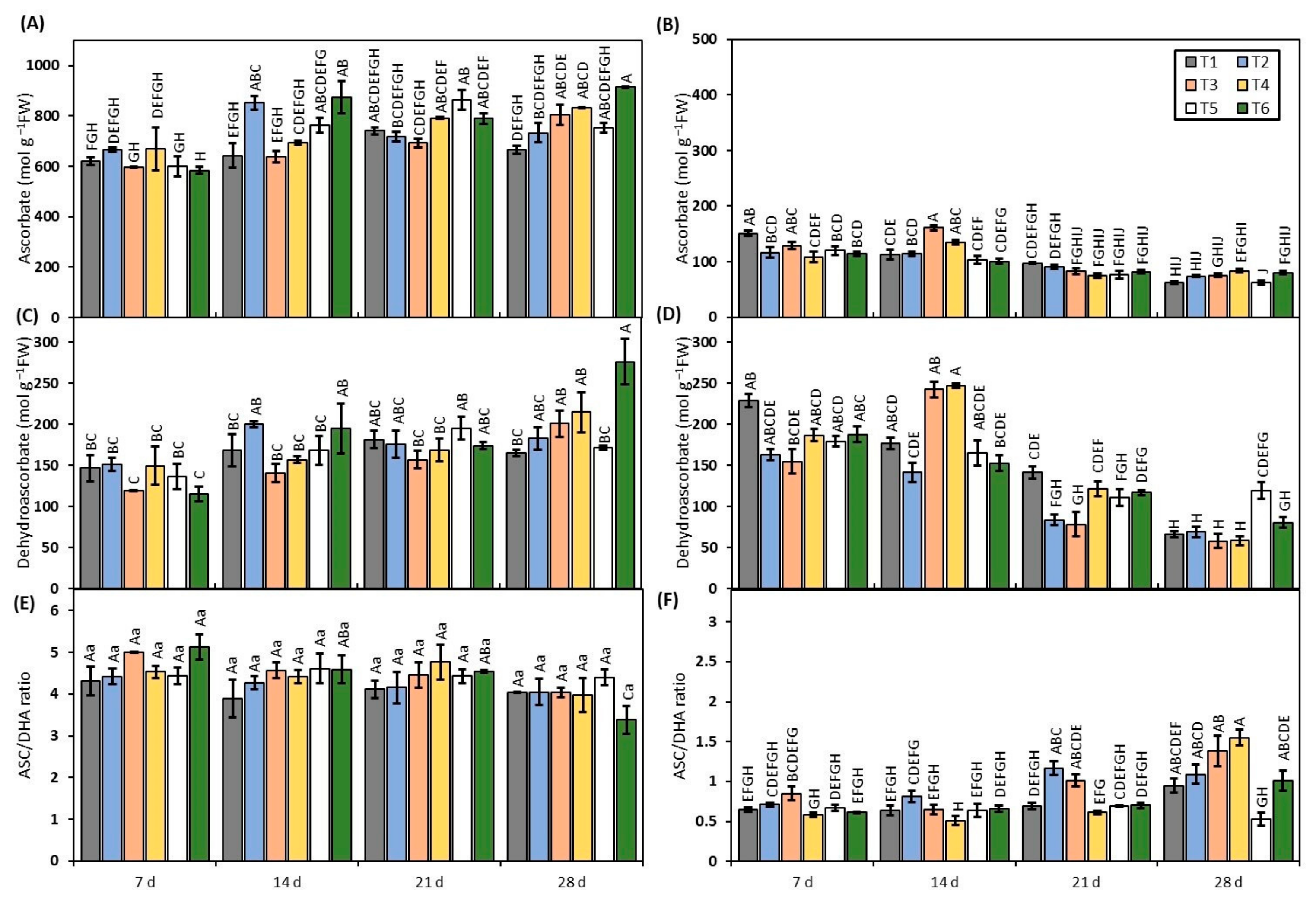
3.6. Ascorbate Determination
3.7. Superoxide Dismutase Activity
3.8. Organic Acid Exudates
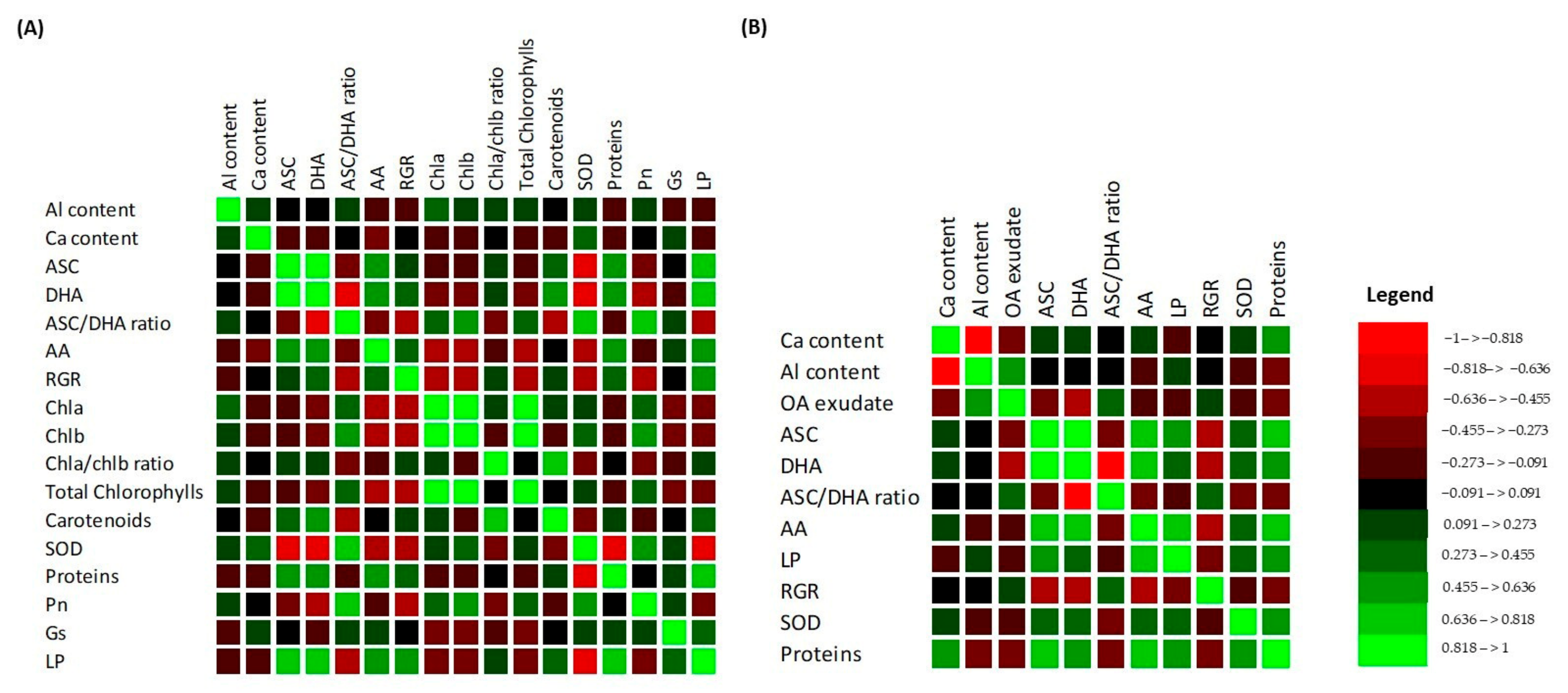
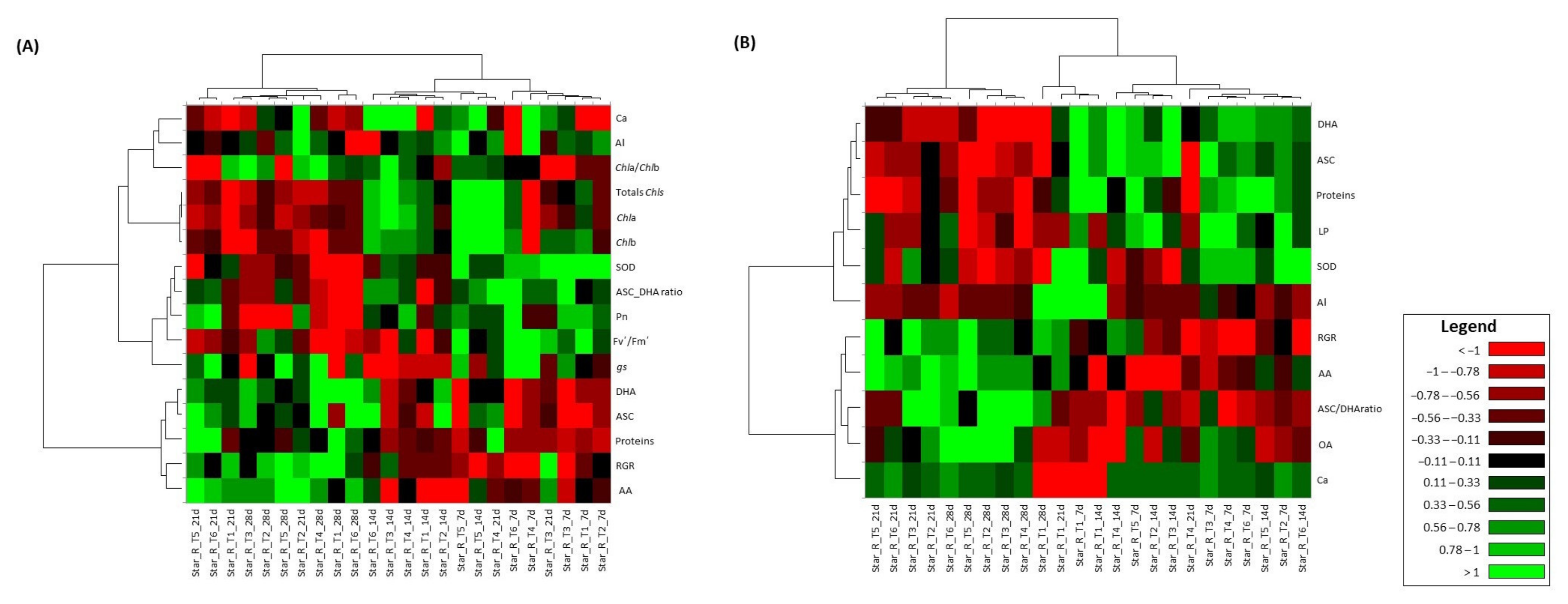
3.9. Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASC | Ascorbic acid/ascorbate |
| DHA | Dehydroascorbate |
| OA | Organic acids |
| LP | Lipid peroxidation |
| AA | Antioxidant activity |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPX | Glutathione peroxidase |
References
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2018, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, L.; Gu, L.; Zhao, W.; Su, H.; Cheng, X. Higher transcription levels in ascorbic acid biosynthetic and recycling genes were associated with higher ascorbic acid accumulation in blueberry. Food Chem. 2015, 188, 399–405. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Farooq, A.; Bukhari, S.A.; Akram, N.A.; Ashraf, M.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Exogenously applied ascorbic acid-mediated changes in osmoprotection and oxidative defense system enhanced water stress tolerance in different cultivars of safflower (Carthamus tinctorious L.). Plants 2020, 9, 104. [Google Scholar] [CrossRef]
- Celi, G.E.A.; Gratão, P.L.; Lanza, M.G.D.B.; dos Reis, A.R. Physiological and biochemical roles of ascorbic acid on mitigation of abiotic stresses in plants. Plant Physiol. Biochem. 2023, 202, 107970. [Google Scholar] [CrossRef]
- Shapiguzov, A.; Vainonen, J.P.; Wrzaczek, M.; Kangasjärvi, J. ROS-talk—How the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 2012, 3, 292. [Google Scholar] [CrossRef]
- Farvardin, A.; González-Hernández, A.I.; Llorens, E.; García-Agustín, P.; Scalschi, L.; Vicedo, B. The apoplast: A key player in plant survival. Antioxidants 2020, 9, 604. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid—A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Foy, C.; Scott, B.; Fisher, J. Genetic differences in plant tolerance to manganese toxicity. In Manganese in Soil and Plants; Graham, R.D., Hannam, R.J., Uren, N.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 293–307. [Google Scholar] [CrossRef]
- Silvia, S. Aluminium toxicity targets in plants. J. Bot. 2012, 219462. [Google Scholar] [CrossRef]
