Abstract
The quantitative composition of phenolic compounds and the antioxidant activity of Serbian and Bulgarian red wines from various vintages were analyzed and compared in this study. Phenolic profiling was conducted using high-performance liquid chromatography coupled with a diode array detector (HPLC-DAD), revealing a total of 29 identified phenolic compounds, including 16 anthocyanins, 7 flavonols, and 6 hydroxycinnamic acids and their derivatives. The antioxidant potential of the red wines was assessed using four distinct analytical methods: 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, ferric-reducing/antioxidant power (FRAP), cupric-ion-reducing capacity in the presence of neocuproine (CUPRAC), and total reducing power (TPR). The correlation of the examined results was monitored, and the results showed that the antioxidant qualities of wines are most strongly correlated with the total content of phenols and flavonoids, while the correlations are weakest for their total anthocyanins. Compositions of phenolics varied from 1016 mg/L to 4115 mg/L, while the value of flavanols was in a wide range from 438 mg/L to 2890 mg/L, whereby the average proportion of flavonoids to total phenols was 52.4%. The wine named Ruen 2019 showed the presence of the highest amount of total phenolics and total flavonoids present, followed by Prokupac and Evita cultivars. Of the tested monoglucoside anthocyanins in all wines, malvidin-3-O-glucoside was the most abundant.
1. Introduction
According to the International Organization for Vine and Wine (OIV), wine is the beverage resulting exclusively from the partial or complete alcoholic fermentation of fresh, crushed, or uncrushed grapes or grape must [1].
Wine is very complex in its chemical composition and consists of several hundred different compounds, some of which are synthesized during the formation of the berries, while some of these compounds are formed by complex biochemical processes during the fermentation of the grapes—the formation of the wine and during wine aging [2,3]. Phenols are the predominant compounds whose concentration influences the formation of color, flavor, bitterness, and astringency, while certain phenols have a strong antioxidant potential, thus protecting the wine from harmful oxidative processes during aging [4,5]. The most important phenols in wine are non-flavonoids: hydroxycinnamates, hydroxybenzoates, and stilbenes, followed by flavonoids: flavan-3-ols, flavonols, and anthocyanins [6,7].
Polyphenols are widely distributed in the human diet [8,9]. An important source of these very important biological antioxidants can be the berries skins and pith of the berries, i.e., the seeds and skins of the berries that are a by-product after the crushing of the grapes or the completion of fermentation [10,11,12].
Serbia and Bulgaria are countries with a long tradition of wine production. Therefore, both countries share many similarities in the production of traditional foods and have a rich cultural heritage of winemaking whose practices have been passed down through generations. This tradition is not only an important economic sector but also a cultural and gastronomic trademark. A special feature of Serbian viticulture is that in the last decade there has been a significant development of the wine sector, in which small family wineries are the mainstay of production. With the development of the wine market, there has been a spreading wave of popularization and a greater demand for wines from autochthonous and newly created grape cultivars compared to the previously widespread international cultivars ‘Cabernet Sauvignon’ and ‘Merlot’. Both countries have a long tradition of producing wines from autochthonous grape cultivars that can be categorized as premium wines [13,14].
Through monitoring and research, examining the presence of phenolic substances and anthocyanins, which characterize wines, still deserves the most attention and interest. Understanding these parameters can provide winemakers with valuable insights to improve production processes and produce wines that meet both traditional standards and modern consumer expectations.
The aim of the work is to analyze and identify phenolic components of red wines made from the same Serbian/Bulgarian regional, autochthonous cultivars using spectrophotometry and HPLC techniques and examine differences in their composition and antioxidant potential. The objective of studies also is to compare phenolic content and level of antioxidant properties as well as in representative samples of some important autochthonous regional grape cultivars from Serbia and Bulgaria with international (‘Cabernet Sauvignon’ and ‘Blaufrankishe’), newly created (‘Evita’) and hybrid (‘Saibel’) cultivars grown in this region. Wines from the 2019 to 2022 vintages were used to compare and contrast the parameters tested. The results presented will contribute to a better understanding of the qualitative composition and enological potential of wines from autochthonous cultivars that have been insufficiently studied to date and wines from international commercial cultivars, especially in view of the increasing request from consumers for these wines, which were less represented on the market in the previous period.
2. Materials and Methods
2.1. Wine Samples
The phenolic composition and antioxidant capacity of monocomponent wines of different vintages produced from autochthonous and newly created cultivars from Serbia and Bulgaria as well as from hybrid and international cultivars from France were investigated (Table 1). The wines of the selected cultivars are of great commercial importance and are recognized on the wine market as increasingly sought-after wines with strong enological potential. International cultivars were used in the work as a comparative control in order to draw relevant conclusions about the position of the autochthonous and newly created cultivars of Serbia and Bulgaria in relation to the already widespread international cultivars.

Table 1.
Selected cultivars and analyzed wine samples.
The microvinification process was carried out for all tested samples according to the standard enological procedures for red wines. The grapes were harvested at the stage of technological ripeness, then destemmed, crushed, and subjected to the following phased fermentation treatments: sulphurization of the crushed grapes with potassium metabisulphite (0.1 g/kg crushed grapes), addition of a selected pure culture of the yeast strain Saccharomyces cerevisae (0.2 g/kg crushed grapes), which tolerates a high sugar content and initiation of alcoholic fermentation, pressing of the fermented crushed grapes, sulphitization of the young wine (0.1 g/kg crushed grapes), settling of the young wine, separation of the wine from the lees and first racking, second racking of the wine after 30 days, and bottling.
Controlled fermentation was carried out in vessels with a controlled temperature of 15–18 °C at the start of fermentation and 23–26 °C during intensive fermentation. Fermentation lasted an average of 15–25 days (depending on the cultivar). All wines were stored in their original packaging at room temperature and protected from light for one year until the time of analysis to avoid any negative impact on their quality.
2.2. Chemicals and Reagents
The following reagents were used for the experiment: 2,2′-diphenyl-1-picrylhydrazyl (DPPH), phenol reagent according to Folin–Ciocalteu, neocuproine, (NH4)2Fe(SO4)2·6H2O, FeCl3, K3[Fe(CN)6], Na2CO3, formic acid-CH2O2, and acetonitrile (available from Merck, Darmstadt, Germany). In addition, certified standards, such as p-coumaric acid, caffeic acid, chlorogenic acid, ferulic acid, quercetin, quercetin-3-O-glucoside (isoquercetin), kaempferol, myricetin, gallic acid, and catechin, were purchased from Sigma Aldrich (Steinheim, Germany). 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was procured from Acros Organics (Morris Plains, NJ, USA). Cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, malvidin-3-O-glucoside, and peonidin-3-O-glucoside were purchased from Extrasyntese (Genay, France). All chemical components listed were HPLC or analytical reagent grade. All solutions were prepared with deionized water with a specific conductivity of 0.05 μS/cm produced with MicroMed ultrapure water systems (TKA Wasseraufbereitungssysteme GmbH, Niederelbert, Germany). Methanol, acetonitrile, and formic acid were purchased from Thermo Fisher Scientific (Hanover Park, IL, USA) and were HPLC-pure.
