Abstract
The study and definition of synergistic, additive and antagonistic effects among biostimulants of microbial and nonmicrobial origin represents one of the most interesting prospects for future research. As part of the SO.MI.PR.O.N regional project, we evaluated the effects of the single and combined applications of three different biostimulants [a plant-derived protein hydrolysate (PH), a tropical plant extract (PE) and a microbial biostimulant based on Trichoderma atroviride (Tricho)] on tomatoes (Solanum lycopersicum L.) grown in a protected environment. From the analysis of our results, we observed that compared with the control conditions, all combinations containing Trichoderma atroviride (Tricho+PH, Tricho+PE and Tricho+PE+PH) significantly increased the marketable fruit production. For the latter parameter, the combined application of all tested biostimulants ensured the much-aspired-for synergistic effect. The combined application of all tested biostimulants (Tricho+PE+PH) significantly improved the quality traits (lycopene content, total polyphenols and total soluble solids) of the tomatoes. Although the understanding of the mechanisms activated by the combined application of the different biostimulants still remains complex to define, the results obtained underscore their potential. Not least, it will be necessary to assess the economic feasibility of the combined applications of biostimulants in order to have a more real picture that fully considers the sustainability of this strategy.
1. Introduction
The tomato (Solanum lycopersicum L.), a plant belonging to the Solanaceae family and native to Mexican and Peruvian regions [1], is considered one of most important vegetables cultivated all over the world, second only to the potato [2]. On the world level, China is the most important producer, with an invested area of over 1 million hectares and a production of almost 70 million tons [2], followed by India (20 million tons) and Turkey (13 million tons). Worldwide, Italy is the sixth biggest producer (around 6 million tons) and seventh in terms of area invested (around 100,000 ha) [2]. In Italy, tomatoes are cultivated in 90,000 hectares of open fields, with a production of 5 million tons, and in 7000 hectares of protected environments, producing around 520,000 tons [3]. The Italian regions most involved in tomato cultivation are Apulia, Campania, Emilia-Romagna and Sicily [3]. The success and spread of this crop worldwide are mainly due to its great versatility, since tomatoes can be employed in many culinary preparations, offering freshness to salads, soups and pasta dressing [4]. Moreover, tomatoes are consumed for their health benefits, since they contain high amounts of vitamin C, potassium, lycopene and fiber [5,6,7,8]. Today, there are hundreds of genotypes (local landraces, varieties and F1 hybrids) on the market, differentiated by shapes (from round to oval), sizes (from 10 to 400 g) and colors (from deep red to yellow or green). However, the most established ones for professional uses are the F1 hybrids that guarantee high performances in different growing conditions [9,10,11].
In Sicily, tomatoes are often cultivated in protected environments, following a long cycle that lasts from September to May. During this long cultivation period, the plants are exposed to several abiotic and biotic stresses that can jeopardize crop yield and quality. In this regard, there are innovative farming techniques, such as soilless cultivation, that can aid in reducing these stresses. Moreover, different green agronomic solutions have been proposed to reduce the ecological impact of intensive greenhouse cultivation [12,13,14]. In this scenario, the employment of biostimulants could represent a promising way to increase crop performance in an environmentally sustainable manner. Biostimulants are substances or microorganisms, different from fertilizers, that can stimulate plant growth, nutrient uptake and tolerance to several abiotic and biotic distresses.
Among nonmicrobial biostimulants, natural substances, such as protein hydrolysates (PHs) and plant extracts (PEs), are well-known for agricultural purposes. Protein hydrolysates are products obtained by the chemical or enzymatic hydrolysis of an organic matrix (animal or vegetable origin) and are made up of amino acids, nitrogen compounds and peptides [15]. Peptides and amino acids are the main compounds that confer biostimulatory action to PHs. Amino acids are used by plants for different purposes, such as protein biosynthesis and energy production, and for the synthesis of biologically active molecules [16,17], whereas peptides play a significant role in cell signaling, in the regulation of plant growth and development and in the response to stressful plant conditions [18]. A direct effect of PH application is the modulation of nitrogen uptake and assimilation through key enzymes involved in nitrogen metabolism [19,20]. Moreover, as reported by Martín et al. [21] and Sokar et al. [22], hormone-like activities, chelating effects and antioxidant action are also described. Plant extracts (PEs) are products obtained by different plant species; they contain bioactive components, secondary metabolites and amino acids, showing positive influences on plant growth, resource use efficiency and biotic/abiotic distress tolerance [23,24].
Trichoderma-based products are very well-known and appreciated in agriculture for their ability to control phytopathogenic fungi [25,26]. However, the Trichoderma genus also encloses many fungus species with interesting biostimulatory properties. Indeed, as reported by Li et al. [27] and Adnan et al. [28], plants inoculated with this microbial biostimulant showed increases in plant growth and yield, nutrient uptake and stress tolerance. The possibility of using Trichoderma as a biofertilizer thanks to its ability to modulate plant root architecture and to increase nutrient availability, which in turn influences plant nutritional status, is also interesting [29,30,31]. Moreover, it was shown that inoculation with Trichoderma triggered increases in phytohormone and phenol concentrations in plant tissues, which contribute to boosting plant tolerance to environmental stresses [25,32].
The use of soilless cultivation techniques and the integration of nonmicrobial or microbial biostimulants into agricultural practices can contribute to the development of more sustainable and efficient farming systems, such as that of Sicilian greenhouses. In this regard, the future of biostimulants lies in the combined application of microbial and nonmicrobial biostimulants since synergistic biostimulant actions have been recorded.
In the light of the above, we hypothesize that the combined application of PHs, PEs and Trichoderma will result in an interactive effect with positive outcomes on soilless tomato crop performance. We have already performed a study with the same treatments and species in the same soil conditions [33], obtaining promising data. Consequently, based on that previous experiment and considering that soilless cultures could be the future for vegetables cultivation, the aim of the present research was to evaluate the impact of the individual and mutual application of PHs, PEs and Trichoderma atroviride on the growth, yield and fruit quality of a cherry tomato F1 hybrid grown in a soilless system. We believe that the knowledge gap that this study fills will be useful, with a view to having more resilient and eco-sustainable soilless tomato cultivation, with relevant benefits for growers, consumers and the environment.
