Effects of Selenium/Iodine Foliar Application and Seasonal Conditions on Yield and Quality of Perennial Wall Rocket
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Protocol and Growing Conditions
2.2. Yield Determination
2.3. Sample Preparation
2.4. Dry Matter
2.5. Ascorbic Acid
2.6. Total Polyphenols (TPs)
2.7. Total Antioxidant Activity (AOA)
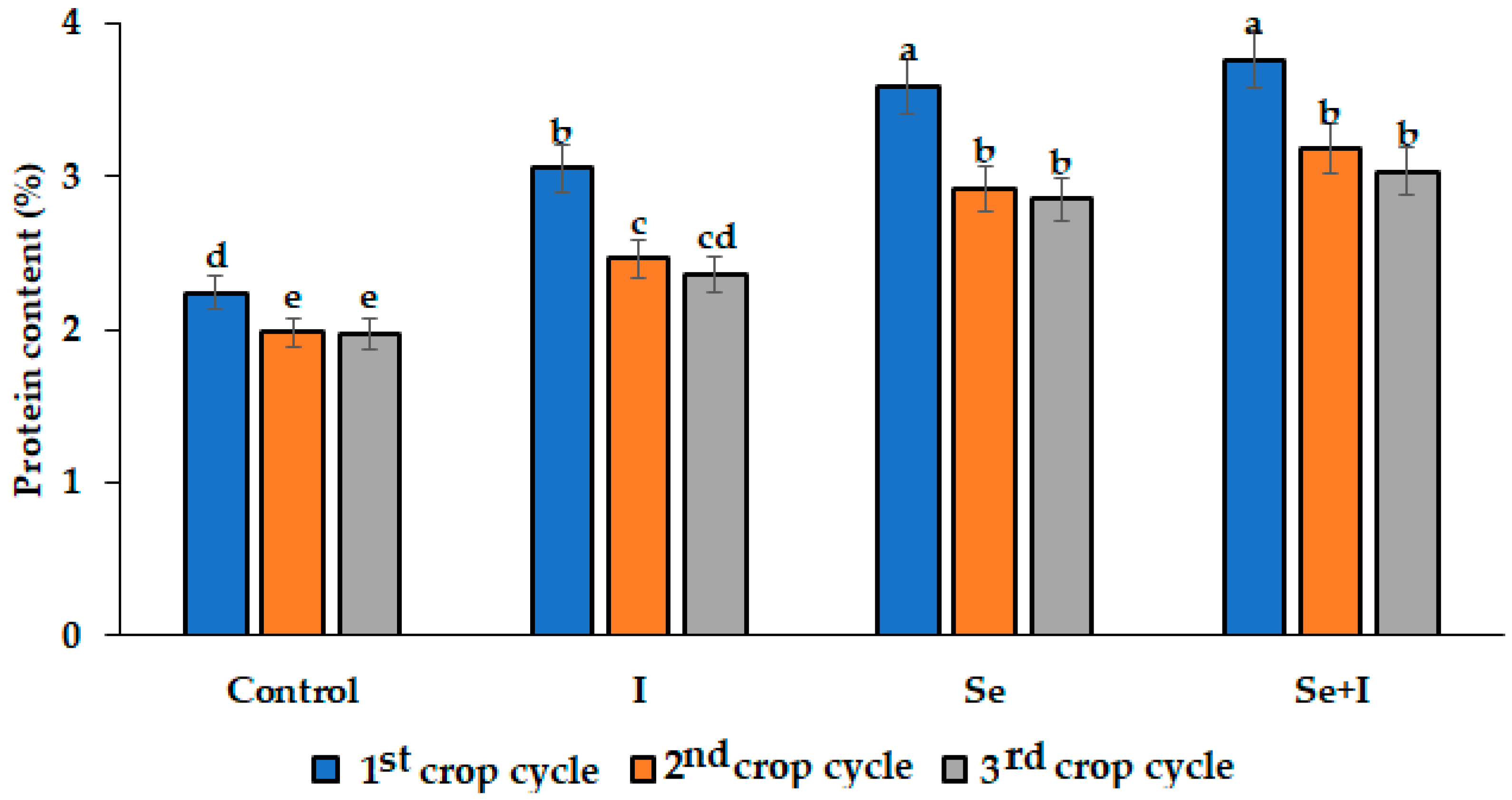
2.8. Proteins
2.9. Soluble Solids
2.10. Nitrates
2.11. Colour Measurement
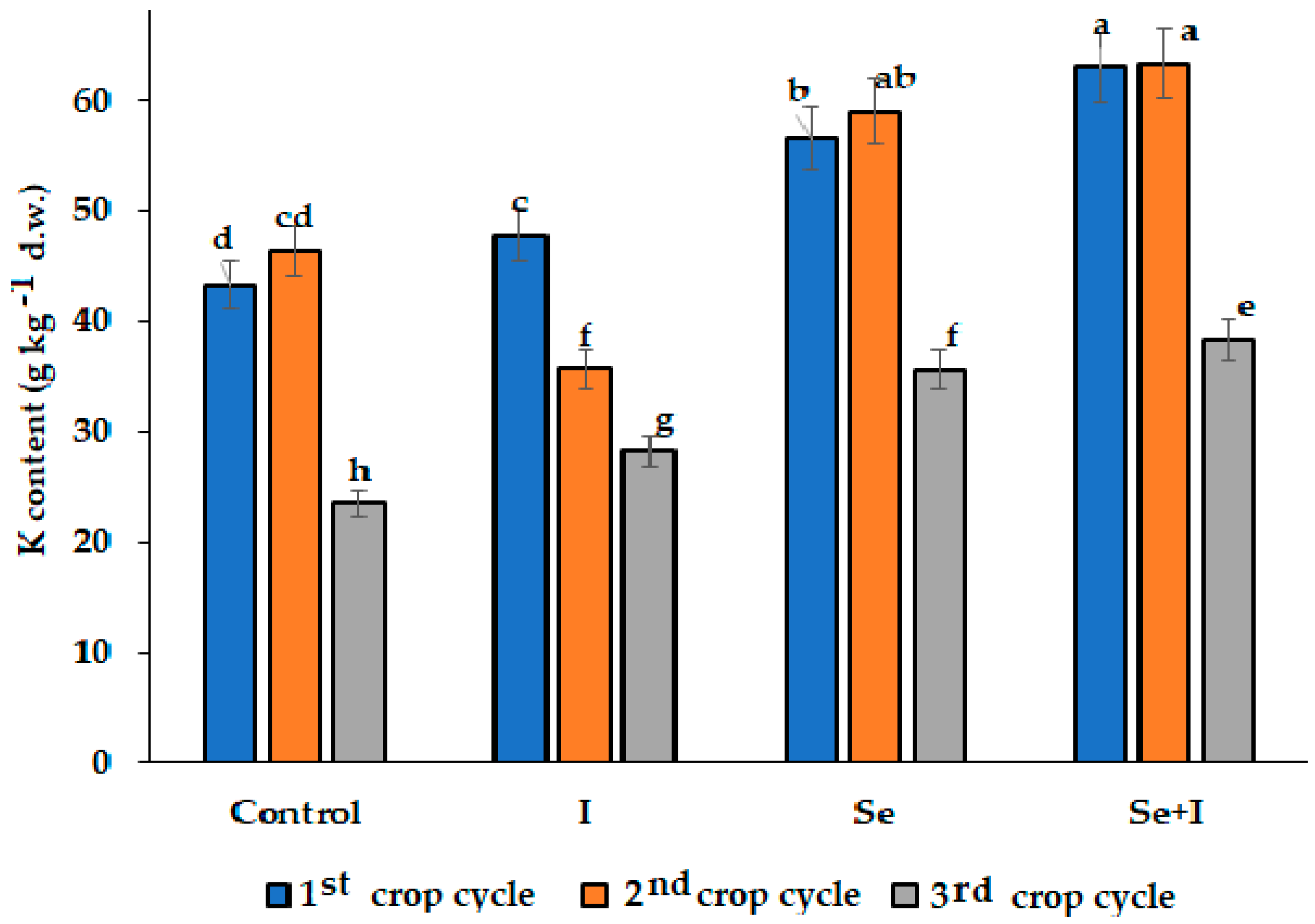
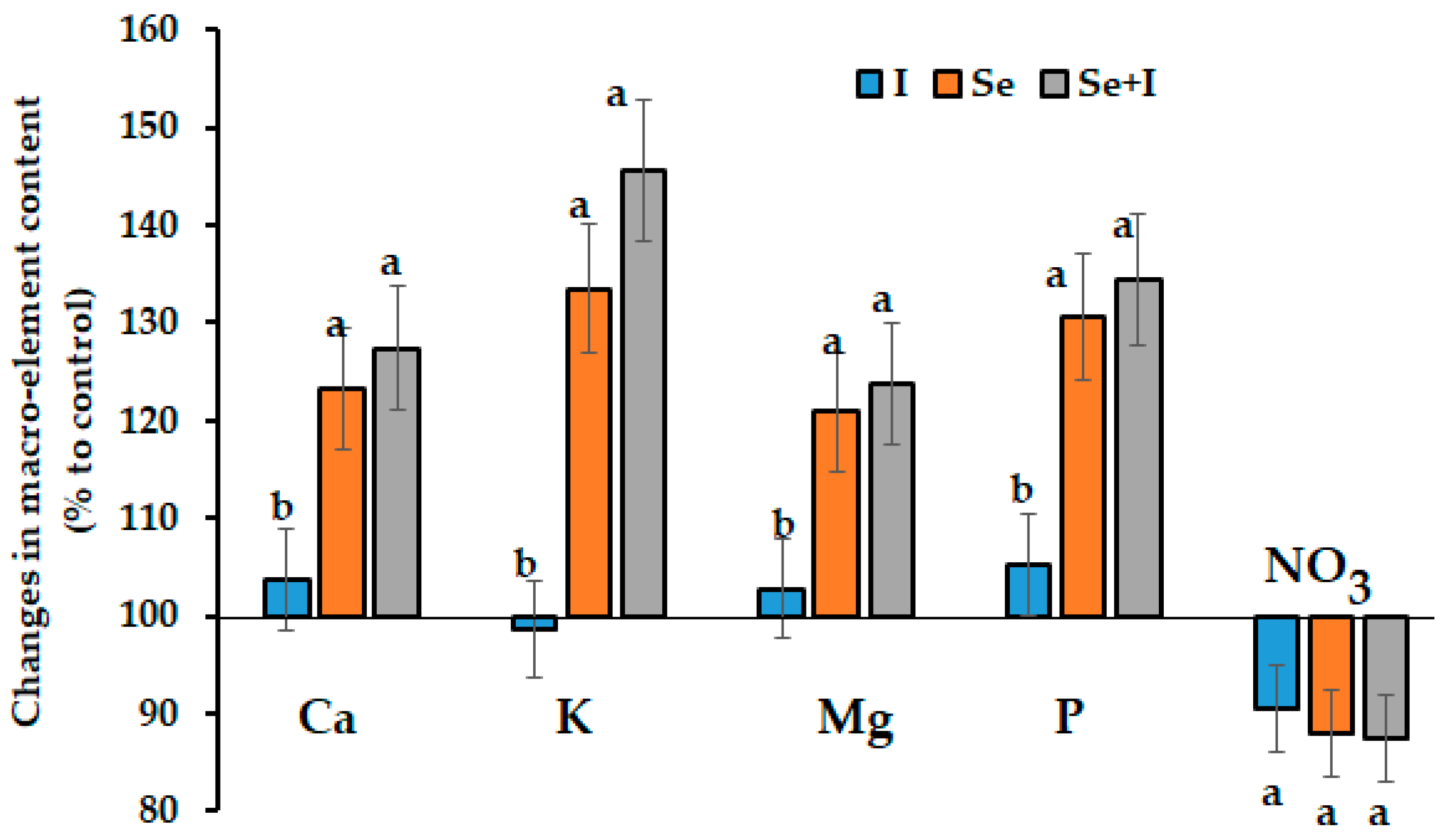
2.12. Mineral Determination
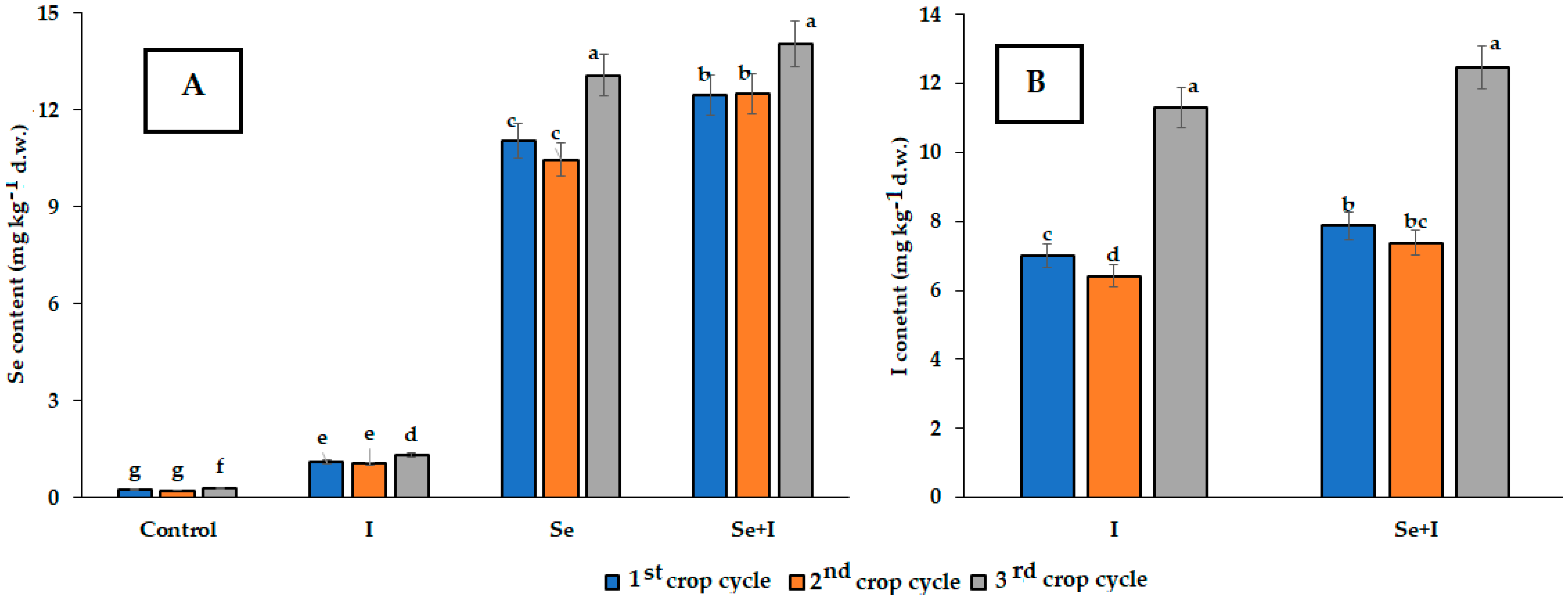
2.13. Selenium Content
2.14. Iodine Content
2.15. Statistical Analysis
3. Results and Discussion
3.1. Yield and Plant Performance
3.2. Quality, Colour, and Antioxidant Parameters
3.3. Mineral Composition
3.4. Correlations Between the Variables
3.5. Mineral Consumption Levels
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Golubkina, N.; Moldovan, A.; Kekina, H.; Kharchenko, V.; Sekara, A.; Vasileva, V.; Skrypnik, L.; Tallarita, A.; Caruso, G. Joint Biofortification of Plants with Selenium and Iodine: New Field of Discoveries. Plants 2021, 10, 1352. [Google Scholar] [CrossRef]
- The Iodine Global Network. Global Scorecard of Iodine Nutrition in 2020 in the General Population Based on School-Age Children (SAC); IGN: Ottawa, ON, Canada, 2021. [Google Scholar]
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Lyons, G. Biofortification of Cereals with Foliar Selenium and Iodine Could Reduce Hypothyroidism. Front. Plant Sci. 2018, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Avnee; Sood, S.; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An approach to eradicate micronutrient deficiency. Front. Nutr. 2023, 10, 1233070. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine—A systematic literature review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef] [PubMed]
- Smolén, S.; Kowalska, I.; Sady, W. Assessment of biofortification with iodine and selenium of lettuce cultivated in the NFT hydroponic system. Sci. Hortic. Amst. 2014, 166, 9–16. [Google Scholar] [CrossRef]
- Smolén, S.; Skoczylas, Ł.; Rakoczy, R.; Ledwożyw-Smolen, I.; Kopec, A.; Piatkowska, E. Mineral composition of field-grown lettuce (Lactuca sativa L.) depending on the diversified fertilization with iodine and selenium compounds. Acta Sci. Pol. Hortorum Cultus 2015, 14, 97–114. [Google Scholar]
- Smolén, S.; Skoczylas, Ł.; Ledwożyw-Smolén, I.; Rakoczy, R.; Kopéc, A.; Piatkowska, E.; Kapusta-Duch, J. Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front. Plant Sci. 2016, 7, 730. [Google Scholar] [CrossRef]
- Smolén, S.; Baranski, R.; Ledwożyw-Smoleń, I.; Skoczylas, Ł.; Sady, W. Combined biofortification of carrot with iodine and selenium. Food Chem. 2019, 300, 125202. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Kacjan-Maršić, N.; Kroflič, A.; Jerše, A.; Stibilj, V.; Golob, A. Significant accumulation of iodine and selenium in chicory (Cichorium intybus L. var. foliosum Hegi) leaves after foliar spraying. Plants 2020, 9, 1766. [Google Scholar] [CrossRef] [PubMed]
