Abstract
It is imperative to investigate the impact of irrigation on the microorganisms inhabiting soil in greenhouses, as this understanding is crucial for the implementation of effective water conservation strategies and optimal soil health sustenance in greenhouse tomato production. To this end, a tomato-cultivating experiment was conducted in a greenhouse, with the control group receiving no planting or irrigation (W0), during the years 2021 and 2022 in the Taiyuan region of Shanxi Province, China. The experiment incorporated three irrigation levels: W1 (50–70% of the field capacity), W2 (60–80% of the field capacity), and W3 (70–90% of the field capacity). The objective of our study was to clarify the effects of different irrigation levels on soil bacterial and fungal community compositions and functions, as well as tomato yield and water use efficiencies, by analyzing the changes in community structural characteristics and potential functional composition of soil bacteria and fungi under different irrigation levels. Our results showed that in comparison with the control treatment, the W3 irrigation treatment exhibited the highest bacterial α-diversity, while its fungal diversity was the lowest. The r-strategy microbial community in tomato soil demonstrated increased abundance under the irrigation treatment. The relative abundance of common beneficial tomato bacteria increased by 0.32–1.70%, but that of beneficial soil fungi decreased by 0.09–3.75%. Among the different irrigation treatments, the relative abundances of Bacillus and Plectosphaerella changed the most. The functional structure of the bacteria in the irrigation treatment remained largely unchanged, while the saprotroph functional group of fungi was increased by 14.72–23.28%. With the increase in irrigation volume, the tomato yield of the W3 treatment increased, though the water use efficiency was not the greatest. The W2 treatment did not significantly reduce the yield, but it did increase the pathotroph functional groups of fungi, which may reduce the stress resistance of plants to soil-borne diseases. The findings of this study serve as a valuable reference point for the prediction of greenhouse soil health and the enhancement of tomato yields.
1. Introduction
Tomato (Solanum lycopersicum L.) is a common commercial crop cultivated in greenhouses across numerous countries, thereby ensuring a consistent supply of fresh produce [1,2]. China is the world’s largest producer of tomatoes, with a growing area of more than 1.1 million hectares [3]. However, greenhouse cultivation introduces novel challenges to tomato plant growth and soil health. These challenges are attributable to the conditions present in greenhouses, which often exhibit relatively high temperatures and humidity levels and deficient air circulation.
Irrigation is the primary source of water for greenhouse crops, and soil moisture conditions affect the soil structure and soil nutrient transfer and transportation, which in turn affect crop and microbial growth [4]. It is evident that the quantity of irrigation, in conjunction with the water quality, can exert a significant influence on soil moisture levels [5]. Concurrently, soil microorganisms can modify soil hydrological properties by actively secreting specific compounds, affecting nutrient and metabolite diffusion, and, in turn, indirectly influencing crop growth [6]. In arid and semi-arid regions of China, water scarcity is in direct conflict with the high demand for greenhouse crop’ irrigation. Implementing judicious irrigation strategies is imperative to enhance crop productivity and optimize water utilization [7]. Deficit irrigation has been demonstrated to be an effective method for optimizing irrigation water requirements. It has been shown that irrigation with moderate deficits can improve water use efficiency and minimize adverse effects on the yield [8,9]. However, it should be noted that deficit irrigation-induced water stress can affect soil microbial activity, which in turn may affect soil health and crop growth [10]. Mulched drip irrigation is a water-saving technology that has been shown to increase the temperature, save water, and increase the yield [11]. Various alterations in the soil structure and microbial activity may occur under diverse irrigation management conditions and varying years of practice of mulched drip irrigation. These alterations can result in increased or decreased crop yields [12]. Against that background, further exploration is necessary to investigate the impact of drip irrigation under greenhouse conditions on crop growth and soil microorganisms.
Microorganisms play a pivotal role in plant productivity and health. They are essential for critical functions such as stress tolerance and pathogen suppression [13]. Soil microorganisms affect crop production by directly or indirectly promoting the decomposition of organic matter and inhibiting the growth of pathogens. Soils with greater microbial biodiversity tend to possess higher nutrient potency and the capacity to promote plant growth [14,15]. The capacity of microorganisms to serve as reliable indicators of soil health is attributable to their environmental sensitivity and their rapid response to environmental changes [16]. The composition of microbial communities is sensitive to changes in soil moisture, and the compositions of soil fungal bacteria differ under different moisture conditions [17,18]. Fungi exhibit greater resistance to water stress when compared with bacteria. Drought has been observed to reduce the proportion of bacterial α-diversity and total biomass, while fungal diversity and biomass may exhibit a tendency to increase, decrease, or remain unaffected by drought or irrigation [19,20,21,22]. In one study, the abundance and diversity of both soil bacteria and fungi decrease with increasing drought [23].