2. Materials and Methods
2.1. Experimental Site and Plant Material and Nutrient Solution Management
The research was carried out in the framework of the Sicilian regional project SO.MI.PR.O.N., during the 2023–2024 cultivation period, on an experimental site (36°99′64.8″ N, 14°59′88.3″ E) located in the countryside of Comiso (RG, Sicily). The test was conducted inside an unheated greenhouse covered with a 150 µm-thick polyethylene film. A tomato genotype (Solanum lycopersicum L.) belonging to the round tomato group, the “Creativo” F1 (HM Clause, Davis, CA, USA), was transplanted into growbags containing a substrate of coconut fiber and perlite (Perlite Italiana, Corsico (MI), Italy), using a plant density of 3.3 plants m−2. The transplanting took place on 25 September 2023. The nutrient solution was prepared following the recipe suggested by Sonneveld et al. [34]. The re-integration of the nutrient solution was valued based on the previous day’s solar radiation [35]. A leaching fraction of 40% was imposed, and the drainage solution was collected but not recycled (open-loop management).
2.2. Biostimulant Treatments
The day before transplanting, the roots of the plants were inoculated with a solution of Trichoderma atroviride (Tricho) Condor® (Hello Nature Italia srl, Rivoli Veronese, Italy) using a dose of 6.25 g L−1, soaking the root systems for 15 min. The treatment was repeated 15 and 45 days after transplanting (DAT), suppling 150 mL of solution per plant. Protein hydrolysate (PH) Trainer® (Hello Nature Italia srl, Rivoli Veronese, Italy) and plant extract (PE) Auxym® (Hello Nature Italia srl, Rivoli Veronese, Italy) were applied by foliar spray at concentrations of 3 mL L−1 and 1.5 mL L−1, respectively. Both treatments were performed every 15 days, from the beginning of the flowering stage until the end of the cycle, using 0.5 L m−2 of biostimulating solution. The doses were chosen by considering the indications provided by the manufacturers and information found in the literature. The control plants were treated only with water. The PH biostimulant product used in the present experiment was principally characterized by peptides and amino acids and, to a lesser extent, soluble carbohydrates, mineral elements and phenolic compounds. Trainer® has a density of 1.21 kg L−1, a dry matter of 46% and a pH of 4.0 and contains 310 g kg−1 of free amino acids and soluble peptides. The aminogram of the product (reported in g kg−1) was as follows: Ala (12), Arg (19), Asp (33), Cys (4), Glu (54), Gly (13), His (8), Ile (12), Leu (24), Lys (19), Met (4), Phe (16), Pro (15), Ser (17), Thr (11), Trp (4), Tyr (13) and Val (16). The antioxidant activity of the PH biostimulant, as measured by ferric-reductive antioxidant power (FRAP), was 41.9 mmol Fe2+ g−1 f.w., while the total phenols and flavonoids were 8.93 mg gallic acid equivalent per gram f.w. product and 0.95 mg quercetin equivalent per gram f.w. product, respectively. The soluble sugar content of the Trainer® was 90 g kg−1 f.w., and the elemental composition was as follows (g kg−1 f.w.): N (50.0), P (0.9), K (41.1), Ca (10.9), Mg (0.5), Fe (0.024), Zn (0.010), Mn (0.001), B (0.005) and Cu (0.001). Relative to the N-NO3 and N-NH4 content of the PH were 3.13 and 6.00 μg g−1 f.w., respectively. For the production of the PE biostimulant, a fermentation process of tropical plants such as hibiscus was used, which contained free amino acids/peptides, carbohydrates, mineral nutrients, vitamins and phytohormones with the following percentages: 54%, 17%, 23%, 6% and 0.22%, respectively.
2.3. Plant Growth and Yield
Growth and yield parameters were recorded on all plants. At the end of the cycle, fresh-shoot biomass (g pt−1) and plant height (cm) were recorded. The yield parameters were measured, from the beginning until the end of the experiment, after each harvest and expressed as g plant−1. Particularly, production was divided into total fruit production (g pt−1) and marketable fruit production (g pt−1). The average weight of the marketable fruit (g plant−1) and the number of marketable fruit (N°. pt−1) were also calculated.
2.4. Physiological Observations and Proximal Fruit Quality
Fruit quality analyses were carried out on five fruits randomly selected from each replicate, belonging to the 3rd harvest. The dry matter content was determined by drying the fruit samples via a ventilated oven at a temperature of 80 °C for 48 h, employing 500 g of sample until a constant weight. Values were reported as percentages. A digital penetrometer (Trsnc, Forlì, Italy) was used to measure the firmness of the fruit. The resistance of the fruit to penetration was measured using a stainless-steel cylindrical probe (3 mm) on two opposite sides of the middle part of the fruit. The values were expressed as newtons (N). For the evaluation of total soluble solids (TSSs), a digital refractometer (MTD-045nD; Three-In-One Enterprises Co. Ltd., New Taipei, Taiwan) was used. Briefly, the tomato fruits were squeezed, and the juice was filtered; after that, the measurement was performed. The values were reported as °Brix. Titratable acidity (TA) (g L−1) was determined according to the protocol of Han et al. [36], using a 10 g aliquot of fruit, which was transferred to 50 mL of distilled water and titrated with 0.1 N NaOH to a final pH of 8.1.