- Huang, M.; Xu, Q.; Deng, X.-X. L-ascorbic acid metabolism during fruit development in an ascorbate-rich fruit crop chestnut rose (Rosa roxburghii Tratt). J. Plant Physiol. 2014, 171, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Tai, A.; Fujinami, Y.; Sasaki, K.; Okazaki, S. Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-α-d-glucopyranosyl-l-ascorbic acids, as skin antioxidants. J. Med. Chem. 2002, 45, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Ryan, P.R. Aluminum toxicity. In Encyclopedia of Applied Plant Sciences: Plant Physiology and Development; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 211–218. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef] [PubMed]
- Furlan, F.; Borgo, L.; Rebelo, F.H.S.; Rossi, M.L.; Martinelli, A.P.; Azevedo, R.A.; Lavres, J. Aluminum induced toxicity in Urochloa brizantha genotypes: A first glance into root Al-apoplastic and symplastic compartmentation, Al-translocation and antioxidant performance. Chemosphere 2020, 243, 125362. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Green, M.A.; Fry, S.C. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature 2005, 433, 83–88. [Google Scholar] [CrossRef]
- Melino, V.J.; Soole, K.L.; Ford, C.M. A method for determination of fruit-derived ascorbic, tartaric, oxalic and malic acids, and its application to the study of ascorbic acid catabolism in grapevines. Aust. J. Grape Wine Res. 2009, 15, 293–302. [Google Scholar] [CrossRef]
- Cai, X.; Ge, C.; Xu, C.; Wang, X.; Wang, S.; Wang, Q. Expression analysis of oxalate metabolic pathway genes reveals oxalate regulation patterns in spinach. Molecules 2018, 23, 1286. [Google Scholar] [CrossRef]
- Lo’ay, A.; El-Khateeb, A. Antioxidant enzyme activities and exogenous ascorbic acid treatment of ‘Williams’ banana during long-term cold storage stress. Sci. Hortic. 2018, 234, 210–219. [Google Scholar] [CrossRef]
- Ribeiro, C.W.; Carvalho, F.E.L.; Rosa, S.B.; Alves-Ferreira, M.; Andrade, C.M.B.; Ribeiro-Alves, M.; Silveira, M.J.A.G.; Margis, R.; Margis-Pinheiro, M. Modulation of genes related to specific metabolic pathways in response to cytosolic ascorbate peroxidase knockdown in rice plants. Plant Biol. 2012, 14, 944–955. [Google Scholar] [CrossRef]