2.3. Determination of Phenolic Compounds
2.3.1. Spectrophotometric Analysis of Total Phenolics Content (TPC)
The total phenol content of the wines was determined spectrophotometrically according to the Folin–Ciocalteu method [15], with slight modifications. An aliquot (1 mL) of the diluted red wine (wine: water = 1:10, v/v) and 0.5 mL of the Folin–Ciocalteu reagent were mixed in a calibrated 25 mL flask. After allowing the mixture to stand at room temperature for one minute, 5 mL sodium carbonate (5% v/v) was added, and the volume was adjusted to 25 mL with deionized water. The absorbance was measured at 765 nm after incubation for 1 h in the dark at room temperature. The analysis was performed with a UV–Vis spectrophotometer (LLG uniSpec2, Jinan, China). Deionized water was used as a blank sample. The calibration standard was gallic acid (0–500 mg/L). The total polyphenol concentration was calculated using a calibration curve for standard gallic acid, and the content was expressed as mg gallic acid equivalent (GAE)/L of the wine sample. All analyses were performed in triplicate (n = 3), and the results are expressed as mean values (n = 3) ± standard deviation.
2.3.2. Total Flavonoids Content (TFC)
The measurement of the total flavonoids content in the investigated wines was determined spectrometically by using the method based on the formation of the complex flavonoid–aluminum [16]. In total, 0.5 mL (diluted with methanol) of the wine was placed in a 10 mL volumetric flask, and 5 mL of distilled water and 0.3 mL of 5% NaNO2 were added and mixed. Then, 5 min later, 0.6 mL of 10% AlCl3·6H2O was added. Two milliliters of 1 mol/L NaOH were added 5 min later, and then the volume was made with up to 10 mL of distilled water. The solution was mixed well, and the absorbance was immediately measured at 510 nm. Deionized water was used as a blank sample. The calibration standard was gallic acid (0–250 mg/L). The total flavonoid concentration was calculated using a calibration curve for standard catechin, and the content was expressed as mg catechin equivalent (CE)/L of the wine sample. All analyses were performed in triplicate (n = 3), and the results are expressed as mean values (n = 3) ± standard deviation.
2.3.3. Determination of Total and Monomeric Anthocyanins and the Percentage of Polymeric Color
According to the “single” method [17], the content of total anthocyanins is determined at pH 1, and the recording is carried out at 520 nm and 700 nm.
The content of total anthocyanins (TA) is calculated according to the following equations:
ATA = (A520 − A700) pH=1.0
The content of monomeric anthocyanins (MAs) was determined using the pH differential method [17]. The samples were appropriately diluted and dissolved in the buffers potassium chloride (0.025 mol/L, pH 1.0) and sodium acetate (0.4 mol/L, pH 4.5). The absorbance was measured at two different wavelengths, 520 nm and 700 nm, for each sample. The total monomeric anthocyanin content was expressed as milligrams of malvidin-3-O-glucoside equivalents per 1 L of wine (mg M3G/L) and calculated according to the following equations:
where A is the absorbance, DF is a dilution factor, MW is the molecular weight of malvidin-3-O-glucoside (493.5), and ε is the molar absorptivity of malvidin-3-O-glucoside (28,000).
AMA = (A520 − A700) pH = 1.0 − (A520 − A700) pH = 4.5
(TA or MA) (mg/L) = (A·MW·DF·100)/(ε·l)
Indices of anthocyanin degradation of wine can be derived using the pH differential method described by [17]. The absorbance at 420 nm of the disulfide-treated sample serves as an index for browning. The color densityof the control sample and the polymer color of the disulfide-bleached sample wine are calculated as follows:
Color density = [(A420nm − A700nm) + (A520nm − A700nm)]
The value of polymeric color is calculated as follows:
Polymeric color = [(A420nm − A700nm)/(A max − A700nm)]
The ratio between polymerization color and color density is used to determine the percentage of color accounted for by the polymerized material:
Polymeric color (%) = (polymeric color/color density) × 100
2.4. Measurement Antioxidant Activity
The antioxidant capacity of red wines was investigated in four antioxidant assays: DPPH radical scavenging [18], ferric-reducing antioxidant power (FRAP) assay [19], iron (III) to iron (II) reduction power (FRP) assay [20], and copper-ion-reducing antioxidant capacity (CUPRAC) assay [21].
2.4.1. DPPH Radical Scavenging Activity
The method for scavenging DPPH radicals is based on the reduction in DPPH radicals in the presence of a hydrogen-donating antioxidant. The DPPH radical is a long-lived organic nitrogen radical with a deep purple color [15]. Absorbance was measured at 515 nm, and all determinations were performed in triplicate. In contrast to other tests, a higher absorbance of the reaction mixture indicates a lower antioxidant activity. The reason for this is the lack of antioxidants in the tested sample, which reduced the purple color of the DPPH reagent and thus reduced the absorbance, which would mean a higher activity. The radical scavenging activity was determined from the calibration curve prepared using Trolox as a positive control at a concentration of 1–30 μmol/L and expressed in μmol Trolox equivalents (TEs) per L of red wine (μg TE/L). A DPPH stock solution was prepared by dissolving 24 mg of the DPPH in 100 mL of methanol and stored in the dark at −20 °C until use. The freshly prepared DPPH working solution was combined with 1.425 mL of the diluted-wine sample (sample/methanol = 1:10, v/v) in an amount of 0.075 mL. After a one-hour incubation in the dark at room temperature, the absorbance was measured. Methanol served as a blank.
2.4.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
Ferric-reducing antioxidant power assay was carried out according to the method of [19] and is based on the reduction in ferric iron in the tripyridyltriazine complex to the blue ferrous form at low pH. This reduction is monitored by measuring the color change during absorbance at 595 nm. The final results are expressed as mmol Fe(II) equivalents per L of red wine (mmol Fe/L), obtained by comparing the absorbance change in the test mixture with the doses obtained from the Fe(II) standard calibration curve at the concentration 2.5–25 μmol Fe(II)/L. The FRAP reagent was prepared by combining acetate buffer (300 mM, pH 3.6), iron(III)–chloride (20 mM), and TPTZ solution (10 mM TPTZ in 40 mM HCl) in a mixture of 10:1:1 (v/v/v). The diluted-wine sample (sample/methanol = 1:50, v/v) in a quantity of 0.05 mL was mixed with 1 mL of the FRAP reagent and 0.45 mL of deionized water. After that, the mixture was then incubated for 5 min in the dark at 37 °C, and the absorbance was measured.
2.4.3. Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
The CUPRAC assay is based on the reduction of a copper–neocuproine complex (Cu(II)–Nc) by antioxidants to the copper form (Cu(I)–Nc). The CUPRAC reagent is much more stable and easily accessible than chromogenic radical reagents (e.g., ABTS and DPPH) [20]. After 30 min of incubation at room temperature, the absorbance was measured at 450 nm. Trolox was used as a standard, and the results were expressed as μmol Trolox equivalents per L (μmol TE/L).
