Jatropha curcas Seed Germination: Effect of Seed Imbibition, Aging, Storage, and Salinity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Germination of J. curcas Seeds After Imbibition Process
2.3. Artificial Seed Aging and Seed Storage
2.4. Effect of Controlled Air Humidity and Cooler Temperatures on Stored J. curcas Germination
2.4.1. Seed Germination
2.4.2. Biochemical and Metabolic Analysis in Stored J. curcas Seeds
2.5. Effect of Salinity on J. curcas Germination
2.6. RT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Germination of Jatropha curcas Seed After Imbibition
3.1.1. pH and Electrical Conductivity of the Imbibition Water
3.1.2. Effect of Seed Imbibition on Seed Water Content and Seed Moisture
3.1.3. Effect of Seed Imbibition on Germination Percentage and Mean Germination Time
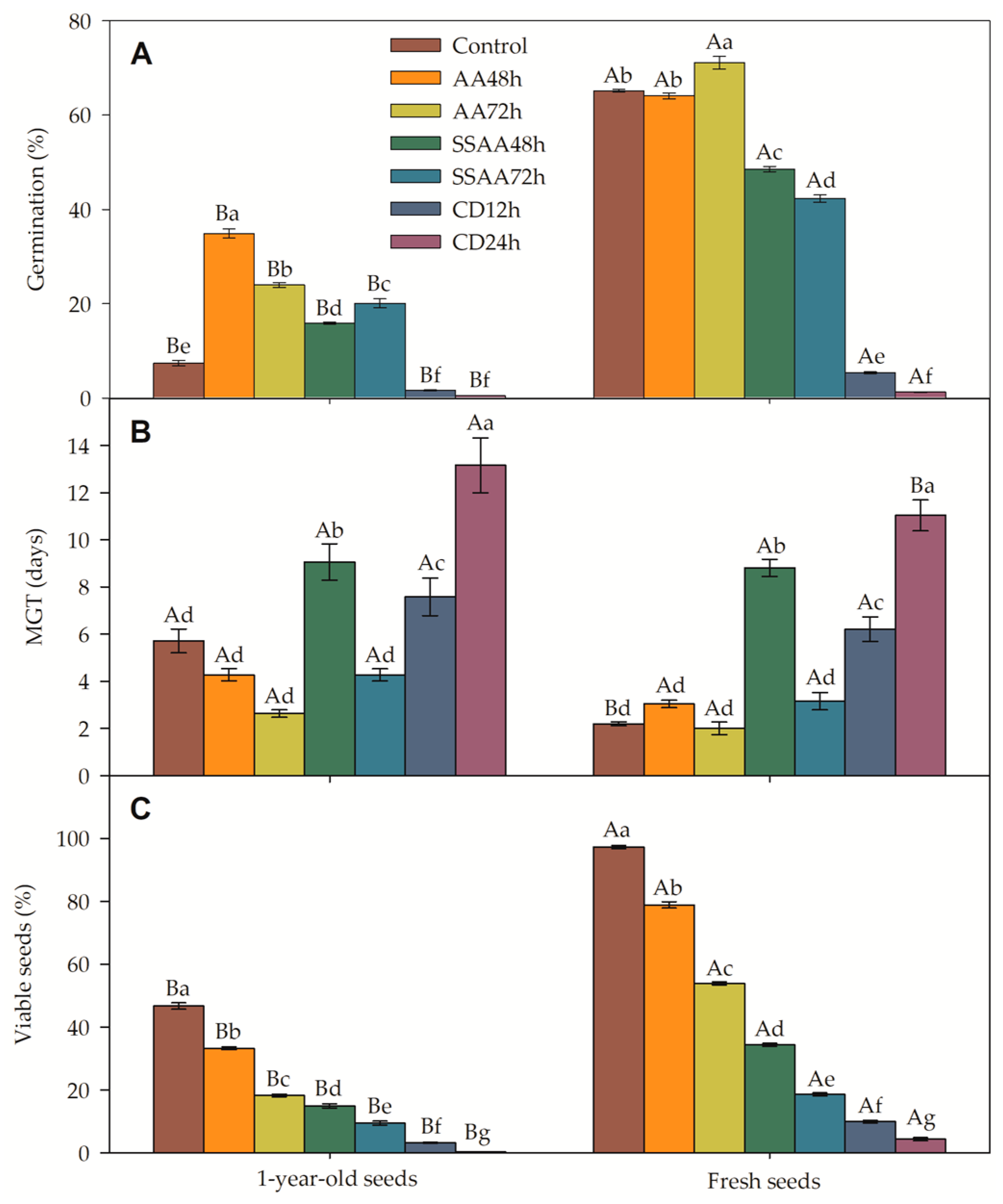
3.2. Germination of Jatropha curcas Seed Under Accelerated Aging (AA), Saturated Salt Accelerated Aging (SSAA), and Controlled Deterioration (CD)
3.2.1. Seed Germination
3.2.2. Mean Germination Time (MGT)
3.2.3. Viable Seeds
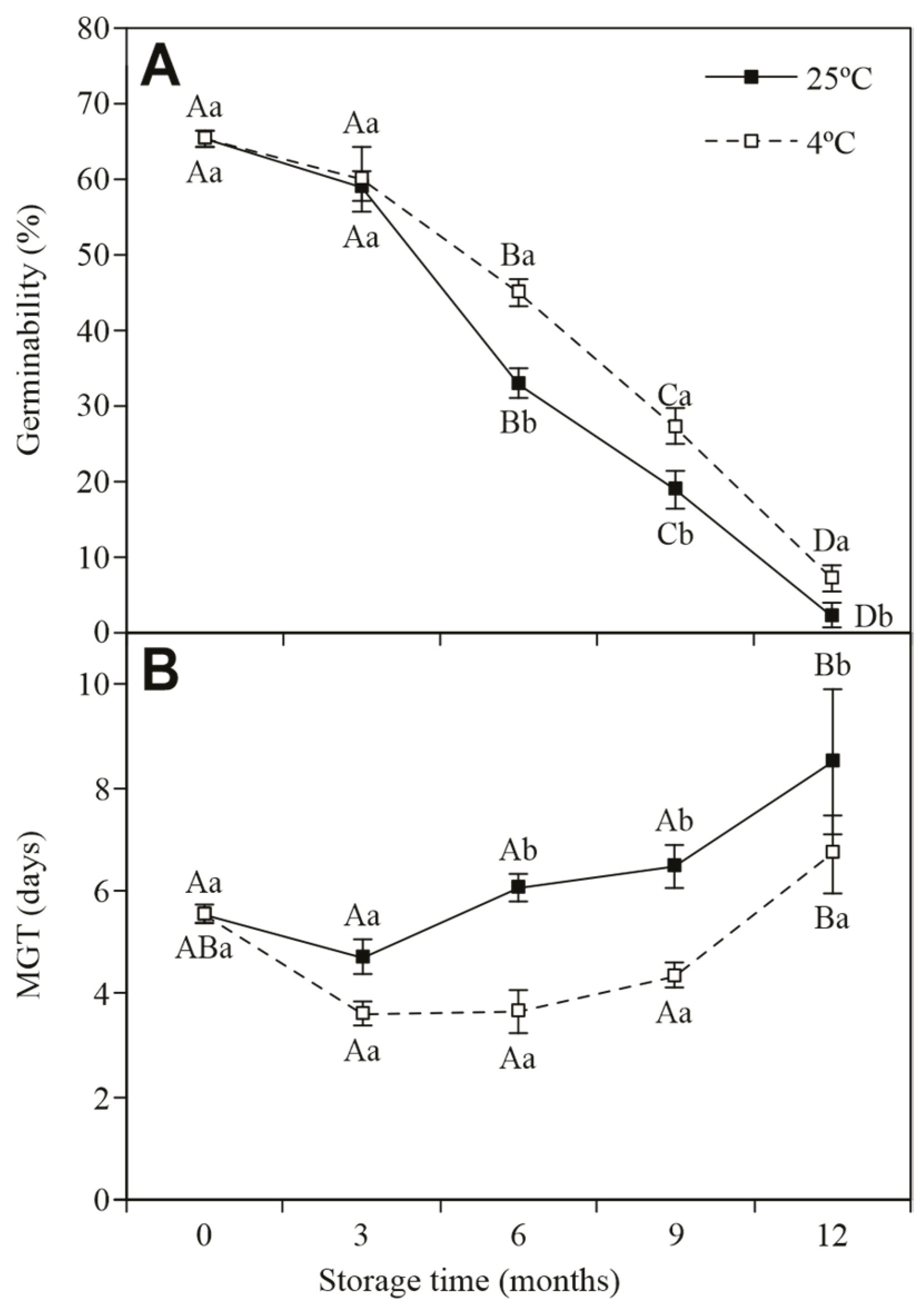
3.3. Germination of Jatropha curcas Stored Under Two Different Temperatures for 12 Months
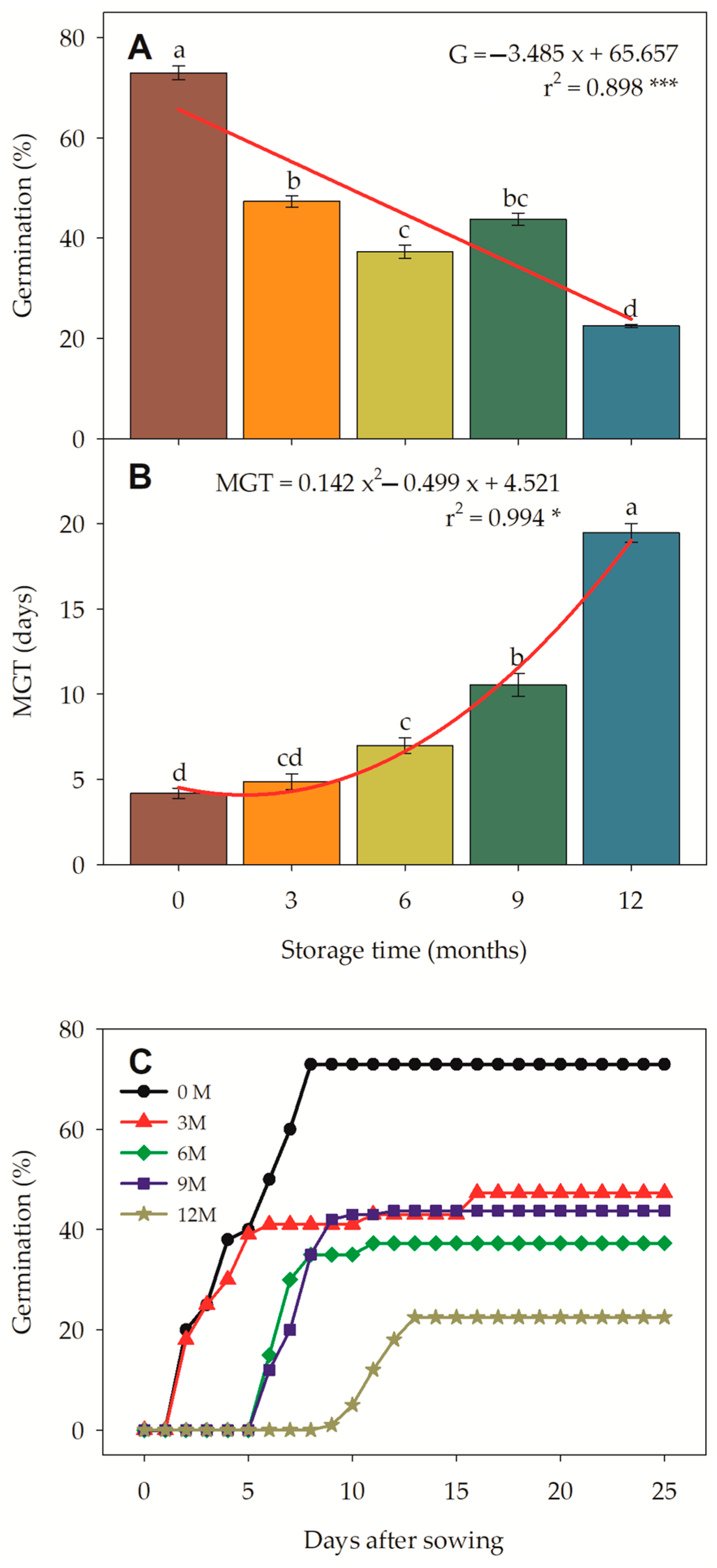
3.4. Germination of Jatropha curcas Seeds Stored Under Controlled Air Humidity and Cooler Temperature
3.4.1. Seed Germination and Mean Germination Time
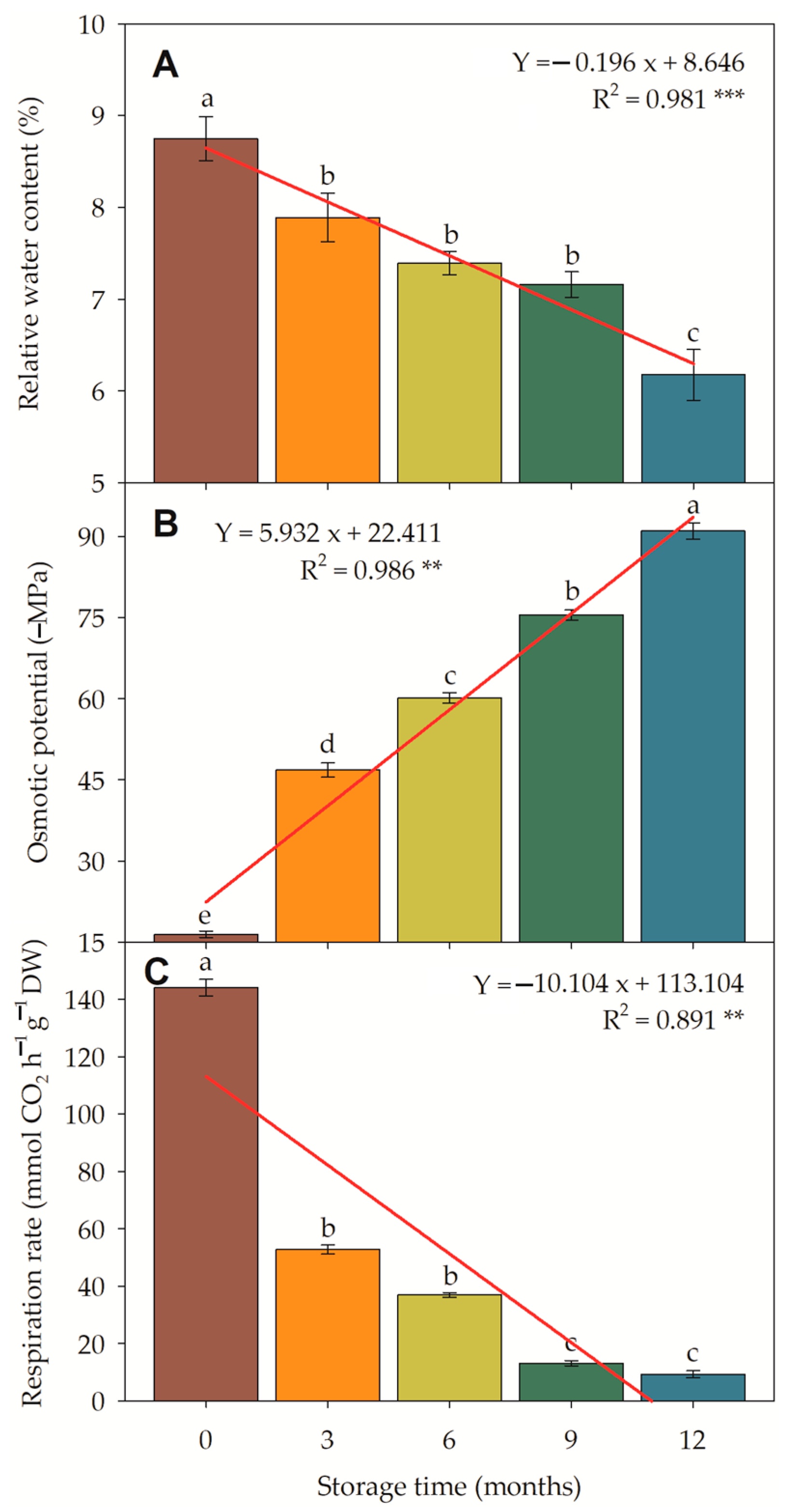
3.4.2. Water Content, Osmotic Potential, and Respiration Rate
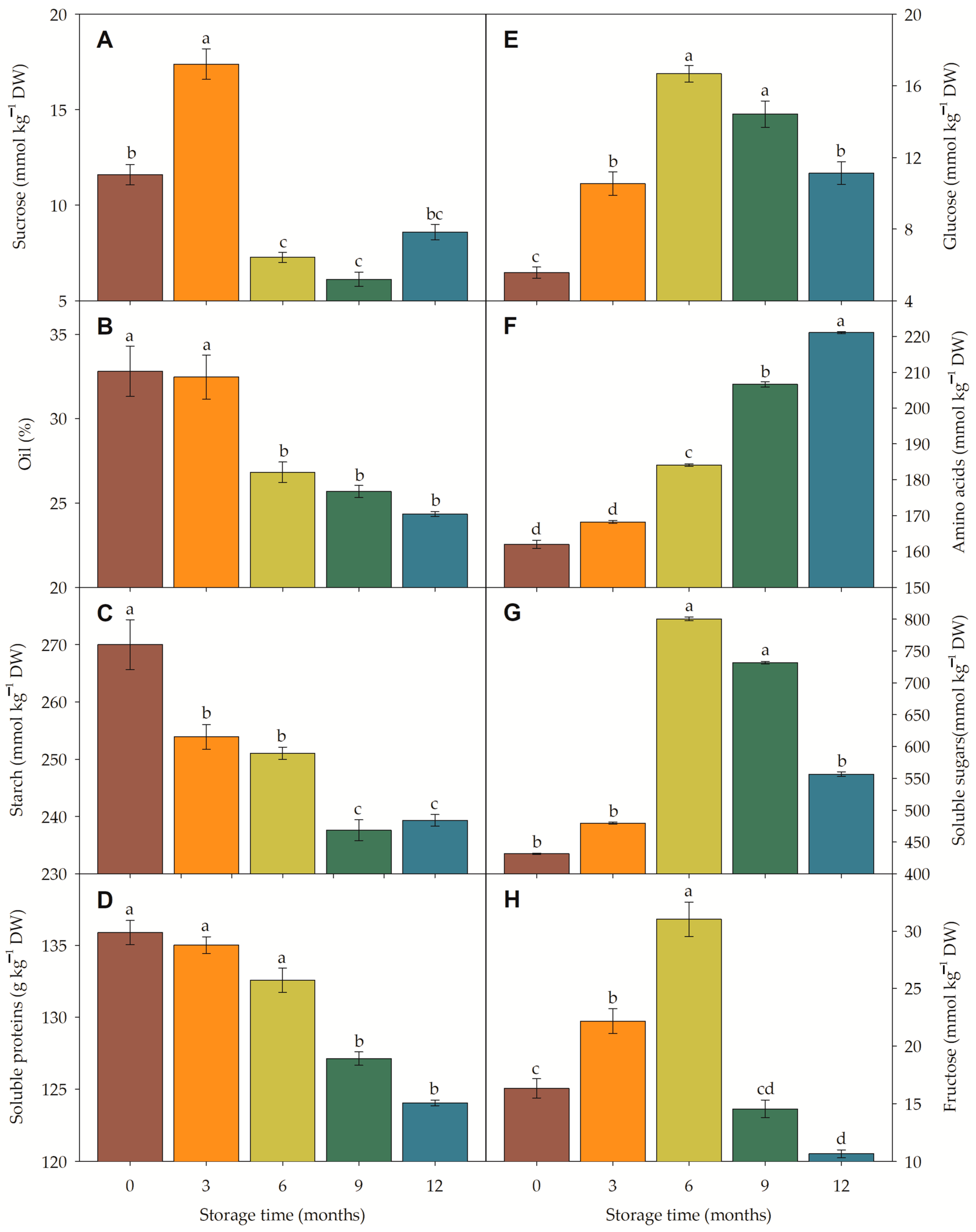
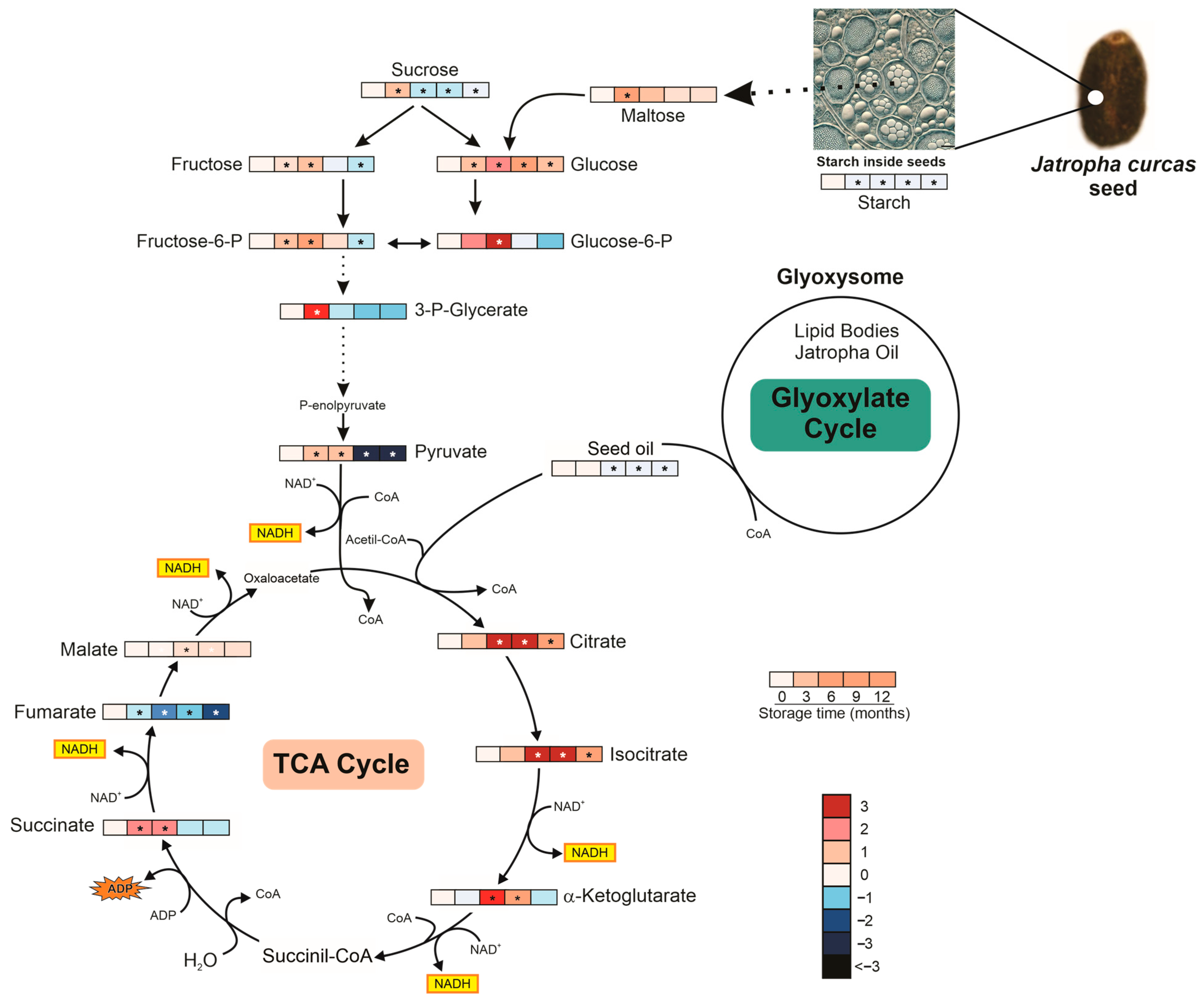
3.4.3. Metabolism and Metabolic Pathway Under Storage
3.4.4. Multivariate Analysis
3.5. Effect of Salinity on Seed Germination Stored by 3 Months Under Controlled Air Humidity and Cooler Temperature
3.5.1. Seed Germination and Mean Germination Time
3.5.2. Mineral Nutrition on Seedlings Germinated Under NaCl
3.6. RT-PCR
4. Discussion
4.1. Germination of Jatropha curcas Seed After Imbibition
4.2. Natural and Accelerated Aging on Seed Germination
4.3. Water Content, Osmotic Potential, and Respiration Rate
4.4. Metabolism and Metabolic Pathway Under Storage
4.5. Effect of NaCl on Seed Germination After Storage Under Air-Humidity Control Container
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behera, S.K.; Srivastava, P.; Tripathi, R.; Singh, J.P.; Singh, N. Evaluation of plant performance of Jatropha curcas L. under different agro-practices for optimizing biomass—A case study. Biomass Bioenerg. 2010, 34, 30–41. [Google Scholar] [CrossRef]
- Alherbawi, M.; McKay, G.; Mackey, H.R.; Al-Ansari, T. Jatropha curcas for jet biofuel production: Current status and future prospects. Renew. Sustain. Energy Rev. 2021, 135, 110396. [Google Scholar] [CrossRef]
