Osmolyte Regulation as an Avocado Crop Management Strategy for Improving Productivity Under High Temperatures
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knox, J.; Hess, T.; Daccache, A.; Wheeler, T. Climate change impacts on crop productivity in Africa and South Asia. Environ. Res. Lett. 2012, 7, 034032. [Google Scholar] [CrossRef]
- Kim, W.; Iizumi, T.; Nishimori, M. Global Patterns of Crop Production Losses Associated with Droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Manousakis, V.; Zervas, G.P.; Koutis, N.; Finos, M.A.; Adamantidi, T.; Panoutsopoulou, E.; Ofrydopoulou, A.; Tsoupras, A. Avocado and Its By-Products as Natural Sources of Valuable Anti-Inflammatory and Antioxidant Bioactives for Functional Foods and Cosmetics with Health-Promoting Properties. Appl. Sci. 2024, 14, 5978. [Google Scholar] [CrossRef]
- Sommuraga, R.; Eldridge, H.M. Avocado Production: Water Footprint and Socio-economic Implications. EuroChoices Agric. Econ. Soc. 2020, 20, 48–53. [Google Scholar] [CrossRef]
- Market Data Forecast. Global Avocado Market Report 2023–2028. Available online: https://www.marketdataforecast.com (accessed on 27 December 2023).
- FAO. Perspectivas Globales del Aguacate; Food and Agriculture Organization: Rome, Italy, 2023. [Google Scholar]
- Hass Avocado Board. Global Avocado Consumption Trends; Hass Avocado Board: Mission Viejo, CA, USA, 2023. [Google Scholar]
- FAO. FAOSTAT-Producción Agrícola. Available online: https://www.fao.org/faostat/en/#data (accessed on 27 December 2022).
- FAO. The State of World Agriculture: The Impact of Climate Change on Food Production; Food and Agriculture Organization: Rome, Italy, 2023. [Google Scholar]
- Sedgley, M. The effect of temperature on floral behaviour, pollen tube growth and fruit set in the avocado. J. Hortic. Sci. 1977, 52, 135–141. [Google Scholar] [CrossRef]
- Tzatzani, T.T.; Morianou, G.; Tül, S.; Kourgialas, N.N. Air Temperature as a Key Indicator of Avocado (Cvs. Fuerte, Zutano, Hass) Maturation Time in Mediterranean Climate Areas: The Case of Western Crete in Greece. Agriculture 2023, 13, 1342. [Google Scholar] [CrossRef]
- Balikian, R.; Genskow, K. Losing Aguacate: What If Water Costs Kill Avocado Farming in San Diego. Case Stud. Environ. 2022, 6, 1559200. [Google Scholar] [CrossRef]
- Shapira, O.; Chernoivanov, S.; Neuberger, I.; Levy, S.; Rubinovich, L. Physiological Characterization of Young ‘Hass’ Avocado Plant Leaves Following Exposure to High Temperatures and Low Light Intensity. Plants 2022, 10, 1562. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, B.N.; Whiley, A.W. What do carbohydrate reserves tell us about avocado orchard management? S. Afr. Avocado Grow. Assoc. Yrbk. 1997, 20, 63–67. [Google Scholar]
- Zamet, D.N. A linear connection between the number of cold nights during flowering and fruit set period and avocado yields as a basis for forecasting seasonal yield. Alon Hanotea 1990, 45, 109–114. [Google Scholar]
- Lomas, J. Analysis of the effect of heat stress during flowering on the yield of avocado under Mediterranean climatic conditions. Agric. Forest Meteorol. 1992, 59, 207–216. [Google Scholar] [CrossRef]
- Pedreschi, R.; Uarrota, V.; Fuentealba, C.; Alvaro, J.; Olmedo, P.; Defilippi, B.; Meneses, C.; Campos-Vargas, R. Primary metabolism in avocado fruit. Front. Plant Sci. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, V.F. Climatic influence on avocado production. In California Avocado Society Yearbooks; California Avocado Society: Ventura, CA, USA, 1935; pp. 111–113. [Google Scholar]