- Ribeiro, C.; de Marcos Lapaz, A.; de Freitas-Silva, L.; Ribeiro, K.V.G.; Yoshida, C.H.P.; Dal-Bianco, M.; Cambraia, J. Aluminum promotes changes in rice root structure and ascorbate and glutathione metabolism. Physiol. Mol. Biol. Plants 2022, 28, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, A.; AbdElgawad, H.; Han, A.; Teixeira, J.; Matos, M.; Fidalgo, F. Oxidative metabolism of rye (Secale cereale L.) after short term exposure to aluminum: Uncovering the glutathione–ascorbate redox network. Front. Plant Sci. 2016, 7, 685. [Google Scholar] [CrossRef]
- Cárcamo-Fincheira, P.; Reyes-Díaz, M.; Omena-García, R.P.; Vargas, J.R.; Alvear, M.; Florez-Sarasa, I.; Rosado-Souza, L.; Rengel, Z.; Fernie, A.R.; Nunes-Nesi, A.; et al. Metabolomic analyses of highbush blueberry (Vaccinium corymbosum L.) cultivars revealed mechanisms of resistance to aluminum toxicity. Environ. Exp. Bot. 2021, 183, 104338. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antioxid. Redox Signal. 2020, 23, 70. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Mellidou, I.; Koukounaras, A.; Kostas, S.; Patelou, E.; Kanellis, A.K. Regulation of vitamin C accumulation for improved tomato fruit quality and alleviation of abiotic stress. Genes 2021, 12, 694. [Google Scholar] [CrossRef]
- MacDonald, M.T.; Kannan, R.; Jayaseelan, R. Ascorbic acid preconditioning effect on broccoli seedling growth and photosynthesis under drought stress. Plants 2022, 11, 1324. [Google Scholar] [CrossRef]
- Elsiddig, M.I.A.; Zhou, G.; Nimir, N.E.A.; Ali, Y.A.A. Effect of exogenous ascorbic acid on two sorghum varieties under different types of salt stress. Chil. J. Agric. Res. 2022, 82, 10–20. [Google Scholar] [CrossRef]
- Loutfy, N.; Azooz, M.M.; Alhamd, M.F. Exogenously-applied salicylic acid and ascorbic acid modulate some physiological traits and antioxidative defense system in Zea mays L. seedlings under drought stress. Egypt. J. Bot. 2020, 60, 313–324. [Google Scholar] [CrossRef]
- El-Afry, M.M.; El-Okkiah Samira, A.F.; El-Kady, E.A.F.; El-Yamanee, G.S.A. Exogenous application of ascorbic acid for alleviation the adverse effects of salinity stress in flax (Linum usitatissimum L.). Middle East J. Agric. Res. 2018, 7, 716–739. [Google Scholar]