The presence of beneficial microorganisms in soil has been shown to enhance disease resistance through various mechanisms, including competition for ecological niches and nutrients, production of antagonistic substances to induce plant systemic resistance, and interference with the pathogenic signals of pathogenic microorganisms. However, the impact of different irrigation conditions, crop species, and other factors impact soil microbial communities, including beneficial microorganisms, with impacts that are non-uniform [24,25,26,27].
Soil microbes serve as sources of rhizosphere microorganisms, which play a pivotal role in the control of soil-borne diseases [28]. Consequently, elucidating changes in the structure of the soil microbial community is imperative for maintaining soil health. Most extant studies have centered on alterations in plant rhizosphere microbial communities and the soil microbiome in response to diverse physicochemical properties and agricultural practices. As a critical step in realizing the potential of the microbiome in agricultural production, further exploration is necessary to investigate the effects of irrigation conditions on soil microbial composition and soil health. Consequently, the present study centered on the response of the tomato soil microbial community structure to varying irrigation levels, with the following primary research objectives: (1) to elucidate the effects of contrasting irrigation levels on the structure and function of tomato soil bacterial and fungal communities and soil health and (2) to investigate the tomato yield and water use efficiency and soil microbial effects on yield and water use efficiency under different irrigation levels.
2. Materials and Methods
2.1. Description of the Study Site
Greenhouse trials were conducted in the tomato industrial park situated in Liujiapu Village (112°24′~112°43′ E, 37°36′~37°49′ N), Xiaodian District, Taiyuan City, Shanxi Province, China. The experiment was designed with four distinct plots for each treatment. The greenhouse was 60 m long and 10 m wide. The test site featured a silty loam soil type. The experiment was conducted in a randomized complete block design with four treatments and three replications for each treatment. Every treatment plot area was 28.8 m2 (8 m × 3.6 m), and each plot comprised 3 ridges and 3 furrows (ridge length × width × height dimensions of 8 m × 0.8 m × 0.1 m, with a furrow width of 0.4 m). The irrigation system employed mulched drip irrigation. Each ridge was meticulously planted with 2 rows of tomato plants, and 2 drip irrigation tapes were laid between the plants. A layer of black mulch 1.5 m wide and 0.008 mm thick was applied to cover the surface of the plot fully. A permeable film was inserted between each sub-division to impede lateral water exchange. The local groundwater had been used for irrigation, and the water quality met the standards for agricultural irrigation.
2.2. Experimental Design
The experiment was meticulously designed with three irrigation levels, employing the upper and lower limits of the diverse soil field water-holding capacity (θf) as follows: low water (W1): 50~70% θf, medium water (W2): 60~80% θf, and high water (W3): 70~90% θf. To compare the effects of whether or not to irrigate on soil microbes, W0 was not irrigated and not planted as a control treatment with an open space of the same area as the irrigated treatment to maintain consistent tomato irrigation at the same level, when the mean value of soil moisture fell to the lower irrigation limit (±5%). The total irrigation volumes for W1, W2, and W3 treatments are 151.2, 226.8, and 340.2 mm and 151.2, 264.6, and 302.4 mm, respectively, in 2021 and 2022; the water volume of a single irrigation event was measured to be 37.8 mm in all cases. The temporal parameters and the volume of irrigation are delineated in Table S1.
The tomato seedlings of variety “Shouyan PT326” were planted on 17 May 2021 and 25 May 2022, and they were harvested on 22 September 2021 and 30 September 2022. The irrigation treatments were the same in 2021 and 2022. The microclimatic data and agrochemical soil analysis data for the 2021 and 2022 test periods are illustrated in Figure S1 and Table S2. Prior to the planting of the tomato, 20,000 kg ha−1 of cow manure was incorporated into a 0–30 cm layer of soil using a rotary tiller, and the equivalent amount of cow manure was applied every year. Other field managements were consistent across all experimental plots and years, including practices such as weeding and the application of pesticides.
2.3. Determine Soil Moisture Content
Prior to the initiation of the experiment, TRIME tubes were buried at a radial distance of 15 cm from the tomato plants. Soil moisture content was measured at approximately 7-day intervals using a time-domain reflectometer (TRIME-PICO-IPH, IMKO, Ettlingen, Germany), with additional measurements taken before and after irrigation. Measurements were taken at depths ranging from 0 to 60 cm, with intervals of 10 cm. To ensure the accuracy of the soil moisture measurements, the time-domain reflectometer was periodically calibrated during the experiment using the drying method.
2.4. High-Throughput Sequencing of Soil Microorganisms
2.4.1. Soil Sample Collection
After two full years, the microbial sampling of bulk soil occurred on 15 May 2023, with each treatment sampled using the 5-point sampling method on the ridges. For the 0–30 cm tillage soil, a diagonal method was employed for soil collection, followed by thorough mixing. Subsequently, 10 g of soil samples were obtained using the quadrat method and placed into sterilized self-sealing bags. These samples were then stored at −80 °C for subsequent molecular analysis.
2.4.2. DNA Extraction and PCR Amplification
Total microbial genomic DNA was extracted from soil samples using the E.Z.N.A.® soil DNA Kit (Omega Biotek, Norcross, GA, USA) according to the manufacturer’s instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA) and kept at −80 °C prior to further use.