2.5. Polyphenols, Anthocyanins, Lycopene and Ascorbic Acid in Tomato Fruits
Fruit quality analyses were carried out on five fruits randomly selected from each replicate belonging to the 3rd harvest. The total polyphenols were evaluated by a spectrophotometric assay following the method of Meda et al. [37]. Briefly, firstly, the total polyphenols were extracted in water and then mixed with Folin e Ciocalteu’s reagent. The values were measured via the assessment of absorbance at 765 nm, comparing them to the gallic acid calibration curve. The values were reported as mg/kg gallic acid of fresh weight. The extraction and evaluation of the anthocyanin concentration were performed by Rabino and Mancinelli [38]. Tomato samples of 2 g were extracted with 50 mL of solvent (95% ethanol, 1.5 N HCl (85/15)); the mixture was stored for 12 h at 4 °C and filtered. The absorbances (at 530 and 657 nm) of the extracts were recorded with the aid of a spectrophotometer (CELL, model CE 1020). The value was expressed as mg of Cya-3-G equivalent per 100 g of f.w. The total lycopene content was determined according to the protocol of Sadler et al. [39] and expressed as mg kg−1 fresh weight. Briefly, homogenized tomato samples (5 g) were extracted, adding 50 mL of a mixture of hexane/acetone/ethanol (2:1:1, v/v/v) for 30 min. The absorbance of the lycopene–hexane solution was measured at 472 nm. For the preparation of the calibration curves, pure lycopene (Sigma, St. Louis, MO, USA) was employed. For the determination of the ascorbic acid content, the tomato fruits were squeezed and then measured with a reflectometer (Merck RQflex* 10) using a reflectoquant Ascorbic Acid Test Strip. The results were expressed as mg of ascorbic acid per 100 g of fresh weight. The taste index (TI) was determined according to Navez et al. [40] from the total sugar content (°Brix; TS) and titratable acidity (TA) values, expressed as percentages, using the formula
2.6. Experimental Setup and Statistics
Eight biostimulant treatments were applied (control, PE, PH, Tricho, PE + PH, PE + Tricho, PH + Tricho, PE + PH + Tricho) to a total of 216 plants arranged in a randomized complete block with three replications per thesis. All the statistical analyses were performed using SPSS V.28 software (StatSoft, Inc.; Chicago, IL, USA). Prior to analysis, the ANOVA assumptions were checked by the Shapiro–Wilk and Levene tests. A one-way ANOVA was performed, setting the biostimulants as the main factor. Tukey’s honestly significant difference (HSD) test (p ≤ 0.05) was used to separate the means. The percentage data were converted to arcsin prior to the ANOVA (Ø = arcsin (p/100)1/2). All plant responses to the single or combined applications of the biostimulants were summarized through a color heatmap generated with the web tool ClustVis (https://biit.cs.ut.ee/clustvis/, accessed 13 January 2025). Euclidean distance was used as a measure of similarity and hierarchical clustering with full-link heatmaps, and the data were normalized and displayed using a scale of false colors (red = increasing values; blue = decreasing values).
3. Results and Discussion
3.1. Yield and Yield Parameters
Based on this information, research currently aims to combine microbial biostimulants with nonmicrobial biostimulants with the goal of achieving superior performance compared to the use of a single biostimulant [41]. However, the combination of biostimulants of different natures would not necessarily guarantee an increase in overall performance. In fact, the combination of biostimulants could produce additive or, even worse, antagonistic effects in addition to the desired synergistic effects. Therefore, it is interesting to analyze the individual and combined action of an endophytic fungus (Trichoderma) and two nonmicrobial biostimulants (PHs and PEs). In our study, a significant increase in fresh-shoot biomass was observed regardless of the biostimulant treatment used (Figure 1A).
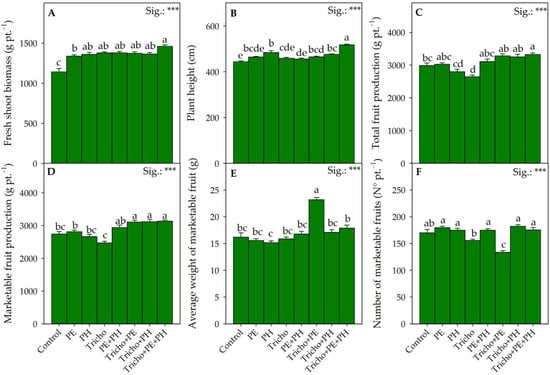
Figure 1.
Effects of single and/or combined applications of three different biostimulants on the vegetative-productive traits of Solanum lycopersicum L: fresh-shoot biomass (A), plant height (B), total fruit production (C), marketable fruit production (D), average weight of marketable fruits (E) and number of marketable fruits (F). Treatment averages within the figure followed by different letters indicate significant differences (p < 0.05) according to the Tukey–Kramer HSD test. Data are mean values ± standard error, n = 3. *** Significant at p ≤ 0.001.
The highest value of the fresh-shoot biomass in absolute terms was recorded from the plants treated with the triple combination Tricho+PE+PH (1461.7 g/pt; Figure 1A).
However, for all the combinations tested (PE+PH, Tricho+PH, Tricho+PE and Tricho+PE+PH), we recorded an antagonistic effect among the different biostimulants under study, since the positive action attributable to the different combinations of biostimulants was found to be no higher than the positive effects obtained following the use of the individual biostimulants [42]. Similar to what was previously described, compared with the control plants, the Tricho+PE+PH treatment showed an increase in plant height of 16.6% (Figure 1B). Still, with respect to the plant height parameter, if on one hand, the PE+PH, Tricho+PE and Tricho+PH combinations exerted an antagonistic action, on the other hand, the triple combination (Tricho+PE+PH) resulted in an additive action. In any case, the increases in plant vigor (higher fresh-shoot biomass) obtained with the PE, PH, Tricho, PE+PH, Tricho+PE and Tricho+PH biostimulant treatments did not then translate into increases in total fruit production compared with the control (Figure 1C). Although it is well-recognized that the application of Trichoderma spp. contributes to increasing crop yield due to several direct and indirect actions that this endophytic fungus performs on plants [43], it is interesting to note that in our study, the Tricho treatment resulted in a decrease in total fruit yield of 11.3% compared with the control (Figure 1C). On the contrary, the Tricho+PE+PH treatment was the only one able to allow a significant increase in fruit yield, with an increase of 11.4% compared with the control condition (Figure 1C). In the present work, the tomatoes were grown in a soilless system using a coconut fiber-based substrate, so it is possible that the individually applied Trichoderma had a depressive effect due to the inadequate growing medium that did not permit the optimal growth and development of this terricolous fungus [44]. In contrast, the co-presence of nonmicrobial biostimulants (PH and PE) may have directly and indirectly exacerbated the positive action of the endophytic fungus (Tricho) even under our experimental conditions. As suggested by Sani and Yong [42], the combined use of microbial and nonmicrobial biostimulants may improve the solubility, absorption and assimilation of nutrients compared with a single application. Also, in the case of marketable fruit production (Figure 1D), the best performance was recorded following the combined application of Tricho and the two nonmicrobial biostimulants used (Tricho+PE, Tricho+PH and Tricho+PE+PH). Unlike the vegetative biometric parameters (fresh-shoot biomass and plant height), where the application of the combined biostimulants had antagonistic effects, for the marketable fruit production parameter, there was a synergistic action for all combinations containing Trichoderma, allowing an average increase of 13.6 percent over the control (Figure 1D). In contrast to the findings of Chrysargyris et al. [45] and Molla et al. [46], in similar studies, applications of the individual biostimulants (PE, PH and Trichoderma) did not result in significant differences compared with the untreated control. Although the Tricho+PE, Tricho+PH and Tricho+PE+PH combinations increased the production of marketable fruit compared with the control, the Tricho+PE treatment resulted in diametrically opposite effects. Specifically, the improved production performance obtained following the application of the endophytic fungus–PE consortium (Tricho+PE) resulted in an increase (+43.2%) in the average weight of the marketable fruit values compared with the control (Figure 1E). In contrast, the lowest absolute values of the number of marketable fruits were recorded by the very plants treated with Tricho+PE (Figure 1D).