- Alhammad, B.A.; Jamal, A.; Carlucci, C.; Saeed, M.F.; Seleiman, M.F.; Pompelli, M.F. Non-conventional oilseeds: Unlocking the global potential for sustainable biofuel production. Catalysts 2023, 13, 1263. [Google Scholar] [CrossRef]
- Gopinathan, M.C.; Babu, C.R. Structural diversity and its adaptive significance in seeds of Vigna minima (Roxb.) Ohwi and Ohashi and its Allies (Leguminosae-Papilionoideae). Ann. Bot. 1985, 56, 723–732. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Anuar, N.; Yaakob, Z. Effect of genotypes and pre-sowing treatmentson seed germination behavior of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodriguez-Paez, L.A. Imbibition and germination of seeds with economic and ecological interest: Physical and biochemical factors involved. Sustainabiity 2023, 15, 5394. [Google Scholar] [CrossRef]
- Garnayak, D.K.; Pradhan, R.C.; Naik, D.N.; Bhatnagar, N. Moisture-dependent physical properties of jatropha seed (Jatropha curcas L.). Ind. Crop Prod. 2008, 27, 123–129. [Google Scholar] [CrossRef]
- Karaj, S.; Müller, J. Determination of physical, mechanical and chemical properties of seeds and kernels of Jatropha curcas L. Ind. Crop Prod. 2010, 32, 129–138. [Google Scholar] [CrossRef]
- Bamgboye, A.I.; Adebayo, S.E. Seed moisture dependent on physical and mechanical properties of Jatropha curcas. J. Agric. Technol. 2012, 8, 13–26. [Google Scholar]
- Corte-Real, N.; Endres, L.; Santos, K.P.O.; Figueirêdo, R.C.B.; Arruda, E.C.P.; Ulisses, C.; Pompelli, M.F. Morphoanatomy and ontogeny of the fruit and seeds of Jatropha curcas L.: A promising biofuel plant. In The Promising Future of Jatropha curcas: Proprieties and Potential Applications; Segura-Campos, M.R., Betancur-Ancova, D., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 141–158. [Google Scholar]
- Moncaleano-Escandon, J.; Silva, B.C.F.; Silva, S.R.S.; Granja, J.A.; Alves, M.C.J.L.; Pompelli, M.F. Germination responses of Jatropha curcas L. seeds to storage and aging. Ind. Crops Prod. 2013, 44, 684–690. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Lozano-Isla, F.; Campos, M.L.O.; Endres, L.; Pompelli, M.F. Effects of seed storage time and salt stress on the germination of Jatropha curcas L. Ind. Crop Prod. 2018, 118, 214–224. [Google Scholar] [CrossRef]
- Rao, R.G.S.; Singh, P.M.; Rai, M. Storability of onion seeds and effects of packaging and storage conditions on viability and vigour. Sci. Hortic-Amst. 2006, 110, 1–6. [Google Scholar] [CrossRef]
- Trethewey, R.N.; Geigenberger, P.; Riedel, K.; Hajirezaei, M.R.; Sonnewald, U.; Stitt, M.; Riesmeier, J.W.; Willmitzer, L. Combined expression of glucokinase and invertase in potato tubers leads to a dramatic reduction in starch accumulation and a stimulation of glycolysis. Plant J. 1998, 15, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; Hassan, M.; Sun, N.; et al. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in Jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef] [PubMed]
