Shoots Regeneration in Brigitta and Duke Blueberry Cultivars from Different Encapsulated Vegetative Propagules
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Culture Conditions
2.3. Encapsulating Procedure and Growing Conditions
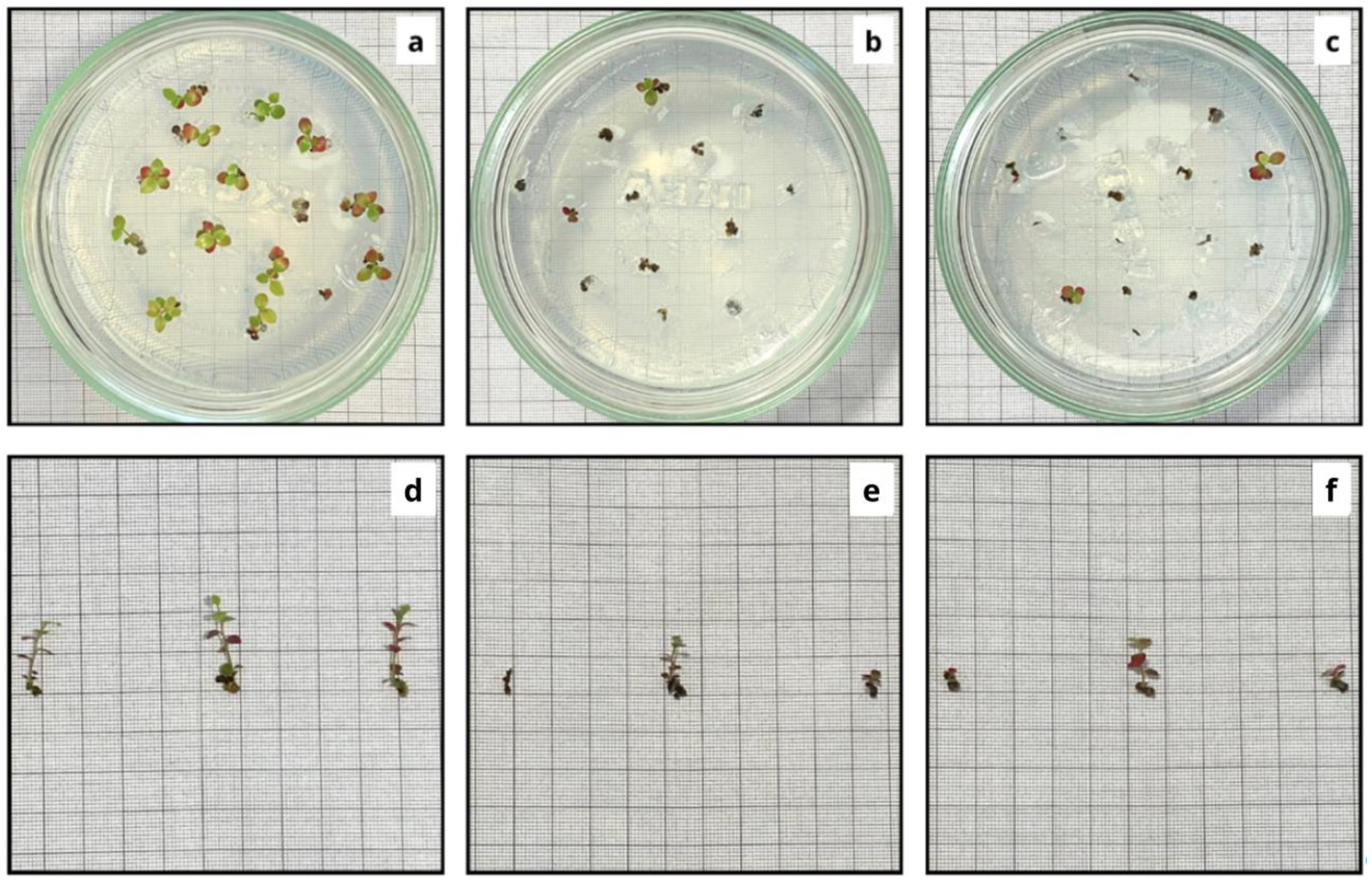
2.4. Data Collection
- -
- Viability (%): incidence of green propagules without browning or necrosis;
- -
- Regrowth (%): incidence of propagules that sprouted or rooted;
- -
- Conversion (%): incidence of propagules that sprouted and rooted at the same time);
- -
- Shoots produced (n) and shoot length (mm): average number of shoots produced per vital explant and their length;
- -
- Roots produced (n) and root length (mm): average roots produced per vital explant and their length;
- -
- Callus (%) and callus fresh weight (mg): incidence of propagules with callus and callus fresh weight;
- -
- Green fresh weight (mg): average fresh weight of vegetative organs (leaves, stems, buds) per vital explant;
- -
- Total dry weight per explant (mg) obtained by keeping the plant material in an oven for three days at 105 °C.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, G.-Q. Blueberry (Vaccinium corymbosum L.). In Agrobacterium Protocols: Volume 2; Springer: New York, NY, USA, 2015; pp. 121–131. [Google Scholar]
- Ehlenfeldt, M. The Delightful Domesticated American Blueberry: Some Research Challenges for Its Next 100 Years. Agric. Res. 2011, 59, 2. [Google Scholar]
- Yu, X.; Yuan, H.; Jin, Y.; Xia, C.; Zhu, J.; Che, J.; Yang, J.; Wang, X.; Zheng, B.; Yang, S.; et al. Establishment of a Breeding Approach Combined with Gamma Ray Irradiation and Tissue Regeneration for Highbush Blueberry. Agronomy 2025, 15, 217. [Google Scholar] [CrossRef]
- Cui, F.; Ye, X.; Li, X.; Yang, Y.; Hu, Z.; Overmyer, K.; Brosché, M.; Yu, H.; Salojärvi, J. Chromosome-Level Genome Assembly of the Diploid Blueberry Vaccinium Darrowii Provides Insights into Its Subtropical Adaptation and Cuticle Synthesis. Plant Commun. 2022, 3, 100307. [Google Scholar] [CrossRef]
- Edger, P.P.; Iorizzo, M.; Bassil, N.V.; Benevenuto, J.; Ferrão, L.F.V.; Giongo, L.; Hummer, K.; Lawas, L.M.F.; Leisner, C.P.; Zalapa, J.; et al. There and back again; historical perspective and future directions for Vaccinium breeding and research studies. Hortic. Res. 2022, 9, uhac083. [Google Scholar]
- Brevis, P.A.; Bassil, N.V.; Ballington, J.R.; Hancock, J.F. Impact of Wide Hybridization on Highbush Blueberry Breeding. J. Am. Soc. Hortic. Sci. 2008, 133, 427–437. [Google Scholar] [CrossRef]
- Ashique, S.; Mukherjee, T.; Mohanty, S.; Garg, A.; Mishra, N.; Kaushik, M.; Bhowmick, M.; Chattaraj, B.; Mohanto, S.; Srivastava, S.; et al. Blueberries in Focus: Exploring the Phytochemical Potentials and Therapeutic Applications. J. Agric. Food Res. 2024, 18, 101300. [Google Scholar] [CrossRef]