- Smolén, S.; Kowalska, I.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Grzanka, M.; Halka, M.; Sady, W. The effect of salicylic acid on biofortification with iodine and selenium and the quality of potato cultivated in the NFT system. Sci. Hort. 2018, 240, 530–543. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Golubkina, N.; Gomez, L.; Kekina, H.; Hallam, R.; Tallarita, A.; Cozzolino, E.; Torino, V.; Koshevarov, A.; Cuciniello, A.; Maiello, R. Joint selenium-iodine supply and arbuscular mycorrhizal fungi inoculation affect yield and quality of chickpea seeds and residual biomass. Plants 2020, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Moldovan, A.; Fedotov, M.; Kekina, H.; Kharchenko, V.; Folmanis, G.; Alpatov, A.; Caruso, G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants 2021, 10, 2528. [Google Scholar] [CrossRef]
- Golob, A.; Kroflič, A.; Jerše, A.; Kacjan Maršić, N.; Šircelj, H.; Stibilj, V.; Germ, M. Response of Pumpkin to Different Concentrations and Forms of Selenium and Iodine, and their Combinations. Plants 2020, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Novak, T.; Kacjan Maršić, N.; Šircelj, H.; Stibilj, V.; Jerše, A.; Kroflič, A.; Germ, M. Biofortification with selenium and iodine changes morphological properties of Brassica oleracea L. var. gongylodes) and increases their contents in tubers. Plant Physiol. Biochem. 2020, 150, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Fan, H.; Wu, D.; Wan, J.; Wang, X.; Huang, R.; Liu, W.; Shen, F. Assessing bioaccessibility of Se and I in dual biofortified radish seedlings using simulated in vitro digestion. Food Res. Int. 2019, 119, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Stibilj, V.; Šircelj, H.; Jerše, A.; Kroflič, A.; Golob, A.; Kacjan Maršić, N. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J. Sci. Food Agric. 2019, 99, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Huang, Y.; Hu, Y.; Liu, Y.; Christie, P. Interactions between selenium and iodine uptake by spinach (Spinacia oleracea L.) in solution culture. Plant Soil 2004, 261, 99–105. [Google Scholar] [CrossRef]
- Jerše, A.; Kacjan Maršić, N.; Kroflič, A.; Germ, M.; Šircelj, H.; Stibilj, V. Is foliar enrichment of pea plants with iodine and selenium appropriate for production of functional food. Food Chem. 2018, 267, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Duborská, E.; Šebesta, M.; Matulová, M.; Zvěřina, O.; Urík, M. Current Strategies for Selenium and Iodine Biofortification in Crop Plants. Nutrients 2022, 14, 4717. [Google Scholar] [CrossRef]
- Nascimento, V.L.; Souza, B.C.O.Q.; Lopes, G.; Guilherme, L.R.G. On the Role of Iodine in Plants: A Commentary on Benefits of This Element. Front. Plant Sci. 2022, 13, 836835. [Google Scholar] [CrossRef]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium accumulation and metabolism in algae. Aq. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Rakoczy-Lelek, R.; Smolen, S.; Grzanka, M.; Ambroziak, K.; Pitala, J.; Skoczylas, Ł.; Liszka-Skoczylas, M.; Kardasz, H. Effectiveness of Foliar Biofortification of Carrot With Iodine and Selenium in a Field Condition. Front. Plant Sci. 2021, 12, 656283. [Google Scholar] [CrossRef] [PubMed]