- Alencar, N.L.M.; Gadelha, C.G.; Gallão, M.I.; Dolder, M.A.H.; Prisco, J.T.; Gomes-Filho, E. Ultrastructural and biochemical changes induced by salt stress in Jatropha curcas seeds during germination and seedling development. Funct. Plant Biol. 2015, 42, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Bezerra-Neto, E.; Coelho, J.B.M.; Jarma-Orozco, A.; Rodríguez-Páez, L.A.; Pompelli, M.F. Modulation of photosynthesis under salinity and the role of mineral nutrients in Jatropha curcas L. J. Agron. Crop Sci. 2021, 208, 314–334. [Google Scholar] [CrossRef]
- Cavalcante, P.G.S.; Santos, C.M.; Filho, H.C.L.W.; Avelino, J.R.L.; Endres, L. Morpho-physiological adaptation of Jatropha curcas L. to salinity stress. Aust. J. Crop Sci. 2018, 12, 563–571. [Google Scholar] [CrossRef]
- Cerqueira, J.V.A.; Silveira, J.A.G.; Carvalho, F.E.L.; Cunha, J.R.; Lima Neto, M.C. The regulation of P700 is an important photoprotective mechanism to NaCl-salinity in Jatropha curcas. Physiol. Plant 2019, 167, 404–417. [Google Scholar] [CrossRef]
- Dorta-Santos, M.A.; Barriola, I.; Wassner, D.F.; Ploschuk, E.L. Photosynthesis, fluorescence and mesophyll conductance responses to increasing salinity levels in Jatropha curcas at early vegetative stages. J. Agron. Crop Sci. 2020, 206, 52–63. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodrígues-Páez, L.A. Salinity in Jatropha curcas: A review of physiological, biochemical, and molecular factors involved. Agriculture 2022, 12, 594. [Google Scholar] [CrossRef]
- Silva, E.N.; Vieira, S.A.; Ribeiro, R.V.; Ponte, L.F.A.; Ferreira-Silva, S.L.; Silveira, J.A.G. Contrasting physiological responses of Jatropha curcas plants to single and combined stresses of salinity and heat. J. Plant Growth Regul. 2013, 32, 159–169. [Google Scholar] [CrossRef]
- Bouaziz, A.; Hicks, D.R. Consumption of wheat seed reserves during germination and early growth as affected by soil water potential. Plant Soil 1990, 128, 161–165. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Chacuttayapong, W.; Enoki, H.; Nabetani, Y.; Matsui, M.; Oguchi, T.; Motohashi, R. Transformation of Jatropha curcas L. for production of larger seeds and increased amount of biodiesel. Plant Biotechnol. 2021, 38, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, Y.; Zhou, H.; Yang, Y.; Chen, M.; Liang, B. Production of biodiesel from Jatropha curcas L. oil. Comput. Chem. Eng. 2009, 33, 1091–1096. [Google Scholar] [CrossRef]
- Sathiyamoorthi, E.; Lee, J.; Devanesan, S.; Priya, S.D. Catalytic biodiesel production from Jatropha curcas oil: A comparative analysis of microchannel, fixed bed, and microwave reactor systems with recycled ZSM-5 catalyst. Environ. Res. 2024, 258, 119474–119481. [Google Scholar] [CrossRef] [PubMed]