2.4.4. Total Reducing Power (TRP) Assay
The total reducing power method is based on the ability of antioxidants to reduce Fe(III) hexacyanate to Fe(II) hexacyanate, resulting in an increase in absorbance of the reaction mixtures. The absorbance was measured at 700 nm against the blank, and the results were expressed as μmol TE per L (μmol TE/L).
2.5. High Performance Liquid Chromatography (HPLC-DAD) Analysis
The quantification of the individual phenolic compounds was carried out using a reversed-phase HPLC analysis. The equipment used was an HPLC Agilent-1200 series (Agilent Technologies, Singapore) with UV–Vis diode array detector (DAD) for multi-wavelength detection. After injecting 5 µL of the sample, the separation was performed in an Agilent-Eclipse XDB C-18 column (4.6 × 150 mm) thermostatted at 25 °C. Two solvents were used for gradient elution: A (Water solution containing 2% of formic acid (HCOOH) and B (80% acetonitrile (ACN) + 2% HCOOH + H2O). The elution program was as follows: from 0 to 10 min 0% B, from 10 to 28 min gradually increased 0–25% B, from 28 to 30 min 25% B, from 30 to 35 min gradually increased 25–50% B, from 35 to 40 min gradually increased 50–80% B, and finally, for the last 5 min, gradually decreased 80–0% B. The phenolic compounds in the samples were identified by comparing their retention times and spectra with the retention times and spectra of the authentic standards for each component. Quantitative data were calculated from the calibration curves using a linear regression of peak area versus concentration. The calibration curve, determination coefficient (R2), limit of detection (LOD), and limit of quantification (LOQ) are shown in Table 2. The concentration of phenolic compounds is given in mg/L red wine.

Table 2.
Validation parameters for 11 phenolic compounds used for HPLC-DAD analysis.
The concentrations of the constituents in the samples were calculated using the equation obtained from the calibration charts prepared for each standard, while the quantification of constituents without the corresponding standard was performed based on a calibration chart prepared for structurally similar standards, i.e., e: p-coumaric acid was used to express the content of trans-coutaric acid (coumaroyltartaric acid); caffeic acid was used as a standard for trans-caftaric acid (caffeoyltartaric acid); ferulic acid was used as a standard for trans-ferric acid; quercetin-3-O-glucoside, myricetin, and kaempferol were used to express the content of quercetin glucuronide, myricetin glucoside, and kaempferol derivative; peonidin-3-O-glucoside was used for petunidin-3-O-glucoside. Delphinidin-O-glucodide, cyanidin-3-O-glucoside, peonidin-3-O-glucoside, and malvidin-3-O-glucoside were used to express the content of acetylglucosides and coum-glucosides. All analyses were performed in triplicate.
2.6. Statistical Analysis
All measurements were performed in triplicate, and data are presented as mean and standard deviation. Statistical analysis was performed using one-way analysis of variance (ANOVA), and significant differences were assessed using Tukey’s HSD post hoc test (p < 0.05). When calculating for determination, a significance test was performed under the assumption of origin (Bulgaria/Serbia).
3. Results and Discussion
3.1. Total Phenolic (TPC) and Total Flavonoid Content (TFC)
The total phenolic content (TPC) varied between the different red wine cultivars, with ‘Ruen’ having the highest concentration, followed by ‘Evita’ and ‘Melnik’ wines (Table 3). For the Bulgarian red wines, the total phenolic content ranged from 2624 to 4115 mg/L, with an average of 3183 mg/L. The Serbian red wines had a total phenolic content between 1016 and 3215 mg/L, with an average value of 1776 mg/L. Comparisons with the existing literature show similar TPC values for red wines from Croatia (2193–3183 mg GAE/L) [22] and Greece (1217–3772 mg GAE/L) [23]. Italian red wines have higher values (3888–5860 mg GAE/L) [24], while Spanish red wines (1262–2389 mg GAE/L) [25], Turkish red wines (1130–2410 mg GAE/L) [26], and South African red wines (2016–2412 mg GAE/L) have similar total phenolic contents. These results underline the variability of phenolic content in the wine samples analyzed.

Table 3.
Total phenolic content (TPC), total flavonoid content (TFC) of red wines, and the TFC/TPC ratio.
The results for the total flavonoid content of the wines using the aluminum chloride assay (Table 3) show similar variations with the results of the TPC assay (Table 1). ‘Ruen’ contained higher amounts of flavonoids compared to other red wines. The content of total flavanols varied from 1782 mg/L to 2890 mg/L, with an average value of 2116 mg/L, from 438 to 1340 mg/L for the Bulgarian red wines, and an average value of 784 mg/L for the Serbian red wines.
The average proportion of flavonoids to total phenols was 52.4%; this value is best represented by the TFC/TPC ratio shown in Table 3. The high flavonoid content of red grape cultivars contributes to their higher antioxidant potential compared to white wine [22]. The amount of flavonoids in red wines ranges from 1941 to 2893 mg GAE/L, while the amount in white wines is significantly lower, from 15.5 to 125.7 mg GAE/L, which is as much as 25 to 125 times smaller [22].
3.2. Spectrophotometric Analysis of Anthocyanins
It was found that red wines from the Balkan region have significantly higher concentrations of anthocyanins [27]. The total anthocyanin content ranged from 61.3 mg/L (‘Prokupac’ 2021) to 286.4 mg/L (‘Seibel’ 2022), with an average value of 145.3 mg/L (Table 4). The results confirm the presence of diversity in the anthocyanin composition of the wine samples analyzed and are consistent with the existing literature [28,29]. Monomeric anthocyanins are the most sensitive phenolic compounds in wine and generally decrease by about 50% annually [30]. The highest proportion of polymeric anthocyanins was determined in the analysis of the following wines: ‘Shiroka Melnik’, ‘Melnik Jubilee 1300’, ‘Ruen’, ‘Evita’ 2019, and ‘Cabernet Sauvignon’ 2021 in amounts of 54.6–65.5%.

Table 4.
Total anthocyanins (TA), monomeric anthocyanins (MA), and percentage of polymeric color (PPC) in different red wines determined via the spectrophotometric method.
When looking at the total amount of anthocyanins, the highest amount was found for the ‘Cabernet Sauvignon’ 2022 and ‘Seibel’ 2022 wines at 239.4 and 286.4 mg/L, respectively. The quantities obtained in the different production years show a clear variation in the content of polymeric anthocyanins and total anthocyanins, which is clearly reflected in the concentration of ‘Cabernet Sauvignon’. On the other hand, the same control parameters for the different production years of ‘Prokupac’ wine show no differences.
The extraction and stability of anthocyanins is influenced by winemaking, with fermentation and maceration generally leading to a decrease in monomeric anthocyanins. Factors such as pH, SO2 content, and acetaldehyde also affect the stability of anthocyanins and interactions with other phenolic compounds. Copigmentation, especially by intramolecular copigmentation facilitated by acylated anthocyanins with multiple aromatic acyl groups, plays a central role in wine aging and maturation [31].
The content of polymeric anthocyanins in the red wine samples of different vintages assessed by the pH differential method [17] ranged from 18.8% to 65.5%. As the wines mature, a greater proportion of the anthocyanin content is polymerized.