- Hassan, B.; Alirezaie, N.; Hossein, N.; Ahmad, N. Exogenous application of ascorbic acid alleviates chilling injury in apricot (Prunus armeniaca L. cv. Shahroudi) flowers. J. Stress Physiol. Biochem. 2013, 9, 199–206. [Google Scholar]
- Namiesnik, J.; Vearasilp, K.; Nemirovski, A.; Leontowicz, H.; Leontowicz, M.; Pasko, P.; Martinez-Ayala, A.L.; González-Aguilar, G.A.; Suhaj, M.; Gorinstein, S. In vitro studies on the relationship between the antioxidant activities of some berry extracts and their binding properties to serum albumin. Appl. Biochem. Biotechnol. 2014, 172, 2849–2865. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rufo, A.; Rodríguez-Solana, R.; Fernández-Recamales, M.Á.; Sayago-Gómez, A.; Weiland-Ardaiz, C.M. Comparative analysis of anatomical characteristics and phenolic compounds of two highbush blueberry (Vaccinium corymbosum L.) cultivars with different rooting ability of semi-hardwood cuttings. Sci. Hortic. 2024, 324, 112591. [Google Scholar] [CrossRef]
- Millaleo, R.; Alvear, M.; Aguilera, P.; González-Villagra, J.; de la Luz Mora, M.; Alberdi, M.; Reyes-Díaz, M. Mn toxicity differentially affects physiological and biochemical features in highbush blueberry (Vaccinium corymbosum L.) cultivars. J. Soil. Sci. Plant Nutr. 2020, 20, 795–805. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Liu, Y.; Zeng, Y.; Jiang, C. Aluminum toxicity could be mitigated with boron by altering the metabolic patterns of amino acids and carbohydrates rather than organic acids in trifoliate orange. Tree Physiol. 2019, 39, 1572–1582. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plant without soil. Calif. Agric. Exp. Stn. 1959, 347, 32. [Google Scholar]
- Hoffmann, W.A.; Poorter, H. Avoiding bias in calculations of relative growth rate. Ann. Bot. 2002, 90, 37–42. [Google Scholar] [CrossRef]
- Sadzawka, A.; Grez, R.; Carrasco, M.; Mora, M. Métodos de Análisis de Tejidos Vegetales; Comisión de Normalización y Acreditación: Santiago, Chile; Sociedad Chilena de la Ciencia del Suelo: Santiago, Chile, 2007; pp. 49–51. [Google Scholar]
- Reyes-Díaz, M.; Meriño-Gergichevich, C.; Alarcón, E.; Alberdi, M.; Horst, W.J. Calcium sulfate ameliorates the effect of aluminum toxicity differentially in genotypes of highbush blueberry (Vaccinium corymbosum L.). J. Soil Sci. Plant Nutr. 2011, 11, 59–78. [Google Scholar] [CrossRef]