- Contran, N.; Chessa, L.; Lubino, M.; Bellavite, D.; Roggero, P.P.; Enne, G. State-of-the art of the Jatropha curcas productive chain: From sowing to biodiesel and by-products. Ind. Crops Prod. 2013, 42, 202–215. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; p. 745. [Google Scholar]
- Maeda, J.A.; Razera, L.F.; Lago, A.A.; Ungaro, M.R.G. Discriminação entre lotes de sementes de girassol através do teste de envelhecimento rápido. Bragantia 1986, 45, 133–141. [Google Scholar] [CrossRef]
- Peñaloza, P.; Ramirez-Rosales, G.; McDonald, M.B.; Bennett, M.A. Lettuce (Lactuca sativa L.) seed quality evaluation using seed physical attributes, saturated salt accelerated aging and the seed vigour imaging system. J. Biotechnol. 2005, 8, 299–307. [Google Scholar] [CrossRef][Green Version]
- Rosseto, C.A.V.; Marcos-Filho, J. Comparação entre os métodos de envelhecimento acelerado e de deterioração controlada para avaliação da qualidade fisiológica de sementes de soja. Sci. Agric. 1995, 52, 123–131. [Google Scholar] [CrossRef]
- Allen, E.; Alvarez, S. International Rules for Seed Testing 2020; The International Seed Testing Association: Zürichstr, Bassersdorf, Switzerland, 2020. [Google Scholar]
- Lozano-Isla, F.; Miranda, P.V.V.C.; Pompelli, M.F. Germination behavior of Jatropha curcas L. after different imbibition times. Per. J. Agron. 2017, 1, 32–38. [Google Scholar] [CrossRef]
- Chidananda, K.P.; Chelladurai, V.; Jayas, D.S.; Alagusundaramb, K.; White, N.D.G.; Fields, P.G. Respiration of pulses stored under different storage conditions. J. Stored Prod. Res. 2014, 59, 42–47. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, D.T.R.G.; Cavalcante, P.P.G.S.; Salvador, T.L.; Hsie, B.S.; Endres, L. Environmental influence on the physico-chemical and physiological properties of Jatropha curcas L. seeds. Aust. J. Bot. 2010, 58, 421–427. [Google Scholar] [CrossRef]
- Fernie, A.R.; Roscher, A.; Ratcliffe, R.G.; Kruger, N.J. Fructose 2,6-bisphosphate activates pyrophosphate: Fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling heterotrophic cells. Planta 2001, 212, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 221, 907–913. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Carrari, F.; Gibon, Y.; Sulpice, R.; Lytovchenko, A.; Fisahn, J.; Graham, J.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J. 2007, 50, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and quantitative method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anual Biochem. 1976, 72, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.G. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Epstein, E.; Bloom, A.J. Mineral Nutrition of Plants: Principles and Perspectives; Sinauer: New York, NY, USA, 2004; p. 380. [Google Scholar]
- Lozano-Isla, F.; Alfaro, O.B.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application ‘GerminaQuant for R’. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Matthews, S.; Hosseini, M.K. Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci. Technol. 2006, 34, 339–347. [Google Scholar] [CrossRef]