3.3. Antioxidant Activities
There are numerous antioxidant assays that can be used to evaluate the antioxidant activity. A considerable number of reactive chemical species with different mechanisms of action can damage the homeostasis of the cell [32]. For this reason, it is impossible to establish a single method for determining antioxidant activity, and it is necessary to choose a combination of several assays based on different principles to detect antioxidant potential through different mechanisms of action such as direct free radicals binding, electron transfer, and inhibition [33]. The results of this study show that the antioxidant activities follow a clear pattern. There is a clear increase in antioxidant activity with an increase in total phenolic content (Table 5).

Table 5.
Antioxidant capacities of selected red wines determined with four different methods.
3.3.1. Free Radical Scavenging Activity (DPPH) Assay
The free radical scavenging assay has mechanisms based on the inhibition of lipid oxidation and is commonly used to assess antioxidant activity. In this study, the free radical scavenging activity of red wines was tested with a methanol solution of the “stable” free radical DPPH. The scavenging activity of the different red wines is significantly different (Table 5). Red wine ‘Ruen’ showed the strongest DPPH radical scavenging effect (8.8 mmol TE/L), while the lowest DPPH radical scavenging activity was obtained from ‘Prokupac’ 2021 (3.39 mmol TE/L).
The total antioxidant capacity of wines from other countries as determined by the DPPH assay is as follows: reported values of 9.51–12.39 mmol TE/L for South African red wines [34], reported values of 4.65–17.41 mmol TE/L for Spanish red wines [25], reported values of 2.6–6.3 mmol TE/L for Brazilian red wines using the DPPH assay [35], reported values of 13.22–17.74 mmol TE/L for Austrian and Slovakian red wines [36], reported values of 4.19–17.17 mmol TE/L for selected Chinese red wines [37], and reported values of 9.2–37.8 mmol TE/L for Croatian red wines [38].
3.3.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
The highest values of FRAP for ‘Ruen’ (13.4 mmol Fe(II)/L) can be explained by the high content of phenolic compounds compared to the other wines analyzed (Table 5). For Macedonian red wines is reported that showed high variations in antioxidant activity measured by the FRAP assay, with a range of 4.45–10.27 mmol TE/L [39].
3.3.3. Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
In the present study, we used the CUPRAC assay, which is based on the reduction in Cu(II) to Cu(I) by antioxidants. All wines analyzed showed significant antioxidant capacity with the CUPRAC test. The values of total antioxidant capacity of the Bulgarian and Serbian red wines in the CUPRAC assay ranged from 13.4 to 50.7 mmol TE/L (Table 5). The Bulgarian red wines had a stronger reducing power (30.1–50.7 mmol TE/L) than the Serbian red wines (13.4–30.1 mmol TE/L). The mean CUPRAC value of the Bulgarian red wines was 38.2 mmol TE/L, and the mean CUPRAC value of the Serbian red wines was 19.8 mmol TE/L.
The total antioxidant capacity of wines from other countries as determined by the CUPRAC assay is as follows: reported values of 16.11–31.93 mmol TE/L for selected Chinese red wines [37], also reported values of 9.19–19.63 mmol TE/L for selected Chinese red wines [40], and reported values of 13.19–31.07 mmol TE/L for selected Turkish red wines [41]. The reducing power of antioxidants of red wines was found in the range of 7.24–15.8 mmol TE/L for North Macedonian wines [39].
3.3.4. Total Reducing Power (TRP) Assay
When comparing the reducing power of 14 different wines, the red wine ‘Ruen’ showed the highest total reducing power (19.4 mmol TE/L), while the ‘Prokupac’ 2021 showed a much lower value (5.6 mmol TE/L).
3.4. Correlation
The total phenolic and flavonoid content of the wines showed the strongest correlation with the antioxidant properties, while the total anthocyanins showed a weaker correlation. Thus, the antioxidant efficiency of the wines studied appears to be largely influenced by the total phenolic and flavonoid content, while anthocyanins play a minor role (Table 6 and Table 7). Apart from the relative amount of flavonoids, other factors affecting the antioxidant activity of flavonoids must also be taken into account, especially the structure of flavonoids, the number of OH groups, the degree of saturation of carbon–carbon bonds, and the substitution of OH groups by methyl and glycoside groups [42].

Table 6.
Linear correlation coefficients I between polyphenolic composition and antioxidant capacity.

Table 7.
Linear correlation coefficients I among the different methods for quantifying the antioxidant capacities.
The monomeric anthocyanins showed weak and non-significant correlations with DPPH, confirming previously found results [43,44]. These authors also found that there was no correlation between the anthocyanins and antioxidant activity measured using the DPPH assay. On the other hand, other authors [45,46] found a significant correlation between total flavonols and anthocyanins and the DPPH scavenging capacity of Spanish and Greek red wines, suggesting that these two flavonoid classes can significantly influence the antioxidant properties of red wines.
Although these recent studies show considerable differences in the antioxidant activity of phenolic compounds, the results remain contradictory. Antioxidant activity depends on many factors other than the phenolic composition of the test material, including the concentration of the free radical, the duration of the assay, and the dilution factor of the sample. In addition, red wine is a complex matrix and contains large amounts of phenolic and non-phenolic compounds, so antioxidant activity cannot be predicted by the content of a particular class or substance alone.
Regarding the different methods, the significant correlation between the methods was confirmed for all four methods (DPPH, FRAP, CUPRAC, and TRP). The relatively close coupling of four parameters (DPPH, FRAP, CUPRAC, and TRP) indicates that each of them can be considered as a relevant and reliable characteristic of the antioxidant capacity of wines.
3.5. HPLC Analysis of Phenolic Compounds in Red Wines
The quantitative evaluation of the phenolic compounds in Serbian and Bulgarian red wines was based on the peak areas in the HPLC-DAD chromatograms, which were recorded at 520 nm (for anthocyanins), 360 nm (for flavonols), and 320 nm (for hydroxycinnamic acids) (Figure 1, Figure 2 and Figure 3).
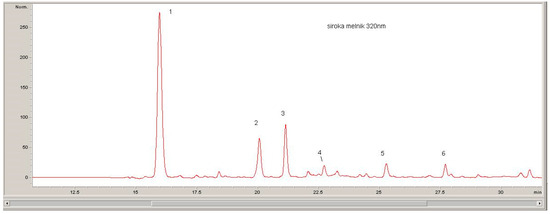
Figure 1.
Chromatograph recorded at 320 nm: 1, trans-cafratic acid; 2, trans-coutaric acid; 3, caffeic acid; 4, trans-fertaric acid; 5, p-coumaric acid; 6, ferulic acid.
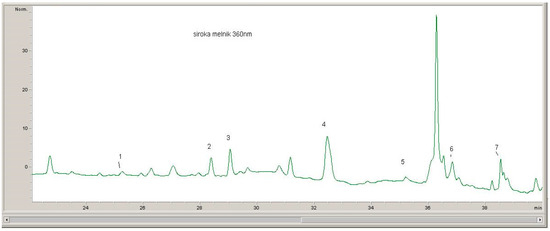
Figure 2.