3.2. Assessment of Key Quality Indexes
Given the increasing consumer demand for high-quality vegetables, special attention was paid in this work to the quality parameters that directly and indirectly influence the organoleptic and sensory characteristics of tomatoes. One of these parameters, namely soluble solid content (TSSs), is mainly influenced by genotype × environment interaction [47,48]. In our study, a round tomato genotype, Creativo F1, was tested, which, under our growing conditions, reported an average TSS content of 6.3 °Brix (Figure 2A), comparable with that reported by Treccarichi et al. [49].
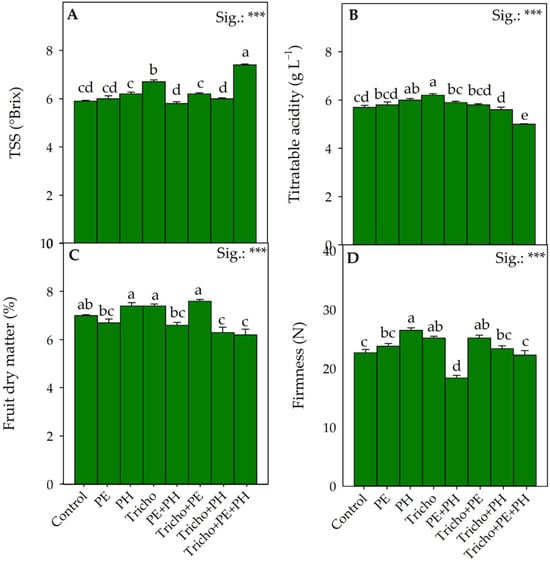
Figure 2.
Effects of single and/or combined applications of three different biostimulants on quality traits of Solanum lycopersicum L.: TSSs (A), titratable acidity (B), fruit dry matter (C) and firmness (D). Treatment averages within the figure followed by different letters indicate significant differences (p < 0.05) according to the Tukey–Kramer HSD test. Data are mean values ± standard error, n = 3. *** Significant at p ≤ 0.001.
In contrast to what was reported by Carillo et al. [50] and Ruiz-Cisneros et al. [51], the application of Trichoderma resulted in a significant increase in the TSS values. Compared to the single application of nonmicrobial biostimulants, the non-significant variation in the TSS values did not confirm the data previously observed in similar studies by Rouphael et al. [52] and Ertani et al. [53]; these contradictory results could be attributable to the different growth conditions, genotypes and types of biostimulants tested in the different studies. It is interesting to note that the application of the Tricho+PE+PH significantly increased the TSS parameter by 25.9% compared with the control (Figure 2A) due to their synergistic action. The synergistic action recorded following the Tricho+PE+PH application could probably be both the consequence of the increased production of primary metabolites due to improved vegetative-productive performance and more efficient transport of these metabolites to the fruits through the phloem. The higher TSS values obtained in the tomatoes from the Tricho+PE+PH-treated plants, regardless of the mechanism of action enacted, would provide consumers with an important improvement in quality characteristics. In any case, the triple combination (Tricho+PE+PH) significantly reduced the titratable acidity compared with the control (Figure 2B). As suggested by Rana et al. [54], the reduction in the acidity of the tomato fruits could be related to more efficient delivery of soluble sugars into the fruit and the concomitant conversion of organic acids to sugars. Formisano et al. [55] recorded a direct correlation between TSS concentration and dry matter content in tomato fruits. The increase in TSSs was attributed to a reduction in water assimilation, which would account for the resulting increase in fruit dry matter. The non-unique modes of action of the biostimulants tested both individually and in the different combinations did not define this direct correlation between TSSs and dry matter in fruits, suggesting a deeper and more complex relationship between the two parameters. It is also interesting to observe that the highest value of TSSs was precisely determined by the Tricho+PE+PH treatment (Figure 2A). This observation supports the hypothesis that the combination of biostimulants of different natures could result in different actions. In fact, the Tricho+PE combination not only affected the aforementioned biometric parameters (number of marketable fruits and average weight of marketable fruit) differently than the other combinations but also the dry matter percentage and fruit firmness, recording the highest values for both parameters (Figure 2C,D). In particular, greater fruit firmness would ensure better tomato storage even during transport, as excessive softening is the main factor that negatively affects shelf life [56,57]. Partially in agreement with our observations, Cozzolino et al. [58], using three different nonmicrobial biostimulants (protein hydrolysate, tropical plant extract and algae extracts), observed a significant increase in tomato fruit firmness. Furthermore, as observed by Shafique et al. [59], the application of Trichoderma alone significantly increased the firmness of tomato fruits compared with the control, probably due to the change in the endogenous biosynthesis of ethylene (a hormone responsible for ripening), probably induced by the activation of plant defense mechanisms. Of all the biostimulant treatments tested, only the triple combination promoted a significant increase in total fruit yield while, at the same time, enhancing organoleptic quality (TSSs). In fact, it is possible that the carbohydrates produced by the photosynthetic process would have been used not to increase the content of organic acids but of TSSs. In this regard, compared with the control conditions, the combined application of all tested biostimulants (Tricho+PE+PH) increased the taste index of the fruits by 13%, calculated according to Navez et al. [40] (Figure 3). Regardless of the application of the biostimulants, the average taste index values (1.13) were comparable with those recorded by Dzhos et al. [60] in an experiment on greenhouse and field-grown tomatoes.