- Cerretani, L.; Comandini, P.; Fumanelli, D.; Scazzina, F.; Chiavaro, E. Evaluation of iodine content and stability in recipes prepared with biofortified potatoes. Int. J. Food Sci. Nutr. 2014, 65, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Tessier, E.; Amouroux, D.; Abril, G.; Lemaire, E.; Donald, O.F.X. Formation and volatilisation of alkyl-iodides and -selenides in macrotidal estuaries. Biogeochemistry 2002, 59, 183–206. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Nie, B.; Lyu, C.; Liu, X. Selenium loss and changes in product quality during cooking of selenium enriched potato tubers. J. Food Comp. Anal. 2021, 96, 103728. [Google Scholar] [CrossRef]
- Bertolino, F.A.; Stege, P.W.; Salinas, E.; Raba, M.G.A.J. Electrochemical Study of the Antioxidant Activity and the Synergic Effect of Selenium with Natural and Synthetic Antioxidants. Anal. Lett. 2010, 43, 2078–2090. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. Int. J. Mol. Sci. 2024, 25, 2600. [Google Scholar] [CrossRef]
- Maia, L.B.; Maiti, B.K.; Moura, I.; Moura, J.J.G. Selenium—More than Just a Fortuitous Sulfur Substitute in Redox Biology. Molecules 2024, 29, 120. [Google Scholar] [CrossRef] [PubMed]
- Finley, G.F. Selenium and glucosinolates in cruciferous vegetables: Metabolic interactions and implications for cancer chemoprevention in humans. Acta Hortic. 2007, 744, 171–180. [Google Scholar] [CrossRef]
- Caruso, G.; Formisano, L.; Cozzolino, E.; Pannico, A.; El-Nakhel, C.; Rouphael, Y.; Tallarita, A.; Cenvinzo, V.; De Pascale, S. Shading Affects Yield, Elemental Composition and Antioxidants of Perennial Wall Rocket Crops Grown from Spring to Summer in Southern Italy. Plants 2020, 9, 933. [Google Scholar] [CrossRef]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. Yield and Quality as Influenced by Cropping Season, Protein Hydrolysates, and Trichoderma Applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Parrella, G.; Giorgini, M.; Nicoletti, R. Crop systems, quality and protection of Diplotaxis tenuifolia. Agriculture 2018, 8, 55. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Consumers acceptance and volatile profile of wall rocket (Diplotaxis erucoides). Food Res. Int. 2020, 132, 109008. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.D.; Jobling, J.J.; Rogers, G.S. Some perspectives on rocket as a vegetable crop a review. Veg. Crops Res. Bull. 2012, 76, 21–41. [Google Scholar] [CrossRef]
- Romano, R.; Pizzolongo, F.; De Luca, L.; Cozzolino, E.; Rippa, M.; Ottaiano, L.; Mormile, P.; Mori, M.; Di Mola, I. Bioactive Compounds and Antioxidant Properties of Wild Rocket (Diplotaxis Tenuifolia L.) Grown under Different Plastic Films and with Different UV-B Radiation Postharvest Treatments. Foods 2022, 11, 4093. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; De Pascale, S.; Nicoletti, R.; Cozzolino, E.; Rouphael, Y. Productivity, nutritional and functional qualities of perennial wall-rocket: Effects of pre-harvest factors. Folia Hort. 2019, 31, 71–80. [Google Scholar] [CrossRef]