1, Myricetin–glucoside; 2, Quercetin–glucuronide; 3, Quercetin–glucoside; 4 Myricetin; 5 Kampferol–glucoside; 6 Quercetin; 7 Kampferol.
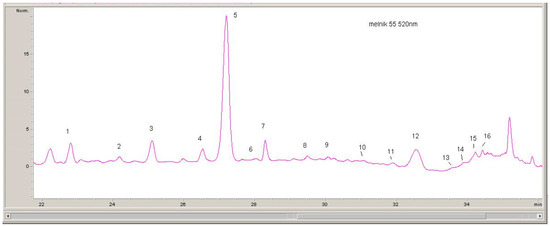
Figure 3.
1, Delphinidin-3-O-glucoside; 2, Cyanidin-3-O-glucoside; 3, Petunidin-3-O-glucoside; 4, Peonidin-3-O-glucoside; 5, Malvidin-3-O-glucoside; 6, Delphinidin-3-O-acetylglucoside; 7, Vitisin A; 8, Cyanidin-3-O-acetylglucoside; 9, Petunidin-3-O-acetylglucoside; 10, Delphinidin-3-O-p-coumaroylglucoside; 11, Peonidin-3-O-acetylglucoside; 12, Malvidin-3-O-acetylglucoside; 13, Cyanidin-3-O-p-coumaroylglucoside; 14, Petunidin-3-O-p-coumaroylglucoside; 15, Peonidin-3-O-p-coumaroylglucoside; 16, Malvidin-3-O-p-coumaroylglucoside.
3.5.1. Anthocyanins
The highest total anthocyanin content was found in the wines ‘Seibel’ 2022 (203.94 mg/L) and ‘Cabernet Sauvignon’ 2022 (169.13 mg/L), while the lowest content was measured in ‘Blaufraenkisch’ 2019 (35.87 mg/L). The red wine samples showed considerable differences in total anthocyanin concentration due to the different aging profiles. In general, younger wines had higher levels of monomeric anthocyanins, which could be detected by liquid chromatography, than older wines, which is the case in this study.
Wines made from ‘Vranac’ had the highest anthocyanin content compared to ‘Cabernet Sauvignon’ and other international wines within the Macedonian wine regions [47], and, on the other hand, anthocyanin levels can vary between 32.5 and 260.3 mg/L in various Italian wines [48]. Therefore, in Greek red wines, anthocyanin contents were in the range of 21–1011 mg/L [49].
The predominant anthocyanin compound in all samples analyzed was malvidin-3-O-glucoside, with concentrations ranging from 21.06 mg/L in ‘Blaufraenkisch’ 2019 to 145.62 mg/L in ‘Seibel’ 2022 (Table 8 and Table 9). This observation is consistent with the widely accepted notion that malvidin-3-O-glucoside is a primary anthocyanin of grapes, a characteristic observed in numerous grape cultivars. However, when looking at the percentage of malvidin-3-O-glucoside in relation to the total anthocyanin content (calculated via HPLC technique), slight differences were observed. ‘Prokupac’, in particular, had the highest proportion of this compound (72.03%), while ‘Evita’ and ‘Melnik’ had lower proportions (58.21% and 53.27%, respectively).

Table 8.
Anthocyanin composition in Bulgarian red wines, (mg/L).

Table 9.
Anthocyanin composition in Serbian red wines, (mg/L).
One hypothesis is that malvidin-3-O-glucoside undergoes additional reactions with pyruvate molecules in the presence of fermenting yeasts, producing vitisin A, a compound that is not found in grapes but is widespread in young wines, although its presence decreases with ripening. In addition, malvidin-3-O-glucoside reacts with caffeic acid to form pinotin A, the concentration of which increases with aging and peaks at around 5 years of age [50].
In addition to malvidin-3-O-glucoside, delfinidin-3-O-glucoside and peonidin-3-O-glucoside were also detected in the tested cultivars, while cyanidin derivatives were absent in all wines. This observation suggests significant enzymatic activity, particularly by the enzymes 3′-O-methyltransferase and flavonoid 3′-O-hydroxylase, which are responsible for the conversion of cyanidin to peonidin and delphinidin, respectively. The concentration of delfinidin-3-O-glucoside peaked in ‘Seibel’ 2022 and ‘Melnik’ 82, while its content in ‘Prokupac’ was comparatively lower.
In ‘Melnik’ wines, the concentration of non-acylated derivatives increased in decreasing order: malvidin-3-O-glucoside > petunidin-3-O-glucoside > delfinidin-3-O-glucoside. In the red wine samples of the ‘Evita’, the order of decreasing concentration was also as follows: malvidin-3-O-glucoside > peonidin-3-O-glucoside > delfinidin-3-O-glucoside.
Genetic factors play a crucial role in determining the specific anthocyanin pattern of each grape cultivar [51,52]. In general, this pattern is assumed to remain constant and is not influenced by environmental conditions, suggesting its potential utility in differentiating between grape cultivars. In addition, fixed ratios between certain anthocyanins have been proposed as parameters for verifying grape identity and ensuring the authenticity of cultivar wines [53].
The ratio of malvidin and peonidin derivatives (ΣMv/ΣPn) provides information on the activity of two enzymes, flavonoid 3′-hydroxylase and O-dihydroxyphenyl-O-transferase, which catalyze the synthesis of disubstituted (cyanidin and peonidin derivatives) and trisubstituted (delphinidin, petunidin, and malvidin derivatives) anthocyanins in grapes. This ratio directly reflects the enzyme activity, with the highest value of ΣMv/ΣPn observed in ‘Ruen’ red wine (13.90) and the lowest in ‘Blaufraenkisch’ 2019 (4.33). As for the ΣDe/ΣPn coefficient, the wines from ‘Melnik’ (except ‘Melnik’ 82) and ‘Ruen’ had the highest values (1.01/1.46 and 1.26, respectively), while the values for the other wines ranged from 0.26 to 0.89. The highest level of the Σgluc/Σcoum coefficient is found for ‘Melnik’ wines (average 19.32), Cabernet Sauvignon’ (average 20.75), and ‘Blaufraenkisch’ (21.14).
3.5.2. Hydroxicinnamic Acid and Flavonols
The analysis of hydroxycinnamic acid derivatives (HCAs) revealed the presence of six compounds: ester forms of HCAs with tartaric acid (trans-caftaric acid, trans-coutaric acid, and trans-fertaric acid) and their free forms (caffeic acid, p-coumaric acid, and ferulic acid). In addition, the ratio of trans-coutaric acid to trans-caftaric acid was determined (Table 10 and Table 11).

Table 10.
Content of hydroxycinnamic acids derivatives and flavonols in Bulgarian wines, (mg/L).

Table 11.
Content of hydroxycinnamic acids derivatives and flavonols in Serbian wines, (mg/L).
Trans-caftaric acid proved to be the predominant hydroxycinnamic acid in Bulgarian red wines, ranging from 25.49 to 106.25 mg/L, with an average concentration of 70.04 mg/L. This was followed by trans-cutaric acid (2.31–14.31 mg/L, average 10.33 mg/L), caffeic acid (5.62–22.41 mg/L, average 9.92 mg/L), and trans-fertaric acid (not detected–10.48 mg/L, average 4.84 mg/L).