Using the above-extracted DNA as a template, the bacterial 16S rRNA gene was amplified with primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by T100 Thermal Cycler PCR thermocycler (BIO-RAD, Hercules, CA, USA). The PCR reaction mixture included 4 μL of 5 × Fast Pfu buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of Fast Pfu polymerase, 10 ng of template DNA, and ddH2O to a final volume of 20 µL. The PCR amplification cycling conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 27 cycles of denaturing at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 45 s, and single extension at 72 °C for 10 min, and ending at 4 °C. The fungal ITS rRNA gene was amplified with primer pairs ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′) by T100 Thermal Cycler PCR thermocycler (BIO-RAD, USA). The PCR reaction mixture included 2 μL of 10 × buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.2 μL of rTaq polymerase, 10 ng of template DNA, and ddH2O to a final volume of 20 µL. The PCR amplification cycling conditions were as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturing at 95 °C for 45 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s, and single extension at 72 °C for 10 min, and ending at 4 °C.
The PCR product was extracted from 2% agarose gel and purified using the PCR Clean-Up Kit (YuHua, Shanghai, China) according to the manufacturer’s instructions and quantified using Qubit 4.0 (Thermo Fisher Scientific, Waltham, MA, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina PE300 platform (Illumina, San Diego, CA, USA) according to the standard protocols of Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw sequencing reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP555291).
2.4.3. Amplicon Sequence Processing and Analysis
After demultiplexing, the resulting sequences were quality-filtered with fastp (0.19.6) and merged with FLASH (v1.2.7). Then, the high-quality sequences were de-noised using the DADA2 plugin in the QIIME2 (version 2022.2) pipeline with the recommended parameters, obtaining a single-nucleotide resolution based on error profiles within samples. DADA2 de-noised sequences are usually called amplicon sequence variants (ASVs). To more accurately assess the microbial diversity and composition, sequence leveling was conducted for each sample, with bacteria leveled to 39,550 sequences per sample and fungi leveled to 73,167 sequences per sample. The ASVs were then analyzed for species taxonomy using the Naive Bayes classifier in QIIME2 software, with the Silva 138/16S Bacteria and Unite 8.0/ITS Fungi databases serving as reference sets.
2.5. Yield and Water Use Efficiency
Yield: Leaving 4 ears of fruit on each tomato plant, the core was picked, and the remaining ripe tomatoes were picked, every 7 days or so, over the entire test plot. They were measured with an accuracy of 0.05 kg according to our electronic balance (A16, Kaifeng, China) to determine the fresh weight of the fruit. After all the fruits were picked, we summed the total yield of tomatoes (t/hm2); WUE: the yield level water use efficiency (kg/m3) is the ratio of tomato yield to the total water consumption during the reproductive period.
2.6. Statistical Analysis
Microbial α-diversity index analysis was performed using mothur (version v.1.30.2); NMDS analysis and graphing were performed using the R language (version 3.3.1) vegan software package (version 2.4.3); one-way analyses of variance (ANOVA) with Duncan tests were performed to discriminate significant differences in α-diversity index, relative abundance of microbial communities, microbial community function, yield, and water use efficiency among treatments using SPSS 27.0; and the linear discriminant analysis (LDA) effect size (accessed on 22 June 2024, http://huttenhower.sph.harvard.edu/LEfSe) was determined to identify the significantly abundant taxa of bacterial and fungal microbial biomarkers among the different groups. Bacterial and fungal function prediction analyses were performed using PICRUSt2 (v2.2.0-b) and FUNGuild (v1.0) software. Graphs were generated using OriginPro 2021 software.
3. Results
3.1. Analysis of Soil Microbial Community Diversity in Different Treatments
The bacterial and fungal coverages were both greater than 0.99, and the soil samples were sequenced at a suitable depth to represent the true condition underground (Table 1). When compared with the W0 treatment, the bacterial Chao index and Shannon index increased while the fungal Chao index and Shannon index decreased in all irrigated treatments, with a significant difference observed between the bacterial Chao index, fungal Chao index, and Shannon index (p < 0.05). The findings suggest that tomato planting and the irrigation regime influenced the α-diversity of the soil microbial community, increasing the bacterial diversity and decreasing the fungal diversity. Furthermore, the fungal diversity exhibited a gradual decline with increasing irrigation levels.

Table 1.
Alpha-diversity index of soil microbial communities in different treatments.
Based on the Bray–Curtis distance, NMDS analysis was conducted to investigate the changes in the bacterial and fungal community structures of soil from tomato plants subject to different drip irrigation treatments under the membrane (Figure 1). The results of the NMDS analysis revealed that the community structure of the irrigation treatments differed significantly from that of the control treatment at the genus level. The investigation demonstrated that the cultivation of tomatoes with irrigation significantly altered the microbial community structure. On the one hand, a statistically significant difference was observed in the bacterial community structure between treatments (p = 0.017). However, the bacterial community structure exhibited greater similarity between irrigation treatments (Figure 1a). On the other hand, a highly significant difference was detected in the fungal community structure (p = 0.001), with this soil fungal community structure demonstrating greater similarity between the W1 and W2 treatments than the W3 treatment (Figure 1b).