- Lobos, G.A.; Hancock, J.F. Breeding Blueberries for a Changing Global Environment: A Review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 17 January 2025).
- Mazurek, M.; Siekierzyńska, A.; Piechowiak, T.; Spinardi, A.; Litwińczuk, W. Comprehensive Analysis of Highbush Blueberry Plants Propagated In Vitro and Conventionally. Int. J. Mol. Sci. 2024, 25, 544. [Google Scholar] [CrossRef]
- Mohamed, G.R.A.; Khusnetdinova, L.Z.; Timofeeva, O.A. Elaboration of micropropagation protocol for Vaccinium corymbosum cv.” Sunt Blue Giant. Asian J. Plant Sci. Res. 2018, 8, 1–11. [Google Scholar]
- Correia, S.; Matos, M.; Leal, F. Advances in Blueberry (Vaccinium spp.) In Vitro Culture: A Review. Horticulturae 2024, 10, 533. [Google Scholar] [CrossRef]
- Regni, L.; Micheli, M.; Del Pino, A.M.; Palmerini, C.A.; D’Amato, R.; Facchin, S.L.; Famiani, F.; Peruzzi, A.; Mairech, H.; Proietti, P. The First Evidence of the Beneficial Effects of Se-Supplementation on In Vitro Cultivated Olive Tree Explants. Plants 2021, 10, 1630. [Google Scholar] [CrossRef]
- Dobránszki, J.; da Silva, J.A.T. Micropropagation of Apple—A Review. Biotechnol. Adv. 2010, 28, 462–488. [Google Scholar] [CrossRef]
- Kavand, S.; Kermani, M.J.; Haghnazari, A.; Khosravi, P.; Azimi, M.R. Micropropagation and Medium-Term Conservation of Rosa Pulverulenta. Acta Sci. Agron. 2011, 33, 297–301. [Google Scholar] [CrossRef]
- Regni, L.; Micheli, M.; Pino, A.M.D.; Facchin, S.L.; Rabica, E.; Camilloni, L.; Cesarini, A.; Proietti, P. Blackberry Synthetic Seeds Storage: Effects of Temperature, Time, and Sowing Substrate. Plant Cell Tissue Organ Cult. 2024, 158, 17. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Kareem, F.; El-Mahrouk, M.E.; Fuller, M.P. Artificial Seeds (Principle, Aspects and Applications). Agronomy 2017, 7, 71. [Google Scholar] [CrossRef]
- Standardi, A.; Micheli, M. Encapsulation of in Vitro-Derived Explants: An Innovative Tool for Nurseries. Methods Mol. Biol. 2013, 11013, 397–418. [Google Scholar] [CrossRef]
- Ahmad, N.; Shahid, A.; Javed, S.B.; Khan, M.I.; Anis, M. Micropropagation of Vitex spp. through in Vitro Manipulation: Current Status and Future Prospectives. J. Appl. Res. Med. Aromat. Plants 2015, 2, 114–123. [Google Scholar] [CrossRef]
- Asmah, N.H.; Hasnida, N.H.; Zaimah, N.A.N.; Noraliza, A.; Salmi, N.N. Synthetic Seed Technology for Encapsulation and Regrowth of in Vitro-Derived Acacia Hyrid Shoot and Axillary Buds. Afr. J. Biotechnol. 2011, 10, 7820–7824. [Google Scholar] [CrossRef]