- Ruttanaruangboworn, A.; Chanprasert, W.; Tobunluepop, P.; Onwimol, D. Effect of seed priming with different concentrations of potassium nitrate on the pattern of seed imbibition and germination of rice (Oryza sativa L.). J. Integr. Agric. 2017, 16, 605–613. [Google Scholar] [CrossRef]
- Vertucci, C.W. The Kinetics of Seed Imbibition: Controlling Factors and Relevance to Seedling Vigor. In Seed Moisture; Stanwood, P.C., McDonald, M.B., Vertucci, C.W., Eds.; CSSA Special Publication: New York, NY, USA, 1989; Volume 14. [Google Scholar]
- Vertucci, C.W.; Leopold, A.C. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiol. 1984, 75, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Marcos-Filho, J. New approaches to seed vigor testing. Sci. Agric. 1998, 55, 27–33. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E.; Kalemba, E. Non-reducing sugar levels in beech (Fagus sylvatica) seeds as related to withstanding desiccation and storage. J. Plant Physiol. 2009, 166, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Fessel, S.A.; Vieira, R.D.; Cruz, M.C.P.; Paula, R.C.; Panobianco, M. Electrical conductivity testing of corn seeds as influenced by temperature and period of storage. Pesqui. Agropecu. Bras. 2006, 41, 1551–1559. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Al-Rawai, A. Effects of seed maturation time and dry storage on light and temperature requirements during germination in invasive Prosopis juliflora. Flora 2006, 201, 135–143. [Google Scholar] [CrossRef]
- Castellión, M.; Matiacevich, S.; Buera, P.; Maldonado, S. Protein deterioration and longevity of quinoa seeds during long-term storage. Food Chem. 2010, 121, 952–958. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, J.; Addo, A.; Kemausuor, F. Influence of storage duration of Jatropha curcas seed on oil yield and free fatty acid content. ARPN J. Agric. Biol. Sci. 2012, 7, 41–45. [Google Scholar]
- Oliveira, F.C.; Coelho, P.H.M.; Neto, M.S.; Almeida, A.C.S.; Santos, F.L.S.; Oliveira, J.P.M.; Oliveira, B.S.; Teixeira, I.R.; Campos, A.J. Logistics and storage of soybean in Brazil. Afr. J. Agric. Res. 2016, 11, 3261–3272. [Google Scholar] [CrossRef]
- Woerfel, J.B. Harvest, Storage, Handling, and Trading of Soybeans. In Practical Handbook of Soybean Processing and Utilization; Erickson, D.R., Ed.; AOCS Press: Bay Harbor, ME, USA, 1995; pp. 39, 55. [Google Scholar]
- Zulauf, C.; Kim, S. Corn vs. soybean storage at the US market level, 1974–2017. Farmdoc Dly. 2020, 10, 10. [Google Scholar]