- Lovatt, C.J. Hass Avocado Nutrition Research in California; University of California: Riverside, CA, USA, 2013. [Google Scholar]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P. Salinity, Osmolytes and Compatible Solutes. In Salinity: Environment—Plants—Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216, ISSN 0098-8472. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Curr. Opin. Plant Biol. 2002, 5, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A Key Organic Osmolyte Effectively Involved in Plant Abiotic Stress Tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Zulfugarov, I.S.; Tovuu, A.; Lee, C.H. Acceleration of cyclic electron flow in rice plants (Oryza sativa L.) deficient in the PsbS protein of Photosystem II. Plant Physiol. Biochem. 2014, 84, 233–239. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species in plant signaling. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.L.; Hormaza, J.I. Polinización y cuajado del fruto del aguacate: Una perspectiva desde España. Anu. Calif. Avocado Soc. 2009, 92, 113–135. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage, and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, J.; Zhang, X.; Wei, H.; Cui, L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ. Exp. Bot. 2006, 56, 274–285. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration—Guidelines for computing crop water requirements. In Irrigation and Drainage; FAO: Rome, Italy, 1998; p. 56. [Google Scholar]
- Schaffer, B.; Wolstenholme, B.N.; Whiley, A.W. El Aguacate (El Palto): Botánica, Producción y Usos; Ediciones Universitarias de Valparaíso: Valparaíso, Chile, 2013. [Google Scholar]
- Alcaraz, M.L.; Thorp, T.G.; Hormaza, J.I. Phenological growth stages of avocado (Persea americana) according to the BBCH scale. Sci. Hortic. 2013, 150, 434–439. [Google Scholar] [CrossRef]
- San Bautista, A.; Fita, D.; Franch, B.; Castiñeira-Ibáñez, S.; Arizo, P.; Sánchez-Torres, M.J.; Rubio, C. Crop Monitoring Strategy Based on Remote Sensing Data (Sentinel-2 and Planet): Study Case in a Rice Field After Applying Glycinebetaine. Agronomy 2022, 12, 708. [Google Scholar] [CrossRef]
- Obreza, T.A.; Rouse, R.E. Fertilizer effects on early growth and yield of 572 Hamlin orange trees. HortScience 1993, 28, 111–114. [Google Scholar] [CrossRef]
- Salazar-García, S. Nutrición del Aguacate: Principios y Aplicaciones; INIFAP, Campo Experimental Santiago Ixcuintla: Santiago Ixcuintla, Mexico, 2002. [Google Scholar]
- Liscovitch, M.; Freese, A.; Blusztajn, J.K.; Wurtman, R.J. High-Performance Liquid Chromatography of Water-Soluble Choline Metabolites. Anal. Biochem. 1985, 151, 182–187. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Agricultural Chemists). Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide, 6th ed.; SAS Institute Inc.: Cary, NC, USA, 1989. [Google Scholar]
- Zhu, J.-K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Connor, D.J.; Loomis, R.S.; Cassman, K.G. Crop Ecology: Productivity and Management in Agricultural Systems; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Lovatt, C.J. Nitrogen Nutrition of the ‘Hass’ Avocado: Where Does All the N Go? In Proceedings of the World Avocado Congress III, Tel Aviv, Israel, 22–27 October 1995; pp. 152–159. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Beaton, J.D.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management; Financial Times Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Fita, D.; Bautista, A.S.; Castiñeira-Ibáñez, S.; Franch, B.; Domingo, C.; Rubio, C. Remote Sensing Dynamics for Analyzing Nitrogen Impact on Rice Yield in Limited Environments. Agriculture 2024, 14, 1753. [Google Scholar] [CrossRef]
- Meyer, J.L.; Yates, M.V.; Stottlemyer, D.E.; Takele, E.; Arpaia, M.L.; Bender, G.S.; Witney, G.W. Irrigation and Fertilization Management of Avocados. In Proceedings of the Second World Avocado Congress, Orange, CA, USA, 21–26 April 1992; pp. 281–288. [Google Scholar]
- Tracy, J.E. A Preliminary Report on Phosphorus Deficiency of Hass Avocado. Calif. Avocado Soc. Yearb. 1985, 69, 145–154. [Google Scholar]
- Chaplin, G.R.; Scott, K.J. Association of Calcium in Chilling Injury Susceptibility of Stored Avocados. HortScience 1980, 15, 514–515. [Google Scholar] [CrossRef]