- Teliban, G.C.; Precupeanu, C.; Munteanu, N.; Burducea, M.; Cojocaru, A.; Vlăduț, N.V.; Popa, L.D.; Stoleru, V. The effect of the crop cycle and differential fertilization on a perennial wall-rocket crop established in polytunnel. Acta Hortic. 2024, 1391, 479–486. [Google Scholar] [CrossRef]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC-MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Nardi, S.; Pilon-Smits, E.A.H.; Dall’Acqua, S. Foliar selenium fertilization alters the content of dietary phytochemicals in two rocket species. Front. Plant Sci. 2022, 13, 987935. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Ertani, A.; Pilon-Smits, E.A.H.; Fabrega-Prats, M.; Schiavon, M. Selenium Biofortification Differentially Affects Sulfur Metabolism and Accumulation of Phytochemicals in Two Rocket Species (Eruca Sativa Mill. and Diplotaxis Tenuifolia) Grown in Hydroponics. Plants 2019, 8, 68. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Nascimento, C.S.; Lopes, G.; Carrasco, G.; Gratão, P.L.; Cecílio Filho, A.B. Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique. Horticulturae 2022, 8, 1088. [Google Scholar] [CrossRef]
- Aksu, G.; Temel, E.; Altay, H. Comparison of iodine application methods in Rocket Plant. Agron. Res. 2017, 15, 5–10. [Google Scholar]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Lawson, P.G.; Daum, D.; Czauderna, R.; Meuser, H.; Härtling, J.W. Soil versus foliar iodine fertilization as a biofortification strategy for field-grown vegetables. Front. Plant Sci. 2015, 6, 450. [Google Scholar] [CrossRef] [PubMed]
- AOAC Association Official Analytical Chemists. The Official Methods of Analysis of AOAC International; 22 ‘Vitamin C’; AOAC: Rockville, MD, USA, 2012. [Google Scholar]
- Golubkina, N.; Kekina, H.; Molchanova, A.; Antoshkina, M.; Nadezhkin, S.; Soldatenko, A. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020. (In Russia) [Google Scholar]
- Walker, J.M. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; Chapter 2; pp. 7–10. [Google Scholar]
- Alfthan, G.V. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Jerše, A.; Kacjan Marši´c, N.; Šircelj, H.; Germ, M.; Kroflic, A.; Stibilj, V. Seed soaking in I and Se solutions increases concentrations of both elements and changes morphological and some physiological parameters of pea sprouts. Plant. Physiol. Biochem. 2017, 118, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Riyazuddin, R.; Singh, K.; Iqbal, N.; Nisha, N.; Rani, A.; Kumar, M.; Khatri, N.; Siddikui, M.H.; Yasheshwar; Kim, S.T.; et al. Iodine: An emerging biostimulant of growth and stress responses in plants. Plant Soil 2022, 486, 119–133. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D. The role of selenium and nano selenium on physiological responses in plant: A review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Bandehagh, A.; Dehghanian, Z.; Gougerdchi, V.; Hossain, M.A. Selenium: A Game Changer in Plant Development, Growth, and Stress Tolerance, via the Modulation in Gene Expression and Secondary Metabolite Biosynthesis. Phyton 2023, 92, 2301–2324. [Google Scholar] [CrossRef]
- Mishra, N.; Jiang, C.; Chen, L.; Paul, A.; Chatterjee, A.; Shen, G. Achieving abiotic stress tolerance in plants through antioxidative defense mechanisms. Front. Plant Sci. 2023, 14, 1110622. [Google Scholar] [CrossRef] [PubMed]
- Ressurreição, S.; Salgueiro, L.; Figueirinha, A. Diplotaxis Genus: A Promising Source of Compounds with Nutritional and Biological Properties. Molecules 2024, 29, 2612. [Google Scholar] [CrossRef]