- Rai, M.K.; Asthana, P.; Singh, S.K.; Jaiswal, V.S.; Jaiswal, U. The Encapsulation Technology in Fruit Plants—A Review. Biotechnol. Adv. 2009, 27, 671–679. [Google Scholar] [CrossRef]
- Magray, M.M.; Wani, K.P.; Chatto, M.A.; Ummyiah, H.M. Synthetic Seed Technology. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 662–674. [Google Scholar] [CrossRef]
- Regni, L.; Micheli, M.; Facchin, S.L.; Del Pino, A.M.; Silvestri, C.; Proietti, P. The Influence of the Explant’s Type on the Performance of Synthetic Seeds of Blackberry (Rubus spp.). Plants 2024, 13, 32. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B.H. Commercially-Feasible Micropropagation of Mountain Laurel, Kalmia Latifolia, by Use of Shoot-Tip Culture. Comb. Proc.-Int. Plant Propagator’s Soc. 1980, 30, 421–427. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Piccioni, E.; Standardi, A. Encapsulation of Micropropagated Buds of Six Woody Species. Plant Cell Tissue Organ Cult. 1995, 42, 221–226. [Google Scholar] [CrossRef]
- Sharma, S.; Shahzad, A.; Teixeira da Silva, J.A. Synseed Technology—A Complete Synthesis. Biotechnol. Adv. 2013, 31, 186–207. [Google Scholar] [CrossRef]
- Benelli, C. Encapsulation of Shoot Tips and Nodal Segments for in Vitro Storage of ‘Kober 5BB’ Grapevine Rootstock. Horticulturae 2016, 2, 10. [Google Scholar] [CrossRef]
- Micheli, M.; Standardi, A.; Fernandes da Silva, D. Encapsulation and Synthetic Seeds of Olive (Olea europaea L.): Experiences and Overview. In Synthetic Seeds: Germplasm Regeneration, Preservation and Prospects; Faisal, M., Alatar, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 347–361. ISBN 978-3-030-24631-0. [Google Scholar]
- Ballester, A.; Janeiro, L.V.; Vieitez, A.M. Cold Storage of Shoot Cultures and Alginate Encapsulation of Shoot Tips of Camellia japonica L. and Camellia reticulata Lindley. Sci. Hortic. 1997, 71, 67–78. [Google Scholar] [CrossRef]
- Cao, H.; Trueman, S. Alginate Encapsulation of Shoot Tips and Nodal Segments for Short-Term Storage and Distribution of the Eucalypt Corymbia torelliana × C. citriodora. Acta Physiol. Plant. 2012, 34, 117–128. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B. Alginate-Encapsulated Shoot Tips and Nodal Segments in Mictopropagation of Medicinal Plands. A Review. Herba Pol. 2011, 57, 45–57. [Google Scholar]
- Dönmez, D. Regeneration of Plants from Alginate-Encapsulated Shoot Tips of Myrtle (Myrtus communis L.). Erwerbs-Obstbau 2022, 64, 307–314. [Google Scholar] [CrossRef]
- Barraco, G.; Sylvestre, I.; Engelmann, F. Comparing Encapsulation-Dehydration and Droplet-Vitrification for Cryopreservation of Sugarcane (Saccharum spp.) shoot tips. Sci. Hortic. 2011, 130, 320–324. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]