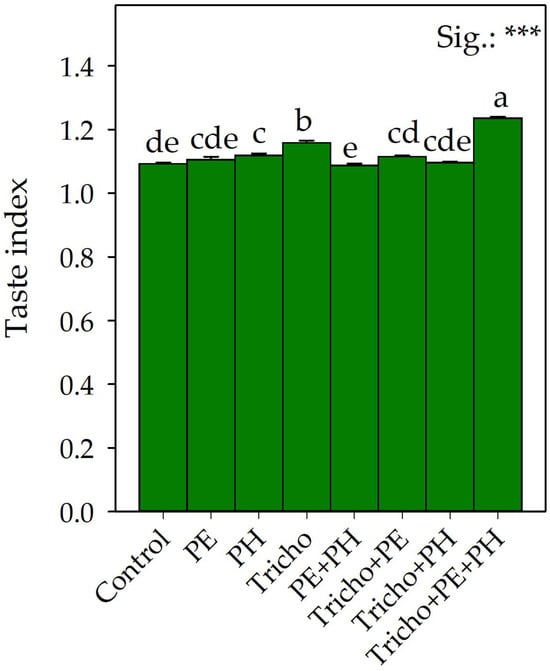
Figure 3.
Effects of single and/or combined applications of three different biostimulants on the taste index (TI) of Solanum lycopersicum L. The averages of the treatments within the figure followed by different letters indicate significant differences (p < 0.05) according to the Tukey–Kramer HSD test. Figures are mean values ± standard error, n = 3. *** Significant at p ≤ 0.001.
In addition, it is noteworthy that the Tricho+PE+PH combination also resulted in improved nutritional characteristics, despite several studies claiming competition in the allocation of common precursors involved in the biosynthesis of plant growth and defense resources [61]. In particular, significant increases in the total phenolic compounds, lycopene, ascorbic acid and anthocyanins were observed compared with the control. These are secondary metabolites known for their antioxidant properties, useful for human health due to their recognized ability in reducing reactive oxygen compounds (ROS), which are responsible for oxidative processes related to various human diseases [62]. The results presented in Figure 4A show, for all combinations containing Trichoderma in addition to the aforementioned increase in fresh-shoot biomass, a significant increase over the untreated control in total phenolic values.
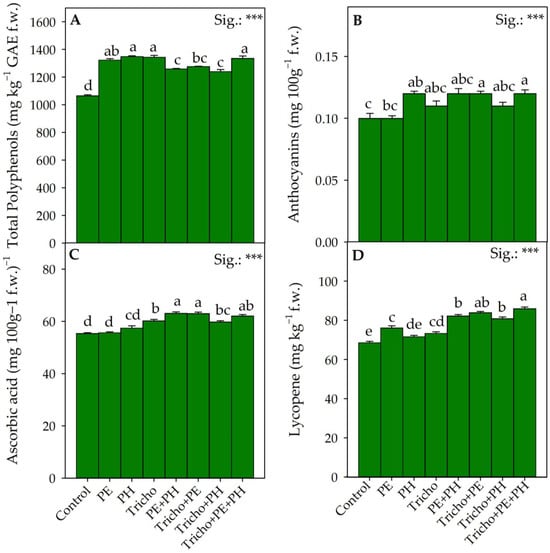
Figure 4.
Effects of single and/or combined applications of three different biostimulants on major secondary metabolites of Solanum lycopersicum L.: total polyphenols (A), anthocyanins (B), ascorbic acid (C) and lycopene (D). Treatment averages within the figure followed by different letters indicate significant differences (p < 0.05) according to the Tukey–Kramer HSD test. Data are mean values ± standard error, n = 3. *** Significant at p ≤ 0.001.
Phenols, in fact, through their antioxidant action, improve tolerance to abiotic and biotic stresses by crops [63,64]. However, the increase in antioxidant compounds induced by biostimulant treatments containing Trichoderma could be justified by a promotion of photosynthetic activity, which in turn promoted the secondary metabolism [65]. Similarly, the Tricho+PE and Tricho+PE+PH combinations resulted in an average 20% increase in the anthocyanin values compared with the control conditions (Figure 4B). Similar to the phenolic compounds, another class of antioxidant molecules that is very important in determining the nutritional quality of tomatoes is ascorbic acid. It has been observed that ascorbic acid could promote the activity of enzymes related to antioxidant homeostasis in cells. In contrast to what was observed by Ertani et al. [53], in our work, the individual applications of PE and PH biostimulants did not result in significant alterations in the ascorbic acid values compared with the control. On the contrary, all combinations (PE+PH, Tricho+PE, Tricho+PH and Tricho+PE+PH) significantly increased the ascorbic acid content in the tomatoes compared with the control plants (Figure 4C). The combination of the microbial and nonmicrobial biostimulants, as suggested by Giordano et al. [66], might have increased the total amount of amino acids by up-regulating the expression of the enzyme L-galactone-1,4-lactone dehydrogenase (L-GalLDH), responsible for ascorbic acid biosynthesis. Among the most studied plant biomolecules for their direct role on human health, we can find carotenoids. The most abundant carotenoid in ripe tomato fruits is undoubtedly lycopene [11]. The interest of the scientific community in this carotenoid is attributed not only to its potent and recognized antioxidant action but mainly to its impact on the coloration that defines the visual quality of the fruits. In our study, a significant increase in this pigment was observed following the application of all biostimulants combined (PE+PH, Tricho+PE, Tricho+PH, Tricho+PE+PH; Figure 4D). Despite Bertin and Génard [67] suggesting that increased accumulation of soluble sugars at the preharvest phenological stage of the fruit could inhibit plastid development and consequently carotenoid accumulation, our results did not show this negative relationship between lycopene content and TSSs in the tomato fruits treated with the different combinations of biostimulants tested.