- Bower, J.P. The Calcium Accumulation Pattern in Avocado Fruit as Influenced by Long-Term Irrigation Regime. S. Afr. Avocado Grow. Assoc. Yearb. 1985, 8, 97–99. [Google Scholar]
- Witney, G.W.; Hoffman, P.J.; Wolstenholme, B.N. Effect of Cultivar, Tree Vigour and Fruit Position on Calcium Accumulation in Avocado Fruit. Sci. Hortic. 1990, 44, 269–278. [Google Scholar] [CrossRef]
- Witney, G.W.; Hoffman, P.J.; Wolstenholme, B.N. Mineral Distribution in Avocado Trees with Reference to Calcium Nutrition and Fruit Quality. Sci. Hortic. 1990, 44, 279–291. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt Stress Effects on Avocado (Persea american Mill.) Plants with and without Seaweed Extract (Ascophyllum nodosum) Application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef]
- Wallihan, E.F.; Embleton, T.W.; Printy, W. Zinc Deficiency in the Avocado. Calif. Avocado Soc. Yearb. 1958, 1, 4–5. [Google Scholar]
- Kadman, A.; Cohen, A. Experiments with Zinc Applications to Avocado Trees. Calif. Avocado Soc. Yearb. 1977, 61, 81–85. [Google Scholar]
- Hapuarachchi, N.S.; Kämper, W.; Wallace, H.M.; Hosseini Bai, S.; Ogbourne, S.M.; Nichols, J.; Trueman, S.J. Boron Effects on Fruit Set, Yield, Quality and Paternity of Hass Avocado. Agronomy 2022, 12, 1479. [Google Scholar] [CrossRef]
- Boldingh, H.L.; Alcaraz, M.L.; Thorp, T.G.; Minchin, P.E.H.; Gould, N.; Hormaza, J.I. Carbohydrate and Boron Content of Styles of ‘Hass’ Avocado (Persea americana Mill.) Flowers at Anthesis Can Affect Final Fruit Set. Sci. Hortic. 2016, 198, 125–131. [Google Scholar] [CrossRef]
- Thorp, T.G.; Barnett, A.M.; Boldingh, H.; Elmsly, T.A.; Minchin, P.E. Is Boron Transport to Avocado Flowers Regulated by Carbohydrate Supply? In Proceedings of the VII World Avocado Congress, Cairns, Australia, 5–9 September 2011. [Google Scholar]
- Arioli, T.; Villalta, O.N.; Hepworth, G.; Farnsworth, B.; Mattner, S.W. Effect of Seaweed Extract on Avocado Root Growth, Yield, and Post-Harvest Quality in Far North Queensland, Australia. J. Appl. Phycol. 2024, 36, 745–755. [Google Scholar] [CrossRef]
- Rojas-Rodríguez, M.L.; Ramírez-Gil, J.G.; González-Concha, L.F.; Balaguera-López, H.E. Biostimulants Improve Yield and Quality in Preharvest without Impinging on the Postharvest Quality of Hass Avocado and Mango Fruit: Evaluation under Organic and Traditional Systems. Agronomy 2023, 13, 1917. [Google Scholar] [CrossRef]
- Trinchant, J.C.; Boscari, A.; Spennato, G.; Van de Sype, G.; Le Rudulier, D. Proline betaine accumulation and metabolism in alfalfa plants under sodium chloride stress. Exploring its compartmentalization in nodules. Plant Physiol. 2004, 135, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.; Critchley, A.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Silber, A.; Israeli, Y.; Levi, M.; Keinan, A.; Shapira, O.; Chudi, G.; Golan, A.; Noy, M.; Levkovitch, I.; Assouline, S. Response of ‘Hass’ Avocado to Temporal Stresses: Role of Irrigation Management and Root Constraint. In Proceedings of the VII World Avocado Congress, Cairns, Australia, 5–9 September 2011. [Google Scholar]
- Rosenzweig, C.; Parry, M.L. Potential Impact of Climate Change on World Food Supply. Nature 1994, 367, 133–138. [Google Scholar] [CrossRef]
- Ameen, M.; Mahmood, A.; Ahmad, M.; Javaid, M.M.; Nadeem, M.A.; Asif, M.; Balal, R.M.; Khan, B.A. Impacts of Climate Change on Fruit Physiology and Quality. Clim. Resilient Agric. 2023, 1, 93–124. [Google Scholar]
- Hakala, M.; Tuominen, I.; Keränen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the Role of the Oxygen-Evolving Manganese Complex in Photoinhibition of Photosystem II. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with Water Stress: Evolution of Osmolyte Systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Jensen, R.G. Strategies for Engineering Water-Stress Tolerance in Plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- MAPA. Anuario de Estadística 2023; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 2023.