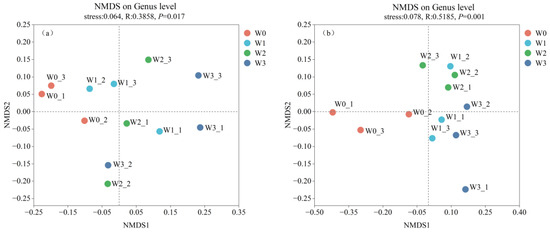
Figure 1.
Utilized a non-metric multidimensional scaling (NMDS) approach to examine the β-diversity of soil microorganisms in distinct treatments: NMDS analyses at the (a) bacterial and (b) fungal genus levels in different treatments.
3.2. Analysis of Soil Microbial Community Composition in Different Treatments
The soil bacterial community structure exhibited variation among treatments (Figure 2a,b). At the phylum level (Figure 2a), the species with a relative species abundance of soil bacteria greater than 1.0% were Proteobacteria (19.08–24.26%), Actinobacteriota (19.76–21.38%), Chloroflexi (12.40–18.47%), and Acidobacteriota (12.40–18.47%), Firmicutes (7.70–16.26%), Bacteroidota (3.01–5.82%), Gemmatimonadota (2.66–4.27%), Myxococcota (2.66–4.27%), unclassified_k_norank_d_Bacteria (1.63–2.40%), and Methylomirabilota (1.00–1.52%). In comparison with the W0 treatment, the relative abundance of Proteobacteria and Bacteroidota increased by 5.05–4.32% and 1.19–2.29%, respectively, in the W2 and W3 treatment; that of Actinobacteriota increased by 0. 86% in the W2 treatment; that of Firmicutes increased by 1.30–8.57% with different irrigation treatments; and those of Acidobacteriota and Myxococcota decreased by 4.19–10.79% and 0.23–0.70%, respectively, with increasing irrigation. Except for the Methylomirabilota, a statistically significant difference was identified between the control treatment and the irrigation treatments (Table S3).
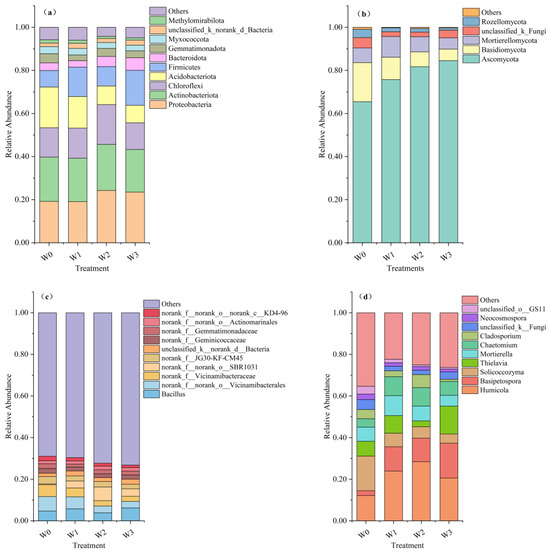
Figure 2.
Structures of soil microbial communities in different treatments: (a) bacterial and (b) fungal phyla and (c) bacterial and (d) fungal genera of tomato soils in different treatments.
The top 10 genera in terms of relative abundance of bacterial communities across treatments were Bacillus (3.87–6.18%), norank_f_norank_o_Vicinamibacterales (3.15–6.98%), norank_f_Vicinamibacteraceae (2.47–5.71%), norank_f_norank_o SBR1031 (0.36–3.39%), norank_f_JG30-KF-CM45 (2.21–3.53%), unclassified_k_norank_d_Bacteria (1.63–2.40%), norank_f_Geminicoccaceae (1.84–2.25%), norank_f_Gemmatimonadaceae (1.43–2.29%), norank_f_norank_o_Actinomarinales (1.47–1.74%), and norank_f_norank_o_norank_c_KD4-96 (1.19–2.15%). In comparison with the W0 treatment, the relative abundances of norank_f_norank_o_Vicinamibacterales, norank_f_Vicinamibacteraceae, and norank_f_norank_o_norank_c_KD4-96 gradually decreased with the increase in irrigation water, respectively, at 1.23–3.84%, 1.46–3.23%, and 0.60–0.95%, while the relative abundance of norank_f_JG30-KF-CM45 was reduced by 0.83–1.32% with irrigation treatment.