3.3. Cluster Heatmap of Yield and Quality Parameters of Tomato Plants Treated with Single and/or Combined Applications of Microbial and Nonmicrobial Biostimulants
A heatmap was performed for all parameters evaluated, with the aim of providing a detailed overview related to the action of the three biostimulants (PE, PH and Tricho) applied individually or in combination. The heatmap analysis of the aggregated data identified two main macro clusters. The first had the control, PH, Tricho, PE+PH, PE and Tricho+PH treatments while the second had the combined Tricho+PE and Tricho+PE+PH treatments (Figure 5). The separation between the two clusters was mainly due to the parameter of the average weight of the marketable fruit. The analyzed parameters were separated into three main clusters, each subdivided into secondary sub-clusters (Figure 5). The clustering of the analyzed parameters shows that the non-application of the biostimulant (control) reduced the total polyphenol content and fresh-shoot biomass compared with the different biostimulant treatments evaluated. In contrast, the combined application of all biostimulants improved the plant height and TSSs. Compared with the other treatments, the application of the Tricho+PE combination improved the average weight of the marketable fruit but contextually reduced the number of marketable fruits.
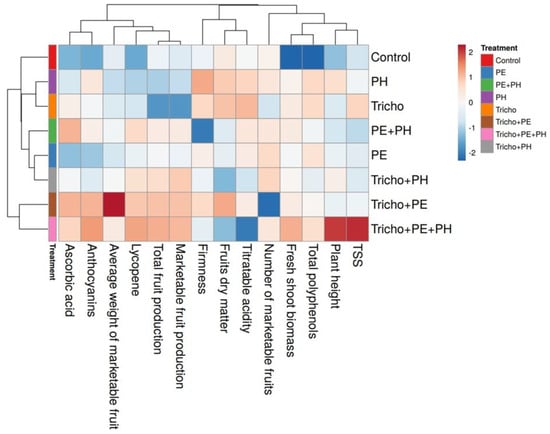
Figure 5.
Analysis of heat maps summarizing the results of biometric, yield and quality parameters of Solanum lycopersicum (L.) fruits grown with single and/or combined biostimulant treatments.
4. Conclusions
The purpose of our work was to explore how the single and/or combined applications of three different biostimulants (plant-derived protein hydrolysates, plant extracts and a product based on Trichoderma atroviride) affect the production and quality response of tomato vegetation grown in a protected environment. Although the triple treatment (Tricho+PE+PH) increased marketable tomato yield compared with the control, the results obtained from the PE+PH, Tricho+PE and Tricho+PH treatments ensured statistically similar values highlighting the non-synergistic action of the tested biostimulants. In contrast, among all combinations tested, only the triple combination resulted in a significant improvement in the total soluble solids and total polyphenol content, suggesting the activation of specific metabolic pathways in plants subjected to the combined application of all biostimulants. Although the triple combination did not promote significantly higher production than what was obtained from the double combinations, the interesting results obtained, especially on the quality parameters, could be useful for the definition of new biostimulant products and agronomic protocols that contextually use microbial and nonmicrobial biostimulating products. In any case, there is a need to better understand the physiological and biochemical mechanisms underlying the responses triggered by combinations of different biostimulants while contextually evaluating the cost-effectiveness of such approaches.
Author Contributions
Conceptualization, E.C., L.V., B.B.C., P.B., F.M., G.G.L.P., M.C., G.C., S.L.B. and L.S.; methodology, E.C., L.V., B.B.C., P.B., F.M., G.G.L.P., M.C., G.C., S.L.B. and L.S.; software, E.C., L.V., B.B.C., P.B., F.M., G.G.L.P., M.C., G.C., S.L.B. and L.S.; validation, E.C., L.V., B.B.C., P.B., F.M., G.G.L.P., M.C., G.C., S.L.B. and L.S.; formal analysis, L.V., M.C., E.C., P.B., B.B.C., F.M. and L.S.; investigation, L.V., M.C., E.C., P.B., B.B.C., F.M. and L.S.; resources, Y.R., G.C. and S.L.B.; data curation, L.V., B.B.C. and L.S.; writing—original draft preparation, E.C., L.V., B.B.C., P.B., F.M., G.G.L.P., M.C., G.C., S.L.B. and L.S.; writing—review and editing, L.V., M.C., E.C., B.B.C., Y.R., G.C., S.L.B. and L.S.; visualization, Y.R., G.C. and L.S.; supervision, Y.R., G.C., S.L.B. and L.S.; project administration, G.C.; funding acquisition, Y.R., G.C. and S.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “SO.MI.PR.O.N.” project (natural products and microorganisms in horticulture for a sustainable vegetable production of high nutraceutical value), Sicilian Region, Programma di Sviluppo Rurale Sicilia (PSR-Sicilia) 2014–2020, CUP G46D20000150009.
Data Availability Statement
The data produced in this research are available upon request from Principal Investigator L.S. due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pavani, K.; Jena, C.; Divya Vani, V.; Mallikarjunarao, K. Cultivation technology of tomato in greenhouse. In Protected Cultivation and Smart Agriculture; Sagar, M., Dinkar, J.G., Tanmoy, S., Eds.; New Delhi Publishers: New Delhi, India, 2020; pp. 121–129. [Google Scholar]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#home (accessed on 26 June 2024).
- ISTAT. 2024. Available online: https://www.istat.it (accessed on 26 June 2024).