- Nie, L.; Liu, H.; Zhang, L.; Wang, W. Enhancement in rice seed germination via improved respiratory metabolism under chilling stress. Food Energy Secur. 2020, 94, e234. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Akimoto, T.; Cho, S.; Yoshida, H.; Furuta, H.; Esashi, Y. Involvement of acetaldehyde in seed deterioration of some recalcitrant woody species through the acceleration of aerobic respiration. Plant Cell Physiol. 2004, 45, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.A.; Smart, D.S.R.; Lee, C.L.R. Biodiesel production from jatropha oil and its characterization. Res. J. Chem. Sci. 2011, 1, 81. [Google Scholar]
- Öpik, H. Respiration rate, mitochondrial activity and mitochondrial structure in the cotyledons of Phaseolus vulgaris L. during germination. J. Exp. Bot. 1965, 16, 667–682. [Google Scholar] [CrossRef]
- Roberts, E.H.; Ellis, R.H. Water and seed survival. Ann. Bot. 1989, 63, 39. [Google Scholar] [CrossRef]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Sew, Y.S.; Ströher, E.; Fenske, R.; Millar, A.H. Loss of mitochondrial malate dehydrogenase activity alters seed metabolism impairing seed maturation and post-germination growth in Arabidopsis. Plant Physiol. 2016, 171, 849–863. [Google Scholar] [CrossRef]
- Kollöffel, C.; Sluys, J.V. Mitochondrial activity in pea cotyledons during germination. Acta Bot. Neerl. 1970, 19, 503–508. [Google Scholar] [CrossRef]
- Khan, A.A.; Tao, K.L.; Knypl, J.S.; Borkowska, B.; Powell, L.E. Osmotic conditioning of seeds: Physiological and biochemical changes. In Symposium on Seed Problems in Horticulture; International Society for Horticultural Science: Leuven, Belgium, 1977; pp. 267–278. [Google Scholar] [CrossRef]
- Braccini, A.D.L.E.; Reis, M.S.; Moreira, M.A.; Sediyama, C.S.; Scapim, C.A. Biochemical changes associated to soybean seeds osmoconditioning during storage. Pesq. Agropec Bras. 2000, 35, 433–447. [Google Scholar] [CrossRef]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum desf.). Plant Soil. 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Meloni, D.; Silva, D.; Ledesma, R.; Bolzón, G. Nutrición mineral y fotosíntesis en plántulas de algarrobo blanco, Prosopis alba (Fabaceae), en estrés salino. Cuad. Investig. UNED 2017, 9, 297–304. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants, 3rd ed.; Elsevier: London, UK, 2012; p. 643. [Google Scholar]
- Silva, E.N.; Silveira, J.A.G.; Rodrigues, C.R.F.; Viégas, R.A. Physiological adjustment to salt stress in Jatropha curcas is associated with accumulation of salt ions, transport and selectivity of K+, osmotic adjustment and K+/Na+ homeostasis. Plant Biol. 2015, 17, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.C.; Mendes, B.S.; Oliveira-Filho, S.; Câmara, T.R.; Willadino, L.G. Crescimento, síntese de solutos orgânicos e equilíbrio iônico de plântulas de pinhão-manso sob estresse salino. Rev. Caatinga 2013, 26, 46–52. [Google Scholar]