The structural composition of soil fungal species exhibited variation across different treatments (Figure 2c,d). The predominant phylum within the soil fungal communities was Ascomycota, accounting for 65.45% to 85.50%, and beyond that, the phyla with relative abundances greater than 1.0% were Basidiomycota (18.13–5.36%), Mortierellomycota (5.23–9.63%), unclassified_k_Fungi (2.20–4.79%), and Rozellomycota (1.01–3.82%). When compared with the W0 treatment, the relative abundance of Ascomycota gradually increased by 10.30–19.05%, and the relative abundance of Basidiomycota gradually decreased by 7.79–12.77% with an increase in irrigation water. A statistically significant difference was identified between the control treatment and the irrigation treatments; however, no statistically significant differences were observed among the latter (Tables S3 and S4).
The following genera were identified as the most prevalent in terms of relative abundance within the fungal communities across various treatments: Humicola (12.14–28.42%), Basipetospora (2.37–16.69%), Solicoccozyma (4.47–16.60%), Thielavia (2.27–13.28%), Mortierella (5.23–9.23%), Chactomium (3.96–9.10%), unclassified_k_Fungi (2.14–4.79%), Necosmospora (1.33–2.66%), unclassified_o_GS11 (0.78–3.70%), and Acremonium (0.53–3.13%). A comparison of the irrigation treatment with the W0 treatment revealed a significant decrease in the relative abundance of Neocosmospora and Acremonium, while the relative abundance of soil Humicola, Basipetospora, and Chactomium increased by 8.49–16.28%, 8.95–14.32%, and 2.72–5.14%, respectively. The relative abundance of Solicoccozyma gradually decreased by 10.10–12.13% with increasing irrigation water. Specifically, the relative abundance of Mortierella among the irrigation treatments exhibited a gradual decline, ranging from 2.48% to 4.39%, with increasing irrigation water.
3.3. Differential Microbial Analysis of Soil Microbial Communities in Different Treatments
Multi-group comparative analysis was employed to identify microbial biomarkers that exhibited significant disparities in soil bacterial and fungal communities across the various treatments (Tables S5 and S6). At the genus level, 52 significantly different genera of bacteria were identified from the various treatments, including 5 highly significantly different genera, and 19 significantly different genera of fungi, including 6 highly significantly different genera. LEfSe analysis of soil fungi and bacteria in the different treatments showed that there were 24 significantly different genera of soil bacteria and 25 significantly different genera of fungi in the four treatments (LDA value > 3.0, p < 0.05) (Tables S7 and S8). Multi-group comparative analysis and LEfSe analysis can be used to identify microbial biomarkers among different groups, and we employed those as our methods to find microbial species. In this study, based on the comparative results of the multi-group analysis, we further excluded the species whose signature microorganisms in the results of the LEfSe analysis did not differ significantly in the multi-group comparative analysis (p > 0.05), and we screened out the significantly enriched bacterial species in the different treatments. Eight bacterial and ten fungal species were identified (Table 2). The fungal biomarkers were exclusively classified as Ascomycota. Some bacterial biomarkers of the irrigation treatments were classified under the Actinobacteriota, and those markers increased. The W0 treatment resulted in a significant enrichment of bacteria norank_f_norank_o_norank_c_JG30-KF-CM66 and Pontibacter and fungi Striaticonidium, Neocosmospora, Cephaliophora, Pyrenochaetopsis, Emericellopsis, and Sarocladium; the W1 treatment resulted in a significant enrichment of bacteria Nonomuraea, Micromonospora, and Romboutsia and fungi Talaromyces; the W2 treatment exhibited a significant enrichment of bacteria Virgispirangium and Devosia and fungi Zygopleurage; the W3 treatment exhibited a significant enrichment of bacterium Thermobifida and fungi unclassified_f_Plectosphaerellaceae and Basipetospora.

Table 2.
Microbial biomarkers of soil bacterial and fungal communities in different treatments.
3.4. Analysis of Beneficial and Harmful Microorganisms in Soil with Different Treatments
Plants can recruit specific rhizosphere microorganisms; however, soil forms the primary source of their rhizosphere microbial communities. Moreover, most soil-borne diseases in plants are caused by pathogenic fungi. Consequently, we conducted a subsequent screening of common tomato soil beneficial bacteria and beneficial fungi and harmful fungi with a species community abundance of more than 0.1% for the different treatments. The relative abundance of cumulative beneficial bacterial communities increased by 0.32–1.70% in the irrigation treatments compared to the W0 treatment, with the largest increase observed in the W1 treatment and the smallest in the W2 treatment (Figure 3a). Bacillus, a prevalent soil biocontrol bacterium, is characterized by its remarkable resilience. Intriguingly, the relative abundance of the bacterial genus Bacillus exhibited increased by 1.11–1.15% in the W1 and W3 treatments, while it decreased by 0.81% in the W2 treatment. Notably, the W2 treatment exhibited the highest cumulative relative abundance of beneficial bacteria, excluding Bacillus.