- Pasini, F.; Verardo, V.; Caboni, M.F.; D’Antuono, L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD–MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012, 133, 1025–1033. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and its role in biological functions: An updated review. EXCLI J. 2018, 17, 856–863. [Google Scholar]
- Ciriello, M.; Campana, E.; Colla, G.; Rouphael, Y. An Appraisal of Nonmicrobial Biostimulants’ Impact on the Productivity and Mineral Content of Wild Rocket (Diplotaxis tenuifolia (L.) DC.) Cultivated under Organic Conditions. Plants 2024, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Antoshkina, M.; Bondareva, L.; Sekara, A.; Campagna, E.; Caruso, G. Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae 2023, 9, 535. [Google Scholar] [CrossRef]
- Strzetelski, P.; Smolen, S.; Rożek, S.; Sady, W. The effect of diverse iodine fertilization on nitrate accumulation and content of selected compounds in radish plants (Raphanus sativus L.). Acta Sci. Pol. Hortorum Cultus 2010, 9, 65–73. [Google Scholar]
- Santamaria, P.; Elia, A.; Serio, F. Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes. J. Plant Nutr. 2002, 25, 245–258. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and Glucosinolates as Strong Determinants of the Nutritional Quality in Rocket Leafy Salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission. Regulation (EU) No 1258/2011 of 2 December 2011 amending Regulation (EC) No. 1881/2006 as regards maximum levels for nitrates in foodstuffs. Off. J. Eur. Union 2011, L320, 15–17. [Google Scholar]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- Schlagenhauf, U. On the Role of Dietary Nitrate in the Maintenance of Systemic and Oral Health. Dent. J. 2022, 10, 84. [Google Scholar] [CrossRef]
- Pinaffi-Langley, A.C.D.C.; Dajani, R.M.; Prater, M.C.; Nguyen, H.V.M.; Vrancken, K.; Hays, F.A.; Hord, N.G. Dietary Nitrates from Plant Foods: A Conditionally Essential Nutrient for Cardiovascular Health. Adv. Nutr. 2024, 15, 100158. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Medrano-Macias, J.; Leija-Martinez, P.; Gonzalez-Orales, S.; Juarez-Maldonado, A.; Benavides-Mendoza, A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016, 7, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Johanningsmeier, S.D.; Price, R.; Reynolds, R.; Truong, V.D.; Payton, S.C.; Breidt, F. Evolution of nitrate and nitrite content in pickled fruit and vegetable products. Food Control 2018, 90, 304–311. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and Human Health: Perspectives and Research Directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Golubkina, N.; Kharchenko, V.; Moldovan, A.; Antoshkina, M.; Ushakova, O.; Sękara, A.; Stoleru, V.; Murariu, O.C.; Tallarita, A.V.; Sannino, M.; et al. Effect of Selenium and Garlic Extract Treatments of Seed-Addressed Lettuce Plants on Biofortification Level, Seed Productivity and Mature Plant Yield and Quality. Plants 2024, 13, 1190. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Zhang, M.; Wei, X.; Zhou, Y. Effects of foliar selenium application on Se accumulation, elements uptake, nutrition quality, sensory quality and antioxidant response in summer-autumn tea. Food Res. Int. 2024, 175, 113618. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B. Effect of selenium on selected macronutrients in maize plants. J. Elementol. 2008, 13, 513–519. [Google Scholar]