Serbian red wines, on the other hand, were also dominated by trans-caftaric acid with concentrations between 22.08 and 60.54 mg/L and an average value of 40.88 mg/L. This was followed by trans-cutaric acid (2.32–9.47 mg/L, average 7.73mg/L), caffeic acid (2.15–9.14 mg/L, average 4.77 mg/L), ferulic acid (2.32–5.98 mg/L, average 4.22 mg/L), and p-coumaric acid (2.32–6.77 mg/L, average 4.02 mg/L).
Significant differences were found in the total hydroxycinnamic acid content between wines from different grape cultivars in the different wine-growing regions of the Balkans. Bulgarian wines had the highest average total hydroxycinnamic acid content (102.96 mg/L) compared to Serbian wines (62.86 mg/L).
In addition, the ratio of trans-coutaric acid to trans-caftaric acid varied greatly among the wines tested: ‘Ruen’ (0.22), ‘Evita’ (0.20), and ‘Cabernet Sauvignon’ (0.15). This ratio, as proposed by [54], can be used to differentiate the grape cultivars of Vitis vinifera L. wines. Remarkably, these ratios in the wines largely corresponded to the values determined in previous studies for the respective grape cultivars [55,56]. In line with the results of [47], who found a similar percentage of caftaric acid (69.1%) predominantly in ‘Vranac’ red wines from Macedonia, our results emphasize the usefulness of the relative proportions of HCAs for the classification of grape cultivars, as shown by [56].
All determined chemical parameters observed composition of anthocyanin, but also, hydroxycinnamic acids derivatives and flavonols showed significant variations in the wine over the years. For this reason, they show a significant difference (p < 0.001) in both Bulgarian and Serbian wines. Flavonols, a class of compounds found in red wine, include aglycones such as quercetin, myricetin, and kaempferol as well as their various glycosides (e.g., glucosides, galactosides, and glucuronides) [57,58]. Quercetin is one of the most abundant flavonoids in red wines, and among the flavonoid groups, these compounds are known for their important biological activities [58].
Aglycones such as kaempferol, quercetin, and myricetin were identified in the tested wines, which are present both as original grape glycosides and as released aglycones after hydrolysis [59]. In particular, quercetin-type flavonols predominated in all wines, with myricetin-type and quercetin-type being the most important flavonols. Although kaempferol was detected in all samples, its concentration was comparatively lower. It is noteworthy that flavonol glycosides in wine exhibit uneven hydrolysis, with certain compounds, such as quercetin-3-glucuronide, showing remarkable resistance to hydrolysis [60]. In addition, flavonol aglycones have limited solubility in wine and may precipitate, while myricetin derivatives are susceptible to oxidation. As a result, quercetin-3-glucuronide has emerged as the dominant flavonol compound in many wines.
The amount of free flavonols in red wines is thought to depend on the winemaking processes [61]. Our results show a relatively high content of conjugated myricetin in the wines studied, with Bulgarian red wines and the Serbian red wines ‘Evita’ and ‘Prokupac’ having a higher content of free myricetin than conjugated myricetin. ‘Seibel’ in particular had the highest concentration of conjugated myricetin at 38.6%. In most of the wines tested (with the exception of ‘Ruen’ and ‘Blaufraenkisch’), the concentration of conjugated quercetin exceeded that of free quercetin. Particularly high values of conjugated quercetin were found in ‘Prokupac’ 2019 and 2021 (65.53% and 66.17%, respectively), followed by ‘Melnik 55’ (52.77%), and ‘Melnik 82’ (50.90%). According to some studies [61,62], the average percentage of free flavonols in red wines is typically between 20 and 50% of the total flavonol content. In our study, the percentage of free flavonols in red wines ranged from 16.54% (‘Seibel’ 2022) to 48.33% (‘Melnik Jubilee 1300’). Two wines had a free flavonol content of more than 50% of the total flavonol content, with the ‘Ruen’ containing 55.53% and the ‘Shiroka Melnik’ 54.91% free flavonols.
The examination of all individual parameters was aimed at looking at their quantities and comparing them with international cultivars, which is, at the same time, a very complex analysis. According to the importance of the content of total phenolic compounds, it can be seen that the content in the wines of Ruen, Melnik 82, and Evita 2019 cultivars is higher compared to international cultivars, which gives a lot of uniqueness and enological potential. When observing the most abundant anthocyanin compound, malvidin-3-O-glucoside, as well as the total amount of detected individual anthocyanins, it can be seen that the values of autochthonous cultivars are in a wider range compared to international cultivars, but that they do not lag behind either in terms of the amount or the types of detected anthocyanins. On the other hand, the lowest content of phenols and anthocyanins was detected in Prokupac wine. Also, the amount of total hydroxycinnamic acids and total aglycon type flavonols is significantly lower compared to international varieties. The amount of total hydroxycinnamic acids is significantly lower compared to international cultivars. This only confirms that the wines of these rare cultivars are not significantly behind in terms of the amount of phenol and their antioxidant activity, so it could be said that the quality is also good; for this reason, in the future, more importance should be given to the cultivation and production of wines from these autochthonous cultivars.
4. Conclusions
In the last ten years, the wine sector has been growing, and wines from autochthonous cultivars are very popular in the wine market. Wine is a popular traditional beverage in Serbia and Bulgaria; however, there are no comparative studies and data on the qualitative parameters of wines of autochthonous cultivars. Also, there is no parallel study test on phenolic content in the international cultivars, which are the most common in this region (like ‘Cabernet Sauvignon’ and ‘Merlot’) and rare autochthones cultivars (‘Prokupac’, ‘Melnik’, and ‘Ruen’), which is the main objective of this study.
Analyzing the anthocyanin complex of Bulgarian wines, the highest amount of monoglycoside anthocyanins is found in samples ‘Melnik 55’ (65.72 mg/L) and ‘Melnik 82’ (62.06 mg/L). The same variation trend was recorded in the composition of acetylated and acylated anthocyanin. Of the coumaroylated forms, poeonidin-3-O-p-coum-glucoside and mavidin-3-O-p-coum-glucoside were detected in all examined samples, while other cumulated species were absent.
In Serbian wine samples (‘Prokupac’ 2019 and 2021), significantly lower values of acetylated and acylated forms of anthocyanins were recorded (30.42 and 29.20 mg/L, respectively). By comparing the international cultivars (‘Cabernet Sauvignon’, ‘Merlot’, and ‘Blaufraenkisch’) with the mentioned autochthonous cultivars, it can be concluded that the ‘Cabernet Sauvignon’ stands out for a large number of the examined parameters. This is precisely what speaks of a significant genetic and enological dominant predisposition to the accumulation of the examined phenolic compounds. On the other hand, ‘Seibel 2022’, which served as a control variant in the experiment, stands out in terms of the amount of values obtained, while in practice and in the field, it is not cultivated due to its poor hybrid predispositions. Vitisin A in Bulgarian wines had fairly stable values in the range of 2.55 to 3.83 mg/L for ‘Shiroka Melnik’ and ‘Melnik Jubilee 1300’, respectively. Vitisin A values were significantly lower in Serbian wines, ranging from 0.79 to 0.85 mg/L. Other cultivars (‘Evita’, ‘Merlot’, ‘Cabernet Sauvignon’, and ‘Seibel’) had values similar to Bulgarian wines.