- Gao, F.; Li, H.; Mu, X.; Gao, H.; Zhang, Y.; Li, R.; Cao, K.; Ye, L. Effects of organic fertilizer application on tomato yield and quality: A meta-analysis. Appl. Sci. 2023, 13, 2184. [Google Scholar] [CrossRef]
- Dawid, J. The role of tomato products for human health (Solanum lycopersicum)—A review. J. Health Med. Nurs. 2016, 33, 66–74. [Google Scholar]
- Sattar, S.; Iqbal, A.; Parveen, A.; Fatima, E.; Samdani, A.; Fatima, H.; Shahzad, M. Tomatoes Unveiled: A Comprehensive Exploration from Cultivation to Culinary and Nutritional Significance. Qeios 2024. [Google Scholar] [CrossRef]
- Kurina, A.; Solovieva, A.; Khrapalova, I.; Artemyeva, A. Biochemical composition of tomato fruits of various colors. Vavilov J. Genet. Breed. 2021, 25, 514. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Venkadeswaran, E.; Vethamoni, P.I.; Arumugam, T.; Manivannan, N.; Harish, S.; Sujatha, R.; Rani, E.A. Performance of F1 hybrids in cherry tomato [Solanum lycopersicum (L.) var. cerasiforme Mill.] for yield and quality. Electron. J. Plant Breed. 2021, 12, 366–370. [Google Scholar]
- Sánchez, A.S.; Flores, P.; Hernández, V.; Sánchez, E.; Molina, E.; López, N.; Rodríguez-Burruezo, A.; Fenoll, J.; Hellín, P. Fruit Agronomic and Quality Traits of Tomato F1 Hybrids Derived from Traditional Varieties. Horticulturae 2024, 10, 440. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Socaci, S.A.; Farcas, A.; Plazas, M.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Physico-chemical, nutritional, and sensory evaluation of two new commercial tomato hybrids and their parental lines. Plants 2021, 10, 2480. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Khouloud, M.; Farrie, Y.; Boulif, R.; Kadmiri, I.M.; Bamouh, A.; Douira, A. Improving growth, yield, and quality of tomato plants (Solanum lycopersicum L.) by the application of moroccan seaweed-based biostimulants under greenhouse conditions. Agronomy 2021, 11, 1373. [Google Scholar] [CrossRef]
- Argento, S.; Garcia, G.; Treccarichi, S. Sustainable and low-input techniques in Mediterranean greenhouse vegetable production. Horticulturae 2024, 10, 997. [Google Scholar] [CrossRef]
- De Corato, U. Agricultural waste recycling in horticultural intensive farming systems by on-farm composting and compost-based tea application improves soil quality and plant health: A review under the perspective of a circular economy. Sci. Total Environ. 2020, 738, 139840. [Google Scholar] [CrossRef] [PubMed]
- Malécange, M.; Sergheraert, R.; Teulat, B.; Mounier, E.; Lothier, J.; Sakr, S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int. J. Mol. Sci. 2023, 24, 9714. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, Y.; Kubo, M. Soybean peptide: Novel plant growth promoting peptide from soybean. In Soybean and Nutrition; IntechOpen: London, UK, 2011; Volume 1. [Google Scholar]
- Liao, H.-S.; Chung, Y.-H.; Hsieh, M.-H. Glutamate: A multifunctional amino acid in plants. Plant Sci. 2022, 318, 111238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Jeon, B.W.; Kim, J. Signaling peptides regulating abiotic stress responses in plants. Front. Plant Sci. 2021, 12, 704490. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Martín, M.-H.J.; Ángel, M.-M.M.; Aarón, S.-L.J.; Israel, B.-G. Protein hydrolysates as biostimulants of plant growth and development. In Biostimulants: Exploring Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 141–175. [Google Scholar]
- Sonkar, S.; Pal, P.; Singh, A.K. Role of protein hydrolysates in plants growth and development. In Biostimulants in Plant Protection and Performance; Elsevier: Amsterdam, The Netherlands, 2024; pp. 61–72. [Google Scholar]
- Bulgari, R.; Morgutti, S.; Cocetta, G.; Negrini, N.; Farris, S.; Calcante, A.; Spinardi, A.; Ferrari, E.; Mignani, I.; Oberti, R. Evaluation of borage extracts as potential biostimulant using a phenomic, agronomic, physiological, and biochemical approach. Front. Plant Sci. 2017, 8, 935. [Google Scholar] [CrossRef]
- Arumugam, R.; Rabert, G.A. Plant biostimulants: Overview of categories and effects. In Biostimulants: Exploring Sources and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–29. [Google Scholar]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Li, Y.-T.; Hwang, S.-G.; Huang, Y.-M.; Huang, C.-H. Effects of Trichoderma asperellum on nutrient uptake and Fusarium wilt of tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Chen, H.Y.; Lu, G.-d. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kubheka, B.P.; Ziena, L.W. Trichoderma: A biofertilizer and a bio-fungicide for sustainable crop production. In Trichoderma-Technology and Uses; IntechOpen: London, UK, 2022. [Google Scholar]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plants. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef] [PubMed]
- Rudresh, D.; Shivaprakash, M.; Prasad, R. Tricalcium phosphate solubilizing abilities of Trichoderma spp. in relation to P uptake and growth and yield parameters of chickpea (Cicer arietinum L.). Can. J. Microbiol. 2005, 51, 217–222. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Shahraki-Mojahed, L.; Piri, R.; Sobhanizadeh, A. Trichoderma: Improving growth and tolerance to biotic and abiotic stresses in plants. In Trends of Applied Microbiology for Sustainable Economy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 525–564. [Google Scholar]
- Vultaggio, L.; Ciriello, M.; Campana, E.; Bellitto, P.; Consentino, B.B.; Rouphael, Y.; Colla, G.; Mancuso, F.; La Bella, S.; Napoli, S. Single or blended application of non-microbial plant-based biostimulants and trichoderma atroviride as a new strategy to enhance greenhouse cherry tomato performance. Plants 2024, 13, 3048. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W. Nutrient solutions for soilless cultures. In Plant Nutrition of Greenhouse Crops; Springer: Berlin/Heidelberg, Germany, 2009; pp. 257–275. [Google Scholar]