- Medeiros, D.B.; Barros, K.A.; Barros, J.A.S.; Omena-Garcia, R.P.; Arrivault, S.; Sanglard, L.M.V.P.; Detmann, K.C.; Silva, W.B.; Daloso, D.M.; DaMatta, F.M.; et al. Impaired malate and fumarate accumulation due to the mutation of the tonoplast dicarboxylate transporter has little effects on stomatal behavior. Plant Physiol. 2017, 175, 1068–1081. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid peroxidation in sugar-rich plant tissue extracts. J. Agric. Food Chem. 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Chinnici, F.; Bendini, A.A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef] [PubMed]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Cárcamo-Fincheira, P.; Reyes-Díaz, M.; Omena-García, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Physiological and metabolic responses to aluminum toxicity reveal differing resistance mechanism to long-term exposure in highbush blueberry cultivars. Sci. Hortic. 2023, 309, 111665. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Rosas, A.; Rengel, Z.; Mora, M.L. Manganese supply and pH influence growth, carboxylate exudation and peroxidase activity of ryegrass and white clover. J. Plant Nutr. 2007, 30, 253–270. [Google Scholar] [CrossRef]
- Slessarev, E.W.; Lin, Y.; Bingham, N.L.; Johnson, J.E.; Dai, Y.; Schimel, J.P.; Chadwick, O.A. Water balance creates a threshold in soil pH at the global scale. Nature 2016, 540, 567–569. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Sun, C.; Lu, L.; Liu, L.; Liu, W.; Yu, Y.; Liu, X.; Hu, Y.; Jin, C.; Lin, X. Nitrate reductase-mediated early nitric oxide burst alleviates oxidative damage induced by aluminum through enhancement of antioxidant defenses in roots of wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, G.; Liu, Y.; Zhang, A.; Li, Y.; Liu, R.; Shi, L.; Jia, L.; Zhang, J. Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J. Exp. Bot. 2012, 63, 1809–1822. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Elhamid, E.M.A.; Mostafa, H.M. Alleviation of adverse effects of salt stress in wheat cultivars by foliar treatment with antioxidants I. changes in growth, some biochemical aspects and yield quantity and quality. Am.-Eur. J. Agric. Environ. Sci. 2013, 13, 1476–1487. Available online: https://www.idosi.org/aejaes/jaes13(11)13/5.pdf (accessed on 7 March 2025).