- Pompelli, M.F.; Ferreira, P.P.B.; Chaves, A.R.M.; Figueiredo, R.C.Q.Q.; Martins, A.O.; Jarma-Orozco, A.; Batista-Silva, W.; Endres, L.; Araújo, W.L. Physiological, metabolic, and stomatal adjustments in response to salt stress in Jatropha curcas. Plant Physiol. Bioch. 2021, 168, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Han, Y.; Cheng, Z.; Lv, Q.; Pompelli, M.F.; Pereira, J.D.; Araújo, W.L. Long exposure to salt stress in Jatropha curcas leads to stronger damage to the chloroplast ultrastructure and its functionality than the stomatal function. Forest 2023, 14, 1868. [Google Scholar] [CrossRef]
- Matos, F.S.; Ribeiro, R.P.; Neves, T.G.; Dos Anjos, R.A.; Da Silveira, P.S.; Cruvinel, C.K.L.; Juen, L.; Torres Júnior, H.D. Growth of Jatropha curcas L. plants under salt and nutrition stress. Afr. J. Agric. Res. 2017, 12, 2468–2474. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; Câmara, C.A.; Cabral, F.F.; Oliveira, J.F.; Carvalho, L.W.; Santos, J.M.; Santos Filho, B.G. Leaf gas exchange, chloroplastic pigments, and dry matter accumulation in castor bean (Ricinus communis L.) seedlings subjected to salt stress conditions. Ind. Crop Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Huang, F.; Jiang, Y.; Zhang, S.; Liu, S.; Eh, T.-J.; Meng, F.; Lei, P. A comparative analysis on morphological and physiological characteristics between castor varieties (Ricinus communis L.) under salt stress. Sustainability 2022, 14, 10032. [Google Scholar] [CrossRef]
- Li, Y.; Chu, Y.; Yao, K.; Shi, C.; Deng, X.; Lin, J. Sugar metabolism of castor under salinity. Plant Biol. 2023, 25, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Li, H.; Teng, R.; Wang, Y.; Wang, W.; Zhuang, J. Genomic and transcriptomic analyses of HD-Zip family transcription factors and their responses to abiotic stress in tea plant (Camellia sinensis). Genomics 2018, 111, 1142–1151. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Global analysis of gene expression profiles in physic nut (Jatropha curcas L.) seedlings exposed to salt stress. PLoS ONE 2014, 9, e97878. [Google Scholar] [CrossRef]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim. Biophys. Acta 2013, 1831, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.S.; Ma, G.J.; Yang, C.C.; Lee, P.D. Cloning, expression, site-directed mutagenesis and immunolocalization of phenylalanine ammonia-lyase in Bambusa oldhamii. Phytochemistry 2010, 71, 1999–2009. [Google Scholar] [CrossRef] [PubMed]




| NaCl | Potassium (g kg−1 DW) | Iron (g kg−1 DW) | Phosphorus (g kg−1 DW) | Manganese (mg kg−1 DW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 33.5 | ± | 2.7 | a | 54.8 | ± | 3.2 | c | 8.6 | ± | 0.5 | a | 239.7 | ± | 5.4 | a |
| 20 | 20.4 | ± | 1.3 | b | 58.6 | ± | 2.6 | c | 7.9 | ± | 0.4 | ab | 242.7 | ± | 4.6 | a |
| 40 | 11.3 | ± | 0.5 | c | 62.3 | ± | 2.2 | c | 7.1 | ± | 0.4 | b | 245.7 | ± | 8.8 | a |
| 60 | 11.1 | ± | 0.7 | c | 74.7 | ± | 1.4 | b | 6.7 | ± | 0.4 | b | 223.4 | ± | 8.0 | ab |
| 80 | 10.3 | ± | 0.4 | c | 81.4 | ± | 1.7 | ab | 6.6 | ± | 0.2 | b | 205.0 | ± | 7.3 | b |