- Ducsay, L.; Zapletalova, A.; Hozlar, P.; Cerny, I.; Varga, L.; Slepcan, M. Effects of selenium on macro- and micro nutrients and selected qualitative parameters of oat (Avena sativa L.). Potravinarstvo 2020, 14, 619–624. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of Selenium Application Differentially Modulate Plant Growth, Selenium Accumulation and Speciation, Protein, Anthocyanins and Concentrations of Mineral Elements in Purple-Grained Wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef]
- van Wonderen, D.; Melse-Boonstra, A.; Gerdessen, J.C. Iron Bioavailability Should be Considered when Modeling Omnivorous, Vegetarian, and Vegan Diets. J. Nutr. 2023, 153, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; MacGregor, G.A. Beneficial effects of potassium on human health. Physiol. Plant. 2008, 133, 725–735. [Google Scholar] [CrossRef]
| Crop Cycle | Starting Date | First Se/I Treatment | Second Se/I Treatment | Third Se/I Treatment | Date of Leaf Cutting |
|---|---|---|---|---|---|
| First | 2 November (sowing) | 15 November | 20 November | 25 November | 9 December |
| Second | 9 December | 25 December | 30 December | 4 January | 24 January |
| Third | 24 January | 10 February | 15 February | 20 February | 9 March |
| Treatment | Crop Surface (m2) | Yield (g m−2) | Number of Leaves per m2 | Mean Leaf Weight (g) | Plant Dry Matter (g m−2) |
|---|---|---|---|---|---|
| Crop cycle (means of 4 values: I, Se, Se + I, control) | |||||
| Autumn | 12 | 1536.6 ± 319.8 a | 1442.8 ± 300.8 a | 1.08 ± 0.13 a | 158.7 ± 42.6 a |
| Autumn–winter | 12 | 724.2 ± 163.7 b | 1213.8 ± 208.8 b | 0.59 ± 0.05 b | 76.4 ± 23.4 b |
| Winter | 12 | 640.0 ± 98.6 b | 1266.9 ± 179.2 b | 0.51 ± 0.02 b | 66.1 ± 14.5 c |
| Biofortification treatment (means of the 3 crop cycles) | |||||
| Control | 9 | 743.9 ± 347.2 c | 987.9 ± 63.3 c | 0.75 ± 0.34 ab | 67.0 ± 32.1 c |
| I | 9 | 893.5 ± 357.6 b | 1320.1 ± 146.8 b | 0.66 ± 0.21 b | 89.4 ± 36.2 b |
| Se | 9 | 1020.7 ± 446.5 a | 1391.0 ± 221.0 b | 0.71 ± 0.22 ab | 111.5 ± 47.5 a |
| Se + I | 9 | 1209.6 ± 589.0 a | 1532.3 ± 147.2 a | 0.77 ± 0.31 a | 133.7± 62.4 a |
| Treatment | Crop Surface | Dry Matter | Soluble Solids | Colour Parameters | ||
|---|---|---|---|---|---|---|
| (m2) | (%) | (°Brix) | L | a | b | |
| Crop cycle (means of 4 values: I, Se, Se + I, control) | ||||||
| Autumn | 12 | 10.2 ± 0.8 | 5.7 ± 0.5 | 38.5 ± 1.9 | −13.4 ± 1.1 | 20.0 ± 1.8 |
| Autumn–winter | 12 | 10.4 ± 1.1 | 5.7 ± 0.6 | 39.5 ± 1.0 | −12.2 ± 1.0 | 22.6 ± 1.3 |
| Winter | 12 | 10.2 ± 0.9 | 5.8 ± 0.6 | 40.7 ± 1.0 | −13.2 ± 1.0 | 21.8 ± 1.1 |
| n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Biofortification treatment (means of the 3 crop cycles) | ||||||
| Control | 9 | 8.97 ± 0.16 b | 4.90 ± 0.23 b | 37.9 ± 2.6 | −12.4 ± 1.0 | 23.0 ± 1.6 |
| I | 9 | 9.99 ± 0.29 ab | 5.95 ± 0.23 a | 40.4 ± 1.0 | −13.2 ± 1.4 | 20.9 ± 1.9 |
| Se | 9 | 10.97 ± 0.20 a | 6.00 ± 0.20 a | 40.1 ± 1.6 | −13.4 ± 0.9 | 21.3 ± 1.4 |