- Asseng, S.; Foster, I.; Turner, N.C. The Impact of Temperature Variability on Wheat Yields. Glob. Change Biol. 2011, 17, 997–1012. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef]
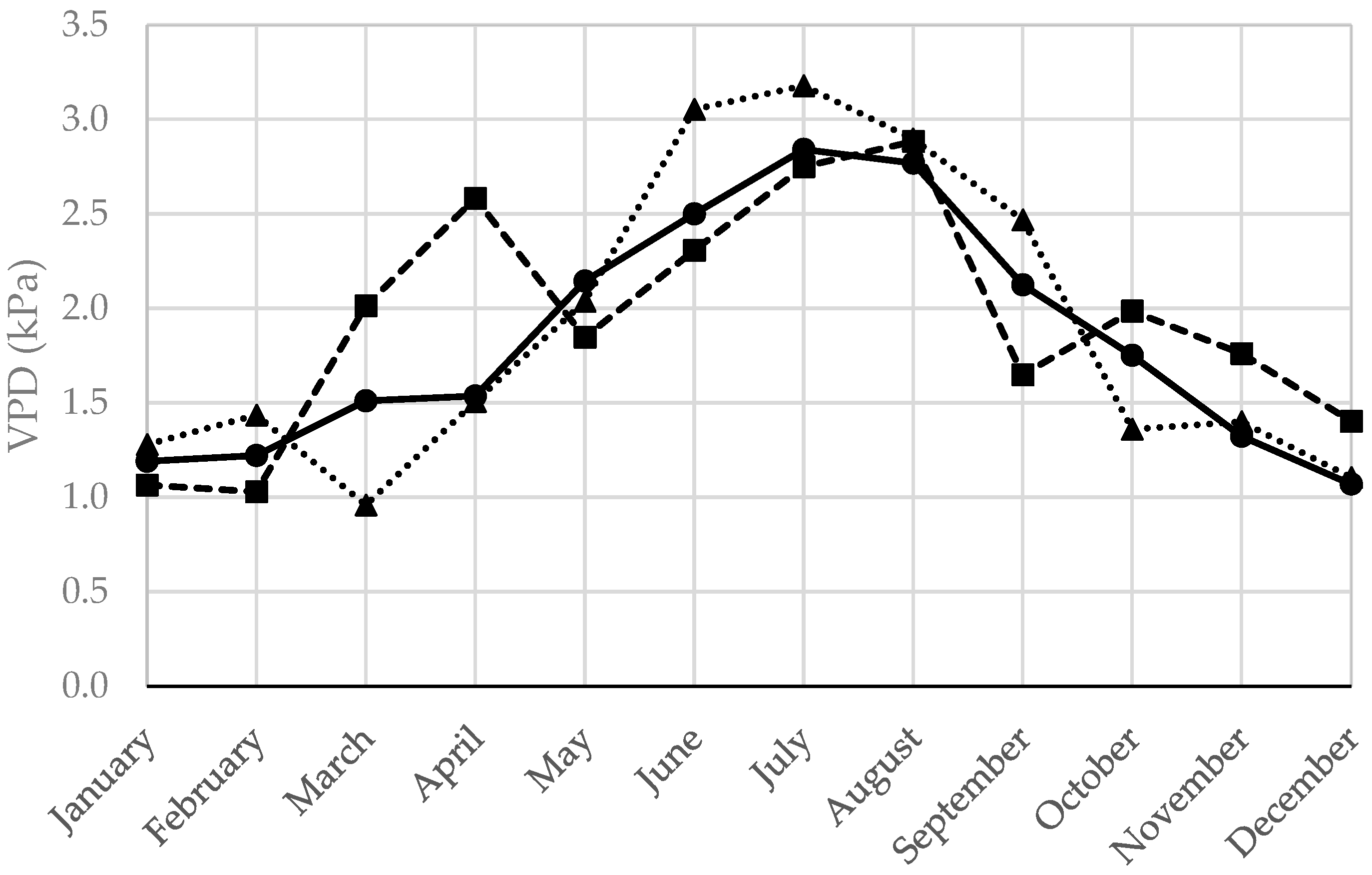
| Climatic Variable | 2022 | 2023 | 2012–2021 | 2022 Deviation * (%) | 2023 Deviation * (%) |
|---|---|---|---|---|---|
| All Seasons | |||||
| T mean (°C) | 18.58 | 18.30 | 17.76 | +4.62 | +3.02 |
| T max (°C) | 30.91 | 31.60 | 30.47 | +1.44 | +3.73 |
| T min (°C) | 7.77 | 6.34 | 6.35 | +22.29 | −0.11 |
| RH mean | 71.94 | 69.74 | 69.15 | +4.04 | +0.86 |
| RH max (%) | 99.59 | 99.84 | 98.72 | +0.88 | +1.13 |
| RH min (%) | 21.13 | 19.92 | 20.98 | +0.74 | −5.04 |
| VPD (kPa) | 1.89 | 1.94 | 1.83 | +3.17 | +5.86 |
| Rainfall (mm) | 894.84 | 373.1 | 637.741 | +40.31 | −41.50 |
| June–September | |||||
| T mean (°C) | 26.19 | 25.26 | 24.54 | +6.69 | +2.93 |
| T max (°C) | 38.97 | 36.68 | 36.89 | +5.66 | −0.56 |
| T min (°C) | 15.24 | 15.08 | 13.88 | +9.75 | +8.60 |
| RH mean | 62.87 | 72.86 | 68.06 | −7.63 | +7.06 |
| RH max (%) | 98.78 | 99.53 | 97.73 | +1.07 | +1.84 |
| RH min (%) | 17.57 | 23.77 | 19.87 | −11.55 | +19.62 |
| VPD (kPa) | 2.90 | 2.40 | 2.56 | +13.29 | −6.32 |
| Rainfall ** (mm) | 499.60 | 128.20 | 148.95 | +235.42 | −13.93 |
| Factor | Trunk Diameter (cm) | Volume (m3) |
|---|---|---|
| Year | ||
| 2022 | 7.24 | 22.4 |
| 2023 | 7.28 | 22.2 |
| Treatment | ||
| Control | 7.25 | 22.1 |
| Antioxidant | 7.27 | 22.4 |
| Analysis of variance Factor (degrees of freedom) | p-value | |
| Year (1) | ns | ns |
| Treatment (1) | ns | ns |
| Year × Treatment (1) | ns | ns |