- Boztok, K.; Hartmann, H.; Zengerle, K. Solar radyasyon esas alinarak yapilan farkli seviyelerde sulamanin bazi sebze turlerinde urune etkileri. Ziraat Fakultesi dergisi-Ege Universitesi 1982, 19, 151–156. [Google Scholar]
- Han, C.; Zhao, Y.; Leonard, S.W.; Traber, M.G. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Postharvest Biol. Technol. 2004, 33, 67–78. [Google Scholar] [CrossRef]
- Lamien-Meda, A.; Lamien, C.E.; Compaoré, M.M.; Meda, R.N.; Kiendrebeogo, M.; Zeba, B.; Millogo, J.F.; Nacoulma, O.G. Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules 2008, 13, 581–594. [Google Scholar] [CrossRef]
- Rabino, I.; Mancinelli, A.L. Light, temperature, and anthocyanin production. Plant Physiol. 1986, 81, 922–924. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Navez, B.; Letard, M.; Grasselly, D.; Jost, M. Les criteres de qualite de la tomate. Infos-Ctifl 1999, 155, 41–47. [Google Scholar]
- Benito, P.; Celdrán, M.; Bellón, J.; Arbona, V.; González-Guzmán, M.; Porcel, R.; Yenush, L.; Mulet, J.M. The combination of a microbial and a non-microbial biostimulant increases yield in lettuce (Lactuca sativa) under salt stress conditions by up-regulating cytokinin biosynthesis. J. Integr. Plant Biol. 2024, 66, 2140–2157. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Yong, J.W. Harnessing synergistic biostimulatory processes: A plausible approach for enhanced crop growth and resilience in organic farming. Biology 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montesinos, B.; Diánez, F.; Moreno-Gavíra, A.; Gea, F.J.; Santos, M. Role of Trichoderma aggressivum f. europaeum as Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 1004. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Marra, R.; Vitale, S.; Pironti, A.; Fiorentino, N.; Mori, M. Yield and quality of processing tomato as improved by biostimulants based on Trichoderma sp. and Ascophyllum nodosum and biodegradable mulching films. Agronomy 2023, 13, 901. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the biostimulant effects of a novel plant-based formulation on tomato crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Molla, A.H.; Manjurul Haque, M.; Amdadul Haque, M.; Ilias, G. Trichoderma-enriched biofertilizer enhances production and nutritional quality of tomato (Lycopersicon esculentum Mill.) and minimizes NPK fertilizer use. Agric. Res. 2012, 1, 265–272. [Google Scholar] [CrossRef]
- Almeida, J.; Perez-Fons, L.; Fraser, P.D. A transcriptomic, metabolomic and cellular approach to the physiological adaptation of tomato fruit to high temperature. Plant Cell Environ. 2021, 44, 2211–2229. [Google Scholar] [CrossRef]
- Alam, Z.; Akter, S.; Khan, M.A.H.; Amin, M.N.; Karim, M.R.; Rahman, M.H.S.; Rashid, M.H.; Rahman, M.M.; Mokarroma, N.; Sabuz, A.A. Multivariate analysis of yield and quality traits in sweet potato genotypes (Ipomoea batatas L.). Sci. Hortic. 2024, 328, 112901. [Google Scholar] [CrossRef]
- Treccarichi, S.; Infurna, M.; Malgioglio, G.; Arena, D.; Ruffino, A.; Prohens, J.; Branca, F. Evaluation of tomato rootstock in Sicilian greenhouse growing conditions. In Proceedings of the III International Organic Fruit Symposium and I International Organic Vegetable Symposium 1354, Catania, Italy, 14–16 December 2021; pp. 129–136. [Google Scholar]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef]
- Ruiz-Cisneros, M.F.; Ornelas-Paz, J.d.J.; Olivas-Orozco, G.I.; Acosta-Muñiz, C.H.; Sepúlveda-Ahumada, D.R.; Pérez-Corral, D.A.; Rios-Velasco, C.; Salas-Marina, M.Á.; Fernández-Pavía, S.P. Efecto de Trichoderma spp. y hongos fitopatógenos sobre el crecimiento vegetal y calidad del fruto de jitomate. Rev. Mex. De Fitopatol. 2018, 36, 444–456. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Sharma, V.; Sharma, S.; Rana, N.; Kumar, V.; Sharma, U.; Almutairi, K.F.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gudeta, K. Seaweed extract as a biostimulant agent to enhance the fruit growth, yield, and quality of kiwifruit. Horticulturae 2023, 9, 432. [Google Scholar] [CrossRef]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Poledica, M.; Starace, G.; Graziani, G.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Pearl grey shading net boosts the accumulation of total carotenoids and phenolic compounds that accentuate the antioxidant activity of processing tomato. Antioxidants 2021, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Dodgson, J.; Weston, A.K.; Marks, D.J. Tomato Firmness and Shelf-Life Increased by Application of Stimulated Calcium. Crops 2023, 3, 251–265. [Google Scholar] [CrossRef]
- Bapary, M.S.; Islam, M.N.; Kumer, N.; Tahery, M.H.; Al Noman, M.A.; Mohi-Ud-Din, M. Postharvest physicochemical and nutritional properties of Tomato fruit at different maturity stages affected by physical impact. Appl. Food Res. 2024, 4, 100636. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Dzhos, E.; Golubkina, N.; Antoshkina, M.; Kondratyeva, I.; Koshevarov, A.; Shkaplerov, A.; Zavarykina, T.; Nechitailo, G.; Caruso, G. Effect of spaceflight on tomato seed quality and biochemical characteristics of mature plants. Horticulturae 2021, 7, 89. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and beyond color: Comparative overview of functional quality of tomato and watermelon fruits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 419–441. [Google Scholar]
- Abd-Elkader, D.Y.; Mohamed, A.A.; Feleafel, M.N.; Al-Huqail, A.A.; Salem, M.Z.; Ali, H.M.; Hassan, H.S. Photosynthetic pigments and biochemical response of zucchini (Cucurbita pepo L.) to plant-derived extracts, microbial, and potassium silicate as biostimulants under greenhouse conditions. Front. Plant Sci. 2022, 13, 879545. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).