- Dinler, B.S.; Demir, E.; Kompe, Y.O. Regulation of auxin, abscisic acid and salicylic acid levels by ascorbate application under heat stress in sensitive and tolerant maize leaves. Acta Biol. Hung. 2014, 65, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Akram, N.A.; Aisha, R.; Shafiq, S.; Ashraf, M. Foliar-applied ascorbic acid enhances antioxidative potential and drought tolerance in cauliflower (Brassica oleracea L. var. Botrytis). Agrochimica 2016, 60, 100–113. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Rahman, M.H.; Haque, K.M.S.; Khan, M.Z.H. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Gad El-Hak, S.H.; Ahmed, A.M.; Moustafa, Y.M.M. Effect of foliar application with two antioxidants and humic acid on growth, yield and yield components of peas (Pisum sativum L.). J. Hortic. Sci. Ornamental Plants 2012, 4, 318–328. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plant and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Mehmood, A.; Naveed, K.; Liu, K.; Harrison, M.T.; Saud, S.; Hassan, S.; Nawaz, T.; Dhara, B.; Dai, D.Q.; Ali, I.; et al. Exogenous application of ascorbic acid improves physiological and productive traits of Nigella sativa. Heliyon 2024, 10, e28766. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Puthur, J.T.; Nagy, V.; Garab, G. Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol. 2009, 149, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zou, J.; Li, S.; Lin, J.; He, L.; Xu, D.; Liao, X.; Li, Q.; Ma, J. Ascorbic acid-enhanced Fe(III)/peracetic acid process for the degradation of diclofenac: Treatment efficiency, mechanism and influencing factors. Sep. Purif. Technol. 2024, 330, 125382. [Google Scholar] [CrossRef]
- Shaikhali, J.; Heiber, I.; Seidel, T.; Ströher, E.; Hiltscher, H.; Birkmann, S.; Dietz, K.-J.; Baier, M. The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol. 2008, 8, 48. [Google Scholar] [CrossRef]
- Plumb, W.; Townsend, A.J.; Rasool, B.; Alomrani, S.; Razak, N.; Karpinska, B.; Ruban, A.V.; Foyer, C.H. Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in Arabidopsis thaliana. J. Exp. Bot. 2018, 69, 2823–2835. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef]
- Rudenko, N.N.; Vetoshkina, D.V.; Marenkova, T.V.; Borisova-Mubarakshina, M.M. Antioxidants of non-enzymatic nature: Their function in higher plant cells and the ways of boosting their biosynthesis. Antioxidants 2023, 12, 2014. [Google Scholar] [CrossRef]
- Mahmood, A.M.; Dunwell, J.M. 2-oxoglutarate-dependent dioxygenases: A renaissance in attention for ascorbic acid in plants. PLoS ONE 2020, 15, e0242833. [Google Scholar] [CrossRef]
- Alamri, S.A.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Khan, M.N.; Ali, H.M.; Alaraidh, I.A.; Alsahli, A.A.; Al-Rabiah, H.; Mateen, M. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J. Plant Interact. 2018, 13, 409–419. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kishor, P.B.K. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit acid soils. Biometals 2016, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Cárcamo-Fincheira, P.; Nunes-Nesi, A.; Soto-Cerda, B.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Ascorbic acid metabolism: New knowledge on mitigation of aluminum stress in plants. Plant Physiol. Biochem. 2024, 217, 109228. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Han, J.C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A.; et al. Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef]