By looking at the results as a whole, we can say that the investigated autochthonous cultivars, in terms of the amount of phenols, their antioxidant capacity, and thus the quality, are not behind the international cultivars, and that in the future we should emphasize the importance of their cultivation, wine production, and further monitoring of the influence of composition, which will also be our goal in the future.
Author Contributions
Conceptualization, M.M. and Z.P.; methodology, M.M.; formal analysis, J.N.; investigation, M.M. and V.S.-J.; resources, N.M. and Z.P.; data curation, M.M., Z.P. and A.T.; writing—original draft preparation, M.M., Z.P. and A.T.; writing—review and editing, M.M., Z.P., A.T. and N.M.; visualization, M.M. and N.M.; supervision, M.M. and Z.P.; funding acquisition, Z.P. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The research was supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia under project number 451-03-137/2025-03/200116 and 451-03-65/2024-03/200124.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Organisation of Vine and Wine (OIV). International Code of Oenological Practices. Resolutions Adopted in Jerez (Spain) 21st G.A.—9 June 2023. Legal Basis: Agreement of 3 April 2001, Resolution AG 3/2004, Resolution 16/70. OIV Code Sheet—Issue 2024/01. Dijon, France. 2024, pp. 1–453. Available online: https://www.oiv.int/sites/default/files/publication/2024-03/CPO%202024%20EN%20.pdf (accessed on 25 December 2024).
- Errichiello, F.; Forino, M.; Picariello, L.; Moio, L.; Gambuti, A. Analysis of Polyphenols During Alcoholic Fermentation of Red Grape Aglianico (Vitis vinifera L.): Potential Winemaking Optimization and Pomace Valorization. Molecules 2024, 29, 5962. [Google Scholar] [CrossRef] [PubMed]
- Jordão, A.M.; Correia, A.C.; Martins, B.; Romão, A.; Oliveira, B. General Physicochemical Parameters, Phenolic Composition, and Varietal Aromatic Potential of Three Red Vitis vinifera Varieties (“Merlot”, “Syrah”, and “Saborinho”) Cultivated on Pico Island—Azores Archipelago. Int. J. Plant Biol. 2024, 15, 1369–1390. [Google Scholar] [CrossRef]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sadeghnezhad, E.; Leng, X.; Pei, D.; Dong, T.; Zhang, P.; Gong, P.; Jia, H.; Fang, J. Assessment of ‘Cabernet Sauvignon’ Grape Quality Half-Véraison to Maturity for Grapevines Grown in Different Regions. Int. J. Mol. Sci. 2023, 24, 4670. [Google Scholar] [CrossRef] [PubMed]
- Chengolova, Z.; Ivanov, Y.; Godjevargova, T. Comparison of Identification and Quantification of Polyphenolic Compounds in Skins and Seeds of Four Grape Varieties. Molecules 2023, 28, 4061. [Google Scholar] [CrossRef] [PubMed]
- Aris, G.; Cuneo, I.F.; Pastenes, C.; Cáceres-Mella, A. Anthocyanin Composition in Cabernet Sauvignon Grape Skins: Effect of Regulated Deficit Irrigation in a Warm Climate. Horticulturae 2022, 8, 96. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yakov, I.; Yashin, Y.Y. Chemical Composition and Polyphenolic Compounds of Red Wines: Their Antioxidant Activities and Effects on Human Health—A Review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, H.; Yang, N.; Cao, J.; Wang, J.; Wang, X.; Xi, Z. Effect of vineyard row orientation on microclimate, phenolic compounds, individual anthocyanins, and free volatile compounds of Cabernet Sauvignon (Vitis vinifera L.) in a high-altitude arid valley. Eur. Food Res. Technol. 2022, 248, 1365–1378. [Google Scholar] [CrossRef]
- Yu, R.; Torres, N.; Kaan Kurtural, S.K. Obtaining Spatial Variations in Cabernet Sauvignon (Vitis vinifera L.) Wine Flavonoid Composition and Aromatic Profiles by Studying Long-Term Plant Water Status in Hyper-Arid Seasons. Horticulturae 2024, 10, 68. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Meade, H.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Investigating the Relation between Skin Cell Wall Composition and Phenolic Extractability in Cabernet Sauvignon Wines. Fermentation 2022, 8, 401. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol Characterization and Antioxidant Activity of Grape Seeds and Skins from Sicily: A Preliminary Study. Sustainability 2022, 14, 702. [Google Scholar] [CrossRef]
- Markovic, N.; Przic, Z. Serbian viticulture from the 19th century to the present day. Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2022, 52, 261–269. [Google Scholar] [CrossRef]
- Przic, Z.; Markovic, N. Agrobiological and technological characteristics of some grapevine varieties and clones grown in Serbia. Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2019, 49, 229–237. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV visible spectroscopy. In Current Protocols in Food Analytical Chemistry, 1st ed.; John Wiley & Sons: New York, NY, USA, 2001; pp. F1.2.1.–F1.2.13. [Google Scholar] [CrossRef]
- Hou, X.; Chen, S.; Pu, Y.; Wang, T.; Xu, H.; Li, H.; Ma, P.; Hou, X. Effect of Winemaking on Phenolic Compounds and Antioxidant Activities of Msalais Wine. Molecules 2023, 28, 1250. [Google Scholar] [CrossRef]
- Benzie, F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘Antioxidant Power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Erçağ, E. The cupric ion reducing antioxidant capacity (CUPRAC) and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Milos, M.; Modun, D.; Music, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, A. Effect of Principal Polyphenolic Components in Relation to Antioxidant Characteristics of Aged red Wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef]
- Cimino, F.; Sulfaro, V.; Trombetta, D.; Saija, A.; Tomaino, A. Radical-scavenging capacity of several Italian red wines. Food Chem. 2007, 103, 73–81. [Google Scholar] [CrossRef]
- Fernandez-Pachon, S.M.; Villano, D.; Garcia-Parrilla, M.C.; Troncoso, A.M. Antioxidant activity of wines and relation with their poliphenolic composition. Anal. Chim. Acta 2004, 513, 113–118. [Google Scholar] [CrossRef]
- Anli, E.; Vural, N. Antioxidant Phenolic Substance of Turkish Red Wines from Different Wine Region. Molecules 2009, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, A.; Radovanović, B.; Jovančičević, B. Free radical scavenging and antibacterial activities of southern Serbian red wines. Food Chem. 2008, 117, 326–333. [Google Scholar] [CrossRef]
- Munoz-Espada, A.C.; Wood, K.V.; Bordelon, B.; Watkins, B.A. Anthocyanin Quantification and radical scavenging capacity of Concord, Norton and Marechal Foch grapes and wines. J. Agric. Food Chem. 2004, 52, 6779–6786. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and purification of anthocyanins by highspeed counter current chromatography and screening for antioxidant activity. J. Agric. Food Chem. 2000, 48, 1063–1072. [Google Scholar] [CrossRef]