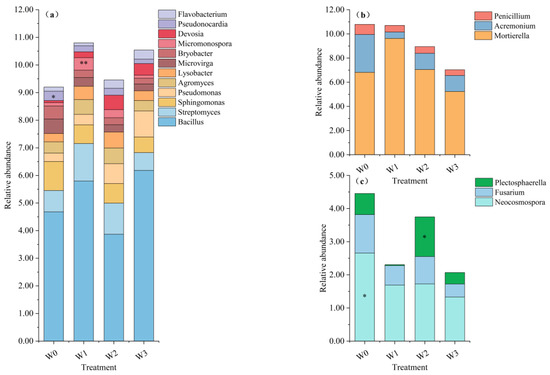
Figure 3.
Beneficial and harmful microbial communities in different treated soils: beneficial soil (a) bacteria and (b) fungi and (c) harmful soil fungi in different treatments; ** highly significant (p < 0.01) and * significant (p < 0.05) differences between groups of species.
The presence of beneficial fungi decreased by 0.09–3.75% in the irrigation treatment in comparison with the W0 treatment (Figure 3b). The greatest relative abundance was observed in Mortierella, within the range of 5.23–9.62%. Irrigation treatments may reduce the relative abundance of harmful fungal communities. Neocosmospora and Plectosphaerella were identified as the primary causal agents of stem and fruit rots. Irrigation treatments were also found to significantly reduce the relative abundance of Neocosmospora (Figure 3c). Furthermore, the relative abundance of harmful fungal communities was observed to be greater in W2 than in the other irrigation treatments, particularly that of Plectosphaerella. Fusarium has been identified as the primary causative agent of fusarium wilt in tomatoes. The present study found that the relative abundance of Fusarium was diminished by 0.33–0.77% in the irrigation treatments, with the W2 treatment exhibiting the least reduction. Collectively, the findings suggest that irrigation treatments curtail the relative abundance of the soil-borne disease causal agent community in tomatoes. However, they imply that the potential for resistance to soil-borne diseases in the W2 irrigation treatment might be diminished relative to the other treatments.
3.5. Predictive Analysis of Soil Microbial Community Function in Different Treatments
Predictive analysis of the COG function via PICRUSt2 revealed the predominant functions of soil bacteria in the various treatments, as follows: function unknown (17.27–17.82%), amino acid transport and metabolism (10.80–10.94%), translation, ribosomal structure and biogenesis (7.55–7.79%), energy production and conversion (7.26–7.42%), cell wall/membrane/envelope biogenesis (6.33–6.70%), and carbon monoxide (6.33–6.70%). Beyond those, carbohydrate transport and metabolism (5.70–5.85%), inorganic ion transport and metabolism (5.67–5.97%), transcription (5.62–6.05%), and replication, recombination, and repair (4.73–4.79%), coenzyme transport and metabolism (4.53–4.64%), and no significant differences were observed among the treatments for the remaining functions, except for a substantial difference between the control and irrigation treatments for coenzyme transport and metabolism (Table S5). The functions of the bacterial community demonstrated no significant alterations in response to alterations in irrigation (Figure 4a).
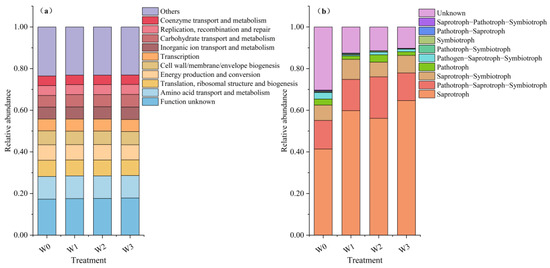
Figure 4.
Functions of soil microbial communities in different treatments: (a) the main function of soil bacteria and (b) the type of function of soil fungi.
FUNGuild functional prediction analysis revealed that the dominant trophic modes of soil fungi under different treatment membranes were saprotroph (64.66–37.06%), pathotroph–saprotroph–symbiotroph (10.79–19.92%), unknown (10.30–30.29%), saprotroph–symbiotroph (7.06–9.63%), pathotroph (1.66–3.54%), and pathogen–saprotroph–symbiotroph (0.53–3.13%). Among them, there were highly significant differences in saprotroph and unknown functions and significant differences in pathotroph–saprotroph–symbiotroph and pathotroph–saprotroph functions among different treatments (Table S9). The pathotroph–saprotroph–symbiotroph and pathotroph–saprotroph functions among the irrigation treatments were significantly different (Table S10). The functional composition of fungi in the irrigation treatment evolved toward a clearer nutrient-functional categorization when compared with the W0 treatment (Figure 4b). The proportion of the unknown functional group gradually decreased by 17.74–20.00% in conjunction with increasing irrigation. Concurrently, the saprotrophic functional group increased by 14.72–23.28% when under irrigation treatment. Consequently, we surmised that saprotrophs were the dominant fungi functioning in the soil of tomato plants under mulched drip irrigation.