- Potters, G.; Horemans, N.; Jansen, M.A.K. The cellular redox state in plant stress biology—A charging concept. Plant Physiol. Biochem. 2010, 48, 292–300. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant. 2021, 173, 1765–1784. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Gill, R.; Hasanuzzaman, M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Tuteja, R.; Tuteja, N. Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma 2014, 251, 1265–1283. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Eltayeb, A.E.; Uddin, M.I.; Yamamoto, Y.; Tsuji, W.; Takeuchi, Y.; Tanaka, K. Overexpression of dehydroascor-bate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tabacco. Planta 2010, 231, 609–621. [Google Scholar] [CrossRef]


| Shoots | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 7 d | 14 d | 21 d | 28 d | ||||||||||||
| Average | SE | Average | SE | Average | SE | Average | SE | |||||||||
| T1 | 1.42 | ± | 0.06 | Aa | 1.44 | ± | 0.15 | Aab | 1.77 | ± | 0.17 | Aa | 1.95 | ± | 0.14 | Aa |
| T2 | 1.54 | ± | 0.13 | Aa | 1.42 | ± | 0.07 | Aab | 1.73 | ± | 0.29 | Aa | 1.72 | ± | 0.13 | Aa |
| T3 | 1.26 | ± | 0.16 | Aa | 1.62 | ± | 0.12 | Aa | 1.78 | ± | 0.08 | Aa | 1.56 | ± | 0.30 | Aa |
| T4 | 1.32 | ± | 0.22 | Aa | 1.41 | ± | 0.21 | Aab | 1.41 | ± | 0.07 | Aa | 2.04 | ± | 0.15 | Aa |
| T5 | 1.41 | ± | 0.10 | Aa | 1.00 | ± | 0.24 | Ab | 1.69 | ± | 0.14 | Aa | 1.80 | ± | 0.27 | Aa |
| T6 | 1.22 | ± | 0.13 | Aa | 1.47 | ± | 0.24 | Aab | 1.54 | ± | 0.15 | Aa | 1.60 | ± | 0.12 | Aa |
| Roots | ||||||||||||||||
| Treatment | 7 d | 14 d | 21 d | 28 d | ||||||||||||
| Average | SE | Average | SE | Average | SE | Average | SE | |||||||||
| T1 | 0.84 | ± | 0.04 | ABCDE | 0.83 | ± | 0.14 | ABCDE | 0.84 | ± | 0.07 | ABCDE | 1.11 | ± | 0.16 | ABCD |
| T2 | 0.63 | ± | 0.03 | DE | 0.75 | ± | 0.08 | BCDE | 1.05 | ± | 0.12 | ABCD | 0.99 | ± | 0.07 | ABCD |
| T3 | 0.83 | ± | 0.06 | ABCDE | 0.30 | ± | 0.02 | F | 1.29 | ± | 0.14 | AB | 1.02 | ± | 0.07 | ABCD |
| T4 | 1.05 | ± | 0.13 | ABCD | 1.2 | ± | 0.15 | ABC | 0.71 | ± | 0.14 | CDE | 1.26 | ± | 0.11 | ABC |
| T5 | 0.87 | ± | 0.06 | ABCDE | 1.1 | ± | 0.09 | ABCD | 1.35 | ± | 0.12 | A | 1.35 | ± | 0.07 | AB |
| T6 | 0.49 | ± | 0.03 | EF | 0.76 | ± | 0.11 | BCDE | 1.02 | ± | 0.13 | ABCD | 1.05 | ± | 0.11 | ABCD |
| Pn (µmol CO2 m−2 s−1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 7 d | 14 d | 21 d | 28 d | ||||||||||||
| Average | SE | Average | SE | Average | SE | Average | SE | |||||||||
| T1 | 2.60 | ± | 0.19 | ABC | 1.82 | ± | 0.08 | DEF | 1.82 | ± | 0.08 | DEF | 1.18 | ± | 0.09 | G |
| T2 | 2.30 | ± | 0.07 | BCD | 2.20 | ± | 0.12 | BCD | 2.50 | ± | 0.07 | ABCD | 1.30 | ± | 0.08 | G |
| T3 | 2.53 | ± | 0.06 | ABC | 2.02 | ± | 0.03 | CDE | 1.94 | ± | 0.12 | CDEF | 1.31 | ± | 0.10 | G |
| T4 | 1.95 | ± | 0.06 | CDEF | 2.61 | ± | 0.09 | BCD | 2.19 | ± | 0.14 | BCD | 1.55 | ± | 0.04 | EFG |
| T5 | 2.41 | ± | 0.18 | BCD | 2.13 | ± | 0.07 | BCDE | 2.66 | ± | 0.25 | BCD | 1.31 | ± | 0.13 | G |