- Lapidot, T.; Harel, S.; Akiri, B.; Granit, R.; Kanner, J. pH-dependent forms of red wine anthocyanins as antioxidants. J. Agric. Food Chem. 1999, 47, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta 2002, 458, 147–155. [Google Scholar] [CrossRef]
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 93–104. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Joubert, E.; Gelderblom, W.C.A.; Manley, M. Antioxidant Activity of South African Red and White Cultivar Wines: Free Radical Scavenging. J. Agric. Food Chem. 2003, 51, 902–909. [Google Scholar] [CrossRef]
- Nixdorf, S.L.; Hermosin-Gutierrez, I. Brazilian Red Wines Made from the Hybrid Grape Cultivar Isabel: Phenolic Composition and Antioxidant Capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Stasko, A.; Brezova, V.; Mazur, M.; Certik, M.; Kalinak, M.; Gescheidt, G. A Comparative Study on the Antioxidant Properties of Slovakian and Austrian Wines. LWT—Food Sci. Technol. 2008, 41, 2126–2135. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.Y.; Li, Y.; Li, P.H.; Wang, H. Polyphenolic Compounds and Antioxidant Properties of Selected China Wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Seruga, M.; Novak, I.; Jakobek, L. Determination of Polyphenols Content and Antioxidant Activity of Some Red Wines by Differential Pulse Voltammetry, HPLC and Spectrophotometric Methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Mitrevska, K.; Grigorakis, S.; Loupassaki, S.; Calokerinos, A. Antioxidant Activity and Polyphenolic Content of North Macedonian Wines. Appl. Sci. 2020, 10, 2010. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.W. Comparison on Phenolic Compounds and Antioxidant Properties of Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Molecules 2012, 17, 8804–8821. [Google Scholar] [CrossRef] [PubMed]
- Büyüktuncel, E.; Porgalı, E.; Çolak, C. Comparison of Total Phenolic Content and Total Antioxidant Activity in Local Red Wines Determined by Spectrophotometric Methods. Food Nutr. Sci. 2014, 5, 1660–1667. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Giovanelli, G. Evaluation of the antioxidant activity of red wines in relationship to their phenolic content. Ital. J. Food Sci. 2005, 17, 381–393. [Google Scholar]
- Zafrilla, P.; Morillas, J.; Mulero, J.; Cayuela, J.M.; Martínez-Cachá, A.; Pardo, F.; Lopes Nicolas, J.M. Changes during storage in conventional and ecological wine: Phenolic content and antioxidant activity. J. Agric. Food Chem. 2003, 51, 4694–4700. [Google Scholar] [CrossRef]
- Alén-Ruiz, F.; García-Falcón, M.S.; Pérez-Lamela, M.C.; Martínez-Carballo, E.; Simal-Gándara, J. Influence of major polyphenols on antioxidant activity in Mencía and Brancellao red wines. Food Chem. 2009, 113, 53–60. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P.; Gkoulioti, A.; Marinos, V.; Tsoupeis, D.; Boutaris, I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Compos. Anal. 2008, 21, 614–621. [Google Scholar] [CrossRef]
- Ivanova-Petroleus, V.; Ricci, A.; Nedelkovski, D.; Dimovska, V.; Parpinello, G.P.; Versari, A. Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem. 2015, 44, 2851–2860. [Google Scholar] [CrossRef]
- Mattivi, F.; Nicolini, G. Analysis of polyphenols and resveratrol in Italian wines. Bio Factors 1997, 6, 445–448. [Google Scholar] [CrossRef]
- Kallithraka, S.; Mohdaly, A.; Makris, D.P.; Kefalas, P. Determination of major anthocyanin pigments in Hellenic native grape varieties (Vitis vinifera sp.): Association with antiradical efficiency. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
- Dimitrovska, M.; Tomovska, E.; Bocevska, M. Characterisation of Vranec, Cabernet Sauvignon and Merlot wines based on their chromatic and anthocyanin profiles. J. Serb. Chem. Soc. 2013, 78, 1269–1460. [Google Scholar] [CrossRef]
- Revilla, E.; García-Beneytez, E.; Cabello, F. Anthocyanin fingerprint of clones of Tempranillo grapes and wines made with them. Aust. J. Grape Wine Res. 2009, 15, 70–78. [Google Scholar] [CrossRef]
- Esteban, M.A.; Villanueva, M.J.; Lissarrague, J.R. Effect of irrigation on changes in the anthocyanin composition of the skin of cv Tempranillo (Vitis vinifera L.) grape berries during ripening. J. Sci. Food Agric. 2001, 81, 4. [Google Scholar] [CrossRef]
- Casavecchia, C.; Magnisi, R.; La Pera, L.; Maisano, R.; Dugo, G. Classification of Sicilian Red Wines from Autochthonous and Allochthonous Cultivars According to Anthocyanin Pattern. Am. J. Enol. Vitic. 2007, 58, 286–290. [Google Scholar] [CrossRef]
- Singleton, V.L.; Zaya, J.; Trousdale, E.K. Caftaric and coutaric acids in fruit of Vitis. Phytochemistry 1986, 25, 2127–2133. [Google Scholar] [CrossRef]
- Pajović, R.; Wendelin, S.; Forneck, A.; Eder, R. Varietal differentiation of grapes cv. ‘Vranac’, ‘Kratošija’ and ‘Cabernet Sauvignon’ from Montenegro according to their polyphenolic composition. Mitt. Klosterneubg. 2014, 64, 9–19. [Google Scholar]
- Pajović-Šćepanović, R.; Wendelin, S.; Eder, R. Phenolic composition and varietal discrimination of Montenegrin red wines (Vitis vinifera var. Vranac, Kratošija, and Cabernet Sauvignon). Eur. Food Res. Technol. 2018, 244, 2243–2254. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno Ottoboni, A.M.M.; Fiorini, A.M.R.; Guiguer, É.L.; Nicolau, C.C.T.; Goulart, R.A.; Flato, U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Munoz, N.; Gomez-Alonso, S.; Garcıa-Romero, E.; Hermosın-Gutierrez, I. Flavonol profiles of vitis vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef]
- Tsanova-Savova, S.; Ribarova, F. Free and Conjugated Myricetin, Quercetin, and Kaempferol in Bulgarian Red Wines. J. Food Compos. Anal. 2002, 15, 639–645. [Google Scholar] [CrossRef]
- Mc Donald, M.S.; Hunhes, M.; Burns, J.; Lean, M.; Matthews, D.; Crozier, A. Survey of the free and conjugated myricetin, and quercetin content of red wines of different geographical origins. J. Agric. Food Chem. 1998, 46, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Tagliafico, L.; Persia, A.; Page, E.; Ottaviani, S.; Cremonini, A.L.; Borgarelli, C.; Pisciotta, L.; Mecocci, P.; Nencioni, A.; et al. The Potential Effects of Red Wine and Its Components on Neurocognitive Disorders: A Narrative Review. Nutrients 2024, 16, 3431. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).