| 100 | 7.5 | ± | 0.1 | c | 88.1 | ± | 2.6 | a | 6.5 | ± | 0.2 | b | 155.7 | ± | 3.2 | c |
| NaCl | Magnesium (g kg−1 DW) | Sodium (mg kg−1 DW) | Chlorine (mg kg−1 DW) | Calcium (g kg−1 DW) | ||||||||||||
| 0 | 2.3 | ± | 0.1 | b | 5.1 | ± | 0.0 | c | 2.7 | ± | 0.1 | c | 11.3 | ± | 0.4 | a |
| 20 | 2.6 | ± | 0.1 | ab | 34.3 | ± | 0.7 | b | 19.1 | ± | 0.7 | b | 10.3 | ± | 0.4 | b |
| 40 | 2.8 | ± | 0.1 | a | 45.8 | ± | 1.7 | a | 26.9 | ± | 2.2 | a | 9.2 | ± | 0.3 | c |
| 60 | 2.8 | ± | 0.1 | a | 52.3 | ± | 1.8 | a | 31.8 | ± | 1.3 | a | 7.4 | ± | 0.5 | d |
| 80 | 2.9 | ± | 0.1 | a | 52.5 | ± | 1.5 | a | 34.2 | ± | 1.3 | a | 6.1 | ± | 0.3 | e |
| 100 | 2.9 | ± | 0.2 | a | 52.7 | ± | 1.5 | a | 33.6 | ± | 1.6 | a | 4.6 | ± | 0.3 | f |
| NaCl Treatment (mM) | SAMe | PAL | SAM | PX | CXE | HD-Zip | NAC | MGL | XTH |
|---|---|---|---|---|---|---|---|---|---|
| 0 (control) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 20 mM | 1.45 | n.s. | 3.20 | 1.78 | 1.30 | 2.40 | 2.10 | 2.85 | 0.90 |
| 40 mM | 1.85 | n.s. | 5.80 | 2.20 | 1.95 | 3.40 | 3.15 | 4.00 | 0.65 |
| 60 mM | 2.00 | n.s. | 7.00 | n.s. | 2.10 | 3.80 | 3.80 | 5.10 | 0.50 |
| 80 mM | 2.20 | n.s. | 9.10 | n.s. | 2.30 | 4.50 | 4.60 | 6.20 | 0.30 |
| 100 mM | 2.36 | n.s. | 10.31 | n.s. | 2.36 | 5.10 | 5.42 | 7.36 | −0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez-Padrón, I.E.; Pompelli, M.F.; Carlucci, C.; Perneth-Montaño, M.J.; Ruiz, A.J.B.; Seleiman, M.F.; Alotaibi, M.; Almutairi, K.F.; Oviedo Zumaque, L.E.; Pineda-Rodríguez, Y.Y.; et al. Jatropha curcas Seed Germination: Effect of Seed Imbibition, Aging, Storage, and Salinity. Horticulturae 2025, 11, 258. https://doi.org/10.3390/horticulturae11030258
Suarez-Padrón IE, Pompelli MF, Carlucci C, Perneth-Montaño MJ, Ruiz AJB, Seleiman MF, Alotaibi M, Almutairi KF, Oviedo Zumaque LE, Pineda-Rodríguez YY, et al. Jatropha curcas Seed Germination: Effect of Seed Imbibition, Aging, Storage, and Salinity. Horticulturae. 2025; 11(3):258. https://doi.org/10.3390/horticulturae11030258
Chicago/Turabian StyleSuarez-Padrón, Isidro Elias, Marcelo F. Pompelli, Claudia Carlucci, Marvin José Perneth-Montaño, Andrés José Betin Ruiz, Mahmoud F. Seleiman, Majed Alotaibi, Khalid F. Almutairi, Luis Eliécer Oviedo Zumaque, Yirlis Yadeth Pineda-Rodríguez, and et al. 2025. "Jatropha curcas Seed Germination: Effect of Seed Imbibition, Aging, Storage, and Salinity" Horticulturae 11, no. 3: 258. https://doi.org/10.3390/horticulturae11030258
APA StyleSuarez-Padrón, I. E., Pompelli, M. F., Carlucci, C., Perneth-Montaño, M. J., Ruiz, A. J. B., Seleiman, M. F., Alotaibi, M., Almutairi, K. F., Oviedo Zumaque, L. E., Pineda-Rodríguez, Y. Y., & Rodríguez-Paez, L. A. (2025). Jatropha curcas Seed Germination: Effect of Seed Imbibition, Aging, Storage, and Salinity. Horticulturae, 11(3), 258. https://doi.org/10.3390/horticulturae11030258