| Factor | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| Year | ||||||
| 2022 | 1.79 | 0.17 | 1.05 | 2.5 | 0.36 | 0.29 |
| 2023 | 1.83 | 0.16 | 1.03 | 2.5 | 0.36 | 0.30 |
| Treatment | ||||||
| Control | 1.93 a | 0.18 a | 0.89 b | 2.3 b | 0.38 | 0.31 |
| Antioxidant | 1.70 b | 0.16 b | 1.19 a | 2.7 a | 0.35 | 0.29 |
| Analysis of variance Factor (degrees of freedom) | p-value | |||||
| Year (1) | ns | ns | ns | ns | ns | ns |
| Treatment (1) | * | ** | ** | * | ns | ns |
| Year × Treatment | ns | ns | ns | ns | ns | ns |
| Factor | Fe | Mn | Zn | Cu | B | Mo |
|---|---|---|---|---|---|---|
| Year | ||||||
| 2022 | 73.41 | 74.60 | 29.38 | 7.44 | 44.95 | 0.08 |
| 2023 | 72.02 | 73.03 | 28.97 | 7.28 | 44.00 | 0.07 |
| Treatment | ||||||
| Control | 72.47 | 40.20 b | 22.57 b | 7.84 a | 44.89 | 0.07 |
| Antioxidant | 72.97 | 107.42 a | 35.78 a | 6.89 b | 44.06 | 0.08 |
| Analysis of Variance Factor (degrees of freedom) | p-value | |||||
| Year (1) | ns | ns | ns | ns | ns | ns |
| Treatment (1) | ns | ** | ** | * | ns | ns |
| Year × Treatment (1) | ns | ns | ns | ns | ns | ns |
| Factor | Fruit Yield (kg ha−1) | Fruit Number (per Plant) | Fruit Weight (g fruit−1) |
|---|---|---|---|
| Year | |||
| 2022 | 10,343.83 | 152.91 a | 202.20 b |
| 2023 | 10,200.50 | 138.60 b | 219.83 a |
| Treatment | |||
| Control | 8245.83 b | 122.58 b | 202.67 b |
| Antioxidant | 12,298.5 a | 168.93 a | 219.37 a |
| Analysis of variance Factor (degrees of freedom) | p-value | ||
| Year (1) | ns | * | * |
| Treatment (1) | ** | * | * |
| Year × Treatment | ns | ns | ns |
| Factor | Fat | Fiber | Protein |
|---|---|---|---|
| Year | |||
| 2022 | 18.56 | 12.97 | 1.93 |