| Explant’s Type | Vitality | Regrowth | Shoot Number | Shoot Length | Shoots with Basal Callus | Callus Weight | Shoot Fresh Weight | Total Dry Weight |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (n) | (mm) | (%) | (mg) | (mg) | (mg) | |
| Shoot tips | 85 ± 5 a | 85 ± 5 a | 1.1 ± 0.05 a | 11.66 ± 1.03 a | 85 ± 5 a | 9.28 ± 2.03 a | 5.89 ± 0.42 a | 4.44 ± 0.62 a |
| Medium node | 30 ± 6 b | 30 ± 6 b | 1.1± 0.1 a | 5.28 ± 1.10 b | 27 ± 6 b | 9.25 ± 2.50 a | 2.49 ± 0.68 b | 2.44 ± 0.87 a |
| Basal node | 40 ± 9 b | 40 ± 9 b | 1.2 ±0.1 a | 5.15 ± 0.68 b | 40 ± 9 b | 9.98 ± 2.67 a | 2.64 ± 0.42 b | 2.93 ± 0.46 a |
| Explant’s Type | Vitality | Regrowth | Shoot Number | Shoot Length | Shoots with Basal Callus | Callus Fresh Weight | Shoot Fresh Weight | Total Dry Weight |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (n) | (mm) | (%) | (mg) | (mg) | (mg) | |
| Shoot tips | 85 ± 3 a | 85 ± 3 a | 1.0 a | 6.73 ± 0.60 a | 82 ± 6 a | 6.08 ± 1.72 a | 5.33 ± 0.91 a | 2.72 ± 0.62 a |
| Medium node | 38 ± 10 b | 38 ± 10 b | 1.0 a | 2.13 ± 0.31 b | 15 ± 8 b | 1.09 ± 0.29 b | 2.25 ± 0.47 b | 0.84 ± 0.17 b |
| Basal node | 12 ± 3 c | 12 ± 3 c | 1.2 ± 0.17 a | 2.50 ± 0.29 b | 0 c | 0 c | 3.83 ± 0.50 ab | 1.23 ± 0.11 b |
| Vitality (%) | Regrowth (%) | Shoot Number (n) | Shoot Length (mm) | Callus (%) | Callus FW (mg) | Green FW (mg) | Total DW (mg) | |
|---|---|---|---|---|---|---|---|---|
| Propagule type (A) | ** | ** | ns | ** | ** | * | ** | * |
| Cultivar (B) | ns | ns | ns | ** | ** | * | ns | ** |
| A × B | ns | ns | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regni, L.; Cesarini, A.; Calisti, S.; Proietti, P.; Micheli, M. Shoots Regeneration in Brigitta and Duke Blueberry Cultivars from Different Encapsulated Vegetative Propagules. Horticulturae 2025, 11, 259. https://doi.org/10.3390/horticulturae11030259
Regni L, Cesarini A, Calisti S, Proietti P, Micheli M. Shoots Regeneration in Brigitta and Duke Blueberry Cultivars from Different Encapsulated Vegetative Propagules. Horticulturae. 2025; 11(3):259. https://doi.org/10.3390/horticulturae11030259
Chicago/Turabian StyleRegni, Luca, Arianna Cesarini, Silvia Calisti, Primo Proietti, and Maurizio Micheli. 2025. "Shoots Regeneration in Brigitta and Duke Blueberry Cultivars from Different Encapsulated Vegetative Propagules" Horticulturae 11, no. 3: 259. https://doi.org/10.3390/horticulturae11030259
APA StyleRegni, L., Cesarini, A., Calisti, S., Proietti, P., & Micheli, M. (2025). Shoots Regeneration in Brigitta and Duke Blueberry Cultivars from Different Encapsulated Vegetative Propagules. Horticulturae, 11(3), 259. https://doi.org/10.3390/horticulturae11030259