| T6 | 3.40 | ± | 0.20 | A | 2.16 | ± | 0.19 | BCD | 2.93 | ± | 0.12 | AB | 1.46 | ± | 0.01 | FG |
| gs (mol H2O m−2 s−1) | ||||||||||||||||
| Treatment | 7 d | 14 d | 21 d | 28 d | ||||||||||||
| Average | SE | Average | SE | Average | SE | Average | SE | |||||||||
| T1 | 0.20 | ± | 0.06 | ABCDE | 0.11 | ± | 0.01 | BCDE | 0.20 | ± | 0.09 | ABCDE | 0.09 | ± | 0.04 | CDE |
| T2 | 0.15 | ± | 0.04 | ABCDE | 0.10 | ± | 0.01 | BCDE | 0.20 | ± | 0.08 | ABCDE | 0.19 | ± | 0.05 | ABCDE |
| T3 | 0.30 | ± | 0.10 | ABCDE | 0.06 | ± | 0.02 | E | 0.14 | ± | 0.04 | ABCDE | 0.09 | ± | 0.03 | BCDE |
| T4 | 0.43 | ± | 0.08 | ABC | 0.10 | ± | 0.03 | BCDE | 0.22 | ± | 0.05 | ABCDE | 0.68 | ± | 0.04 | A |
| T5 | 0.36 | ± | 0.14 | ABCD | 0.11 | ± | 0.04 | BCDE | 0.27 | ± | 0.03 | ABCDE | 0.40 | ± | 0.14 | ABCD |
| T6 | 0.49 | ± | 0.08 | AB | 0.06 | ± | 0.01 | DE | 0.41 | ± | 0.10 | ABC | 0.26 | ± | 0.03 | ABCDE |
| Oxalate (µmol g−1 h−1) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | 7 d | 14 d | 21 d | 28 d | ||||||||||||
| Average | SE | Average | SE | Average | SE | Average | SE | |||||||||
| T1 | 54.2 | ± | 7.7 | Aa | 41.6 | ± | 4.9 | Aa | 51.5 | ± | 6.4 | Aa | 52.0 | ± | 4.4 | Aa |
| T2 | 53.2 | ± | 5.0 | Aa | 52.4 | ± | 7.6 | Aa | 76.0 | ± | 24.8 | Aa | 88.0 | ± | 12.8 | Aa |
| T3 | 75.1 | ± | 10.1 | Aa | 66.0 | ± | 6.9 | Aa | 61.9 | ± | 27.0 | Aa | 87.4 | ± | 17.5 | Aa |
| T4 | 70.1 | ± | 5.2 | Aa | 41.2 | ± | 20.4 | Aa | 57.9 | ± | 9.8 | Aa | 65.8 | ± | 3.2 | Aa |
| T5 | 69.6 | ± | 12.0 | Aab | 51.0 | ± | 4.7 | Ab | 61.2 | ± | 4.6 | Aab | 112.5 | ± | 24.5 | Aa |
| T6 | 66.1 | ± | 13.8 | Aa | 56.2 | ± | 18.5 | Aa | 66.7 | ± | 18.5 | Aa | 93.0 | ± | 10.4 | Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárcamo-Fincheira, P.; Nunes-Nesi, A.; Soto-Cerda, B.; Tighe-Neira, R.; Tranamil-Manquein, J.; Mora-Sanhueza, R.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Ascorbic Acid Mitigates Aluminum Stress Through Improved Antioxidant Mechanism in Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae 2025, 11, 330. https://doi.org/10.3390/horticulturae11030330
Cárcamo-Fincheira P, Nunes-Nesi A, Soto-Cerda B, Tighe-Neira R, Tranamil-Manquein J, Mora-Sanhueza R, Inostroza-Blancheteau C, Reyes-Díaz M. Ascorbic Acid Mitigates Aluminum Stress Through Improved Antioxidant Mechanism in Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae. 2025; 11(3):330. https://doi.org/10.3390/horticulturae11030330
Chicago/Turabian StyleCárcamo-Fincheira, Paz, Adriano Nunes-Nesi, Braulio Soto-Cerda, Ricardo Tighe-Neira, Jaime Tranamil-Manquein, Rodrigo Mora-Sanhueza, Claudio Inostroza-Blancheteau, and Marjorie Reyes-Díaz. 2025. "Ascorbic Acid Mitigates Aluminum Stress Through Improved Antioxidant Mechanism in Highbush Blueberry (Vaccinium corymbosum L.)" Horticulturae 11, no. 3: 330. https://doi.org/10.3390/horticulturae11030330
APA StyleCárcamo-Fincheira, P., Nunes-Nesi, A., Soto-Cerda, B., Tighe-Neira, R., Tranamil-Manquein, J., Mora-Sanhueza, R., Inostroza-Blancheteau, C., & Reyes-Díaz, M. (2025). Ascorbic Acid Mitigates Aluminum Stress Through Improved Antioxidant Mechanism in Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae, 11(3), 330. https://doi.org/10.3390/horticulturae11030330