3.6. Yield, Water Use Efficiency, and Correlation Analysis of Tomatoes in Facilities with Different Irrigation Treatments
The tomato yields of the different mulched drip irrigation treatments gradually decreased in conjunction with the reduction in irrigation volume, while the water use efficiency increased (except for the W2 treatment in 2022) (Figure 5a,b). In 2021, when compared with the W3 treatment, the yields of the W2 and W1 treatments were 4.51% and 33.01% lower, respectively; the difference in water use efficiencies between the W1 and W2 treatments was not significant, while their efficiencies were 21.56% and 19.03% greater than those of the W3 treatment. In 2022, the yields of the W2 and W1 treatments were 9.08% and 31.28% lower, respectively, compared to the W3 treatment. The difference in water efficiencies between the W2 and W3 treatments was not significant, while the water use efficiency of the W1 treatment was 10.95% and 7.54% greater than those of the W2 and W3 treatments, respectively. Notable findings were the declines in the yield and water use efficiency of the W2 treatment, with 6.40% and 14.75% decreases, respectively, compared to 2021. In contrast, the other irrigation treatments maintained their levels.
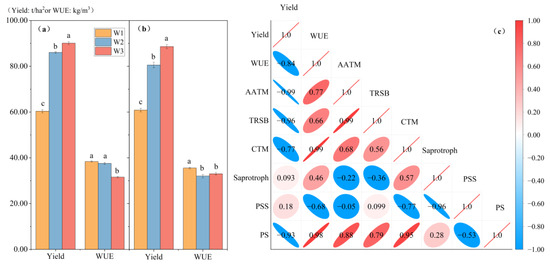
Figure 5.
Correlation analysis was conducted to examine the relationship between yield, water use efficiency, and the microbial function of tomatoes in facilities with different irrigation treatments. The analysis included the following aspects: yields and water use efficiencies in (a) 2021 and (b) 2022; (c) correlation analysis of yield and water use efficiency with microbial function in 2022. AATM: amino acid transport and metabolism; TRSB: translation, ribosomal structure, and biogenesis; CTM: coenzyme transport and metabolism; PSS: pathogen–saprotroph–symbiotroph; PS: pathotroph–saprotroph.
Pearson correlation analysis revealed negative correlations between the yield and the following: water use efficiency; amino acid transport and metabolism; translation, ribosomal structure, and biogenesis; and pathotrophs–saprotrophs (Figure 5c). Conversely, water use efficiency exhibited positive correlations with the following: amino acid transport and metabolism; translation, ribosomal structure, and biogenesis; and pathotrophs–saprotrophs. Nevertheless, a lack of statistical significance was observed between the variables (p > 0.05).
4. Discussion
The findings of our study demonstrate that bacterial α-diversity increased while fungal α-diversity decreased under irrigation treatments in comparison with the control treatment (Table 1). This outcome is generally consistent with the results of previous studies [15,20]. The bacterial community structure is more sensitive to moisture than that of fungal communities. As the water content decreases, the soil environment becomes more favorable for fungal communities than those of bacteria [29]. Under drought conditions, fungi with greater resistance to water limitation outcompete bacteria for carbon and energy. Furthermore, bacteria exhibit superiority over fungi in their capacity to utilize hydrophilic compounds, while fungi demonstrate a greater aptitude for utilizing hydrophobic compounds [1]. Consequently, the high-water W3 treatment resulted in the most pronounced bacterial α-diversity and the least fungal α-diversity.
Irrigation treatments altered soil bacterial and fungal phylum-level and genus-level community abundance. The predominant bacterial phyla identified were Proteobacteria, Actinobacteriota, Chloroflexi, Acidobacteriota, and Firmicutes, while the dominant fungal phyla were Ascomycota and Basidiomycota. Proteobacteria, Firmicutes, and Ascomycota are classified as eutrophic microorganisms (r-strategy). In contrast, Acidobacteriota and Basidiomycota were identified as oligotrophic microorganisms (K-strategy) [30]. Soil nutrient resources were richer in the irrigation treatments due to the presence of root secretions and plant litter. Consequently, the relative abundance of soil r-strategy microbial communities exhibited an upward trend, while the relative abundance of communities utilized by K-strategy microbial communities exhibited a downward trend. This suggests that the r-strategy microbes gradually dominated with increasing irrigation and that their presence contributes to population rebuilding following water disturbances [31].
Our study revealed that microorganisms with distinct signatures were present in the various irrigation treatments and that the number of genera under the Actinobacteriota phylum classification increased. It has been demonstrated that Devosia is associated with the group of beneficial soil bacteria, which have amino acid transport and metabolism functions that promote plant growth and inhibit diseases [32]. Microorganisms of genera under the Actinobacteria classification (Micromonospora, Nonomuraea, Virgisporangium, Thermobifida) are believed to promote nutrient uptake and antimicrobial effects in plants [33,34,35]. Control treatments were significantly enriched with Pyrenochaetopsis, which secretes peptidases that can infect plant cells and lead to plant pathogenicity [36]. Talaromyces secretes secondary metabolites that can inhibit pathological changes in crops and promote tomato growth [37].