| I + Se | 9 | 11.14 ± 0.42 a | 6.08 ± 0.17 a | 39.8 ± 1.2 | −12.7 ± 0.9 | 20.7 ± 1.6 |
| n.s. | n.s. | n.s. | ||||
| Treatment | Crop Surface | Total Antioxidant Activity | Total Polyphenols | Vitamin C |
|---|---|---|---|---|
| (m2) | (mg-GAE g−1 d.w.) | (mg-GAE g−1 d.w.) | (g 100 g−1 f.w.) | |
| Crop cycle (means of 4 values: I, Se, Se + I, control) | ||||
| Autumn | 12 | 25.5 ± 1.9 b | 13.5 ± 1.7 b | 20.8 ± 2.2 a |
| Autumn-winter | 12 | 25.1 ± 2.2 b | 14.4 ± 1.3 ab | 21.1 ± 2.2 a |
| Winter | 12 | 27.2 ± 3.4 a | 15.3 ± 1.8 a | 21.4 ± 2.0 a |
| Biofortification treatment (means of the 3 crop cycles) | ||||
| Control | 9 | 22.9 ± 1.1 b | 12.9 ± 1.0 b | 18.5 ± 0.9 a |
| I | 9 | 25.0 ± 1.6 b | 13.5 ± 1.2 b | 20.3 ± 0.9 a |
| Se | 9 | 27.5 ± 1.9 a | 15.4 ± 1.3 a | 22.4 ± 1.0 a |
| Se + I | 9 | 28.4 ± 1.8 a | 15.8 ± 1.5 a | 23.2 ± 1.0 a |
| Treatment | Ca (g kg−1 d.w.) | Mg (g kg−1 d.w.) | NO3− (mg kg−1 f.w.) | P (g kg−1 d.w.) |
|---|---|---|---|---|
| Crop cycle (means of 4 values: I, Se, Se + I, control) | ||||
| Autumn | 27.4 ± 2.7 b | 3.45 ± 0.32 b | 5150 ± 408 a | 2.84 ± 0.40 b |
| Autumn-winter | 29.0 ± 3.7 ab | 3.59 ± 0.37 ab | 5079 ± 342 a | 2.95 ± 0.42 ab |
| Winter | 31.1 ± 4.2 a | 3.81 ± 0.48 a | 4206 ± 287 b | 3.11 ± 0.45 a |
| Biofortification treatment (means of the 3 crop cycles) | ||||
| Control | 25.7 ± 1.6 b | 3.24 ± 0.17 b | 5260 ± 586 a | 2.52 ± 0.16 b |
| I | 26.6 ± 1.8 b | 3.33 ± 0.12 b | 4763 ± 443 b | 2.65 ± 0.15 b |
| Se | 31.6 ± 2.6 a | 3.88 ± 0.26 a | 4625 ± 478 b | 3.29 ± 0.19 a |
| Se + I | 32.7 ± 1.8 a | 4.01 ± 0.31 a | 4597 ± 494 b | 3.39 ± 0.20 a |
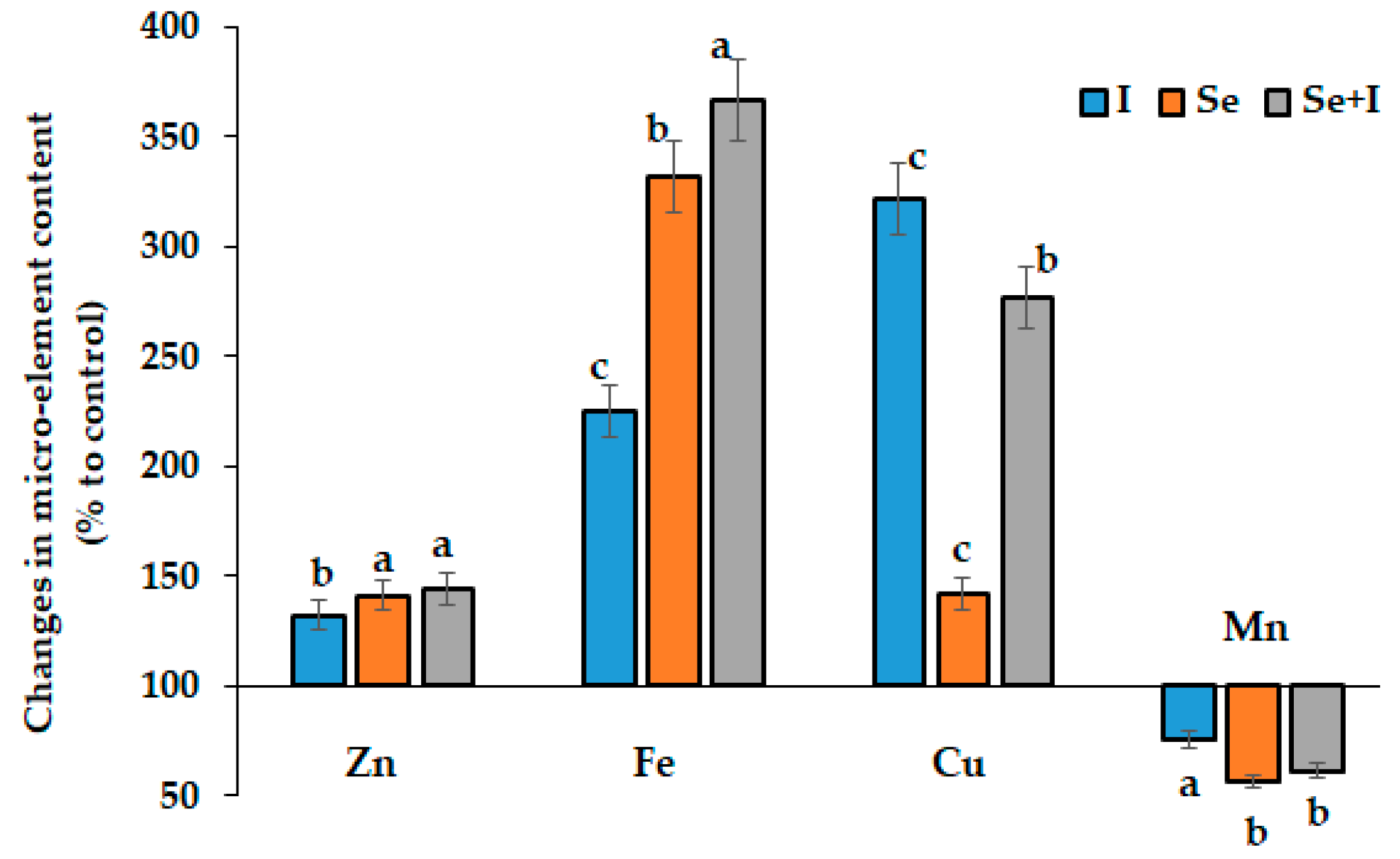
| Treatment | Zn (mg kg−1 d.w.) | Fe (mg kg−1 d.w.) | Cu (mg kg−1 d.w.) | Mn (mg kg−1 d.w.) |
|---|---|---|---|---|
| Crop cycle (means of 4 values: I, Se, Se + I, control) | ||||
| Autumn | 11.9 ± 1.8 a | 239.2 ± 132.7 a | 15.9 ± 7.5 a | 10.8 ± 4.5 b |
| Autumn-winter | 11.9 ± 1.9 a | 233.0 ± 107.6 a | 14.9 ± 7.9 a | 16.6 ± 2.6 a |
| Winter | 14.1 ± 2.4 a | 96.5 ± 21.4 b | 6.6 ± 1.8 b | 7.0 ± 1.9 c |
| Biofortification treatment (means of the 3 crop cycles) | ||||
| Control | 9.7 ± 0.8 b | 77.0 ± 20.8 d | 5.9 ± 1.3 c | 15.6 ± 4.7 a |
| I | 12.9 ± 1.3 a | 173.3 ± 65.9 c | 19.0 ± 7.8 b | 11.8 ± 4.9 b |
| Se | 13.7 ± 2.0 a | 225.5 ± 113.7 b | 8.4 ± 2.1 a | 8.8 ± 4.3 c |
| Se + I | 14.0 ± 1.5 a | 282.4 ± 129.9 a | 16.4 ± 6.7 c | 9.6 ± 4.1 c |
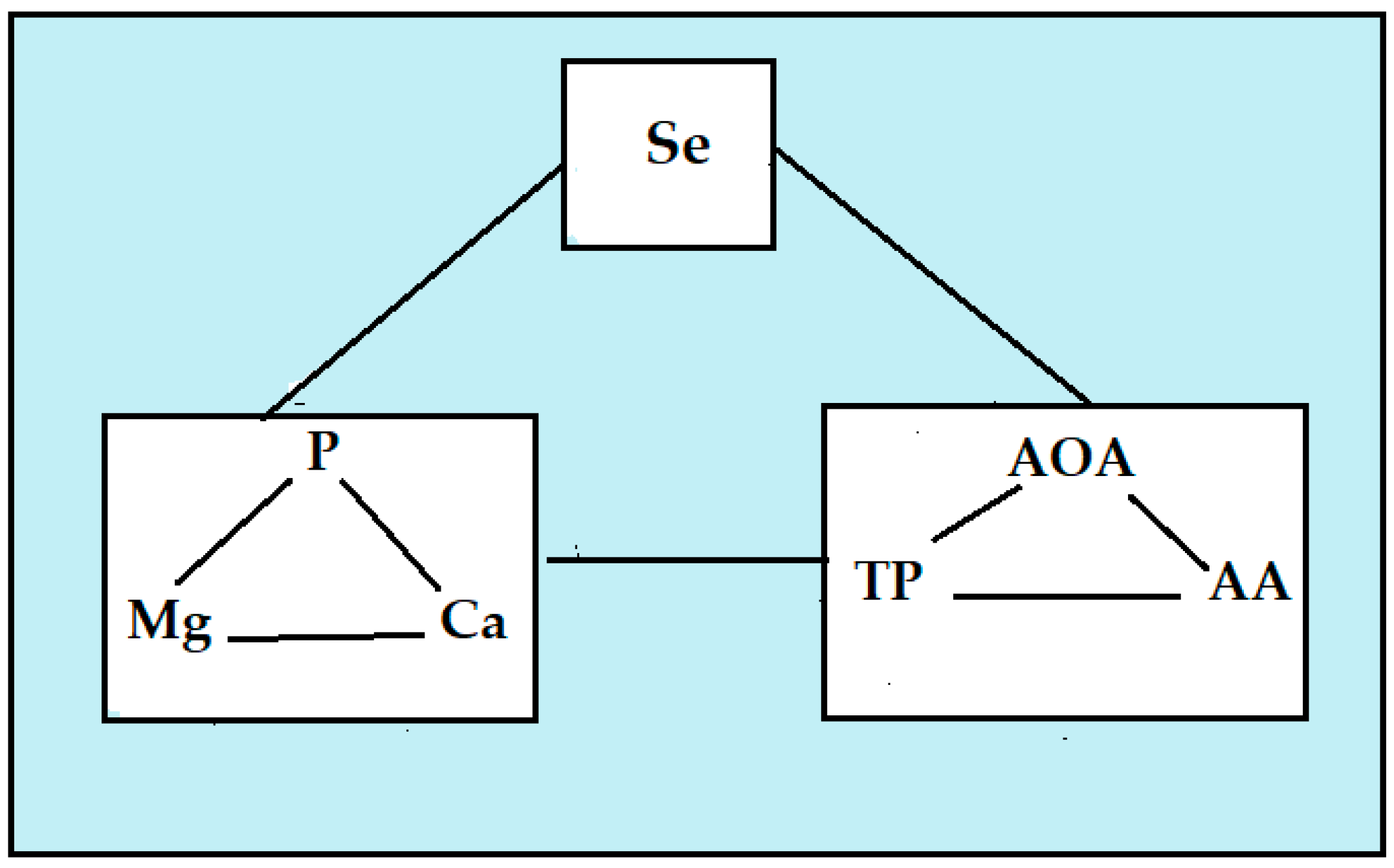
| Ca | Mg | K | NO3 | Se | I | Zn | Fe | Cu | Mn | DM | SS | Protein | AOA | TP | AA | Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | 0.893 a | 0.903 a | 0.367 | −0.553 f | 0.925 a | 0.232 | 0.703 c | 0.420 | −0.046 | −0.509 | 0.826 a | 0.727 c | 0.603 f | 0.797 a | 0.766 a | 0.827 a | 0.117 |
| Ca | 0.848 a | 0.161 | −0.582 f | 0.838 a | 0.273 | 0.751 b | 0.255 | −0.138 | −0.512 | 0.706 c | 0.626 e | 0.453 | 0.800 a | 0.762 a | 0.782 a | −0.041 | |
| Mg | 0.221 | −0.583 f | 0.842 a | 0.258 | 0.717 c | 0.250 | −0.124 | −0.492 | 0.739 b | 0.636 e | 0.491 | 0.797 a | 0.814 a | 0.747 b | −0.009 | ||
| K | 0.361 | 0.617 b | −0.053 | −0.006 | 0.762 a | 0.431 | 0.177 | 0.528 | 0.345 | 0.688 d | 0.182 | 0.140 | 0.385 | 0.617 e | |||