| 2023 | 18.55 | 13.02 | 1.90 |
| Treatment | |||
| Control | 17.95 b | 12.72 | 1.77 |
| Antioxidant | 19.17 a | 13.22 | 2.08 |
| Analysis of variance Factor (degrees of freedom) | p-value | ||
| Year (1) | ns | ns | ns |
| Treatment (1) | * | ns | ns |
| Year × Treatment | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San Bautista, A.; Agenjos-Moreno, A.; Martínez, A.; Escudero, A.I.; Arizo-García, P.; Simeón, R.; Meyer, C.; Kadyampakeni, D.M. Osmolyte Regulation as an Avocado Crop Management Strategy for Improving Productivity Under High Temperatures. Horticulturae 2025, 11, 245. https://doi.org/10.3390/horticulturae11030245
San Bautista A, Agenjos-Moreno A, Martínez A, Escudero AI, Arizo-García P, Simeón R, Meyer C, Kadyampakeni DM. Osmolyte Regulation as an Avocado Crop Management Strategy for Improving Productivity Under High Temperatures. Horticulturae. 2025; 11(3):245. https://doi.org/10.3390/horticulturae11030245
Chicago/Turabian StyleSan Bautista, Alberto, Alba Agenjos-Moreno, Ana Martínez, Ana Isabel Escudero, Patricia Arizo-García, Rubén Simeón, Christian Meyer, and Davie M. Kadyampakeni. 2025. "Osmolyte Regulation as an Avocado Crop Management Strategy for Improving Productivity Under High Temperatures" Horticulturae 11, no. 3: 245. https://doi.org/10.3390/horticulturae11030245
APA StyleSan Bautista, A., Agenjos-Moreno, A., Martínez, A., Escudero, A. I., Arizo-García, P., Simeón, R., Meyer, C., & Kadyampakeni, D. M. (2025). Osmolyte Regulation as an Avocado Crop Management Strategy for Improving Productivity Under High Temperatures. Horticulturae, 11(3), 245. https://doi.org/10.3390/horticulturae11030245