| NO3 | −0.446 | −0.391 | −0.733 b | 0.161 | 0.220 | 0.733 b | −0.442 | −0.510 | −0.175 | −0.600 f | −0.603 f | −0.500 | 0.267 | ||||
| Se | 0.168 | 0.656 e | 0.509 | 0.024 | −0.503 | 0.872 a | 0.686 d | 0.753 b | 0.815 a | 0.750 b | 0.842 a | 0.291 | |||||
| I | 0.515 | 0.159 | 0.443 | −0.300 | 0.324 | 0.515 | 0.270 | 0.415 | 0.247 | 0.345 | 0.08 | ||||||
| Zn | 0.178 | 0.035 | −0.734 b | 0.664 d | 0.747 b | 0.504 | 0.830 a | 0.710 c | 0.670 d | 0.005 | |||||||
| Fe | 0.615 f | −0.006 | 0.612 f | 0.501 | 0.720 c | 0.223 | 0.214 | 0.504 | 0.602 f | ||||||||
| Cu | 0.161 | 0.284 | 0.343 | 0.470 | −0.032 | −0.122 | 0.195 | 0.473 | |||||||||
| Mn | −0.431 | −0.506 | −0.462 | −0.622 f | −0.484 | −0.487 | −0.214 | ||||||||||
| DM | 0.837 a | 0.783 a | 0.760 a | 0.620 f | 0.836 a | 0.271 | |||||||||||
| SS | 0.721 c | 0.727 c | 0.581 f | 0.747 b | 0.284 | ||||||||||||
| Protein | 0.580 f | 0.402 | 0.705 c | 0.739 b | |||||||||||||
| AOA | 0.751 b | 0.788 a | 0.100 | ||||||||||||||
| TP | 0.619 f | −0.123 | |||||||||||||||
| AA | 0.244 |
| Element | RDA (mg) | Treatment | |||
|---|---|---|---|---|---|
| Control | Iodine | Selenium | Iodine + Selenium | ||
| Fe | 8 | 5.7 (3.9) * | 14.2 (6.3) | 22.3 (6.6) | 24.0 (7.3) |
| Cu | 2 | 1.8 (1.1) | 6.1 (2.2) | 2.7 (1.5) | 5.3 (1.9) |
| Zn | 10 | 0.5 (0.6) | 0.6 (0.7) | 0.7 (0.8) | 0.7 (0.8) |
| Mn | 2.3 | 3.9 (2.1) | 2.1 (1.7) | 1.6 (1.1) | 1.7 (1.3) |
| Se | 0.070 | 1.8 (2.1) | 7.9 (9.4) | 78.9 (93.4) | 88.8 (100) |
| I | 0.150 | 0 | 23.4 (37.7) | 0 | 25.9 (41.6) |
| Ca | 1000 | 14.4 (13.4) | 12.7 (14.1) | 14.7 (17.1) | 15.0 (17.7) |
| K | 3510 | 6.2 (3.3) | 6.7 (4.0) | 8.1 (5.1) | 9.0 (5.5) |
| Mg | 350 | 4.5 (4.7) | 4.6 (5.3) | 5.3 (5.9) | 5.4 (6.2) |
| P | 800 | 1.5 (1.6) | 1.6 (1.7) | 2.0 (2.2) | 2.0 (2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallarita, A.V.; Golubkina, N.; De Pascale, S.; Sękara, A.; Pokluda, R.; Murariu, O.C.; Cozzolino, E.; Cenvinzo, V.; Caruso, G. Effects of Selenium/Iodine Foliar Application and Seasonal Conditions on Yield and Quality of Perennial Wall Rocket. Horticulturae 2025, 11, 211. https://doi.org/10.3390/horticulturae11020211
Tallarita AV, Golubkina N, De Pascale S, Sękara A, Pokluda R, Murariu OC, Cozzolino E, Cenvinzo V, Caruso G. Effects of Selenium/Iodine Foliar Application and Seasonal Conditions on Yield and Quality of Perennial Wall Rocket. Horticulturae. 2025; 11(2):211. https://doi.org/10.3390/horticulturae11020211
Chicago/Turabian StyleTallarita, Alessio Vincenzo, Nadezhda Golubkina, Stefania De Pascale, Agnieszka Sękara, Robert Pokluda, Otilia Cristina Murariu, Eugenio Cozzolino, Vincenzo Cenvinzo, and Gianluca Caruso. 2025. "Effects of Selenium/Iodine Foliar Application and Seasonal Conditions on Yield and Quality of Perennial Wall Rocket" Horticulturae 11, no. 2: 211. https://doi.org/10.3390/horticulturae11020211
APA StyleTallarita, A. V., Golubkina, N., De Pascale, S., Sękara, A., Pokluda, R., Murariu, O. C., Cozzolino, E., Cenvinzo, V., & Caruso, G. (2025). Effects of Selenium/Iodine Foliar Application and Seasonal Conditions on Yield and Quality of Perennial Wall Rocket. Horticulturae, 11(2), 211. https://doi.org/10.3390/horticulturae11020211