Soil microorganisms play a pivotal role in the defense against biotic stresses. Beneficial soil microorganisms can maintain soil health through competition for ecological niches and nutrients, production of antagonistic substances to induce plant systemic resistance, and interference with pathogenic microorganisms’ disease-causing signals [38]. Tomato crops in facilities frequently suffer from several major diseases, including fusarium wilt, stem rot, and root rot. In our study, the causal agents of these diseases, namely, Neocosmospora, Plectosphaerella, and Fusarium, were identified in various treatments (Figure 3c). Beneficial microorganisms, such as Streptomyces and Sphingomonas, can inhibit the growth of Fusarium and promote plant growth [18]. Moreover, bacteria, such as Bacillus and Streptomyces, and fungi, including Mortierella, produce or release various compounds that can activate plant immunity against pathogen infection. These organisms are classified as plant growth-promoting bacteria or fungi [39,40,41]. Humicola, a saprophytic bacterium, was found in soil and has the greatest relative abundance in the different irrigation treatments (Figure 2d). It has been observed to produce heat-stable cellulases, among other enzymes, and recent studies have demonstrated that Humicola can effectively suppress Fusarium wilt when utilized in conjunction with microbial agents [42].
The bacterial and fungal functional types exhibited no substantial alterations in response to increasing irrigation volumes. This finding aligns with the conclusions of Wang et al. (2024), who determined that soil microbial communities retained relatively stable metabolic functions across drip irrigation treatments [43]. However, the functional structural composition of fungi underwent a significant change, while that of bacteria remained constant (Figure 4a,b). Bacteria exhibit greater diversity, microbiome complexity, and interspecies associations, as well as overlapping ecological niches and a potentially high degree of functional redundancy between communities [44]. This suggests that the soil bacterial function remained relatively stable under varying irrigation volumes. In contrast, the increase in soil microbiome abundance of Ascomycota in the irrigation treatment resulted in a greater proportion of saprotroph in the fungi functions. Concurrently, we observed an increase in pathotroph–saprotroph–symbiotroph, pathotroph–saprotroph, and pathotroph fungi functional groups in the W2 treatment in comparison to the other irrigation treatments. The type of pathogenic fungi can obtain nutrients from the host plant and cause plant disease [45]. This observation supports the hypothesis that the W2 treatment soils have diminished the capacity to resist soil-borne diseases.
The findings of the present study demonstrated that a reduction in irrigation significantly diminished the tomato yield under drip irrigation beneath a film, except for the W2 treatment in 2022. In that case, the water use efficiency (WUE) was diminished, whereas the remainder of the treatments exhibited enhanced WUEs (Figure 5a,b). Our results are generally in line with the results of a previous study, which found that increased irrigation favored the yield rather than the water use efficiency [46], but it was also found that a severe water deficit decreased the WUE, whereas slight water stress promoted the WUE [47]. In the present study, our anomalous finding may have arisen because the different tomato planting seasons resulted in different water consumption levels of tomatoes during the growing season. It has been demonstrated that mild water stress reduces the yield but improves the fruit quality and WUE to a certain extent, representing the optimal balance of the tomato yield, fruit quality, and WUE [48]. Notably, our findings revealed that, while the W2 treatment did not substantially reduce the yield compared to the W3 treatment, there was a decline in the tomato yield and WUE with continuing planting years. Furthermore, the beneficial soil microorganism Bacillus decreased while the pathomycete Plectosphaerella significantly increased under the W2 treatment. This phenomenon may be attributed to the enhanced soil water, air, and heat conditions under mulched drip irrigation, which have been shown to stimulate soil microbial activity [12]. Additionally, the improvement in the soil structure and aeration under optimal water stress has been demonstrated to be conducive to the survival of aerobic bacteria and fungi [49]. In our study, however, the W2 treatment promoted the growth of some harmful fungi. Under stress conditions such as pathogen invasion, plants have “cry for help” strategies, i.e., plants can inhibit soil-borne pathogens by actively releasing non-volatile root secretions (e.g., amino acids, nucleotides, and long-chain organic acids), and these beneficial soil microorganisms’ transfer to the rhizosphere, rhizome, or aerial portions of the plant to inhibit soil-borne pathogens [33]. Furthermore, the transfer of beneficial soil microorganisms into the rhizosphere and rhizome zone of the plant is necessary to maintain normal plant growth, which may decrease the beneficial bacteria in non-rhizosphere soils. Concurrently, monocropping over an extended period has been observed to diminish the abundance of certain beneficial bacteria and augment the relative abundance of potentially harmful fungi [50], thereby influencing crop growth. The experimental findings demonstrated that under greenhouse mulched drip irrigation conditions, moderate irrigation maintained crop yield and water use efficiency. However, maintaining an adequate water supply throughout the life cycle of the tomatoes under continuous cropping conditions increased the growth of soil pathogenic fungi, which was detrimental to long-term crop growth and soil health. Therefore, water deficit regulation during different growth periods should be considered to further optimize the soil microbial composition and function under mulched drip irrigation. It has been demonstrated in prior research that the composition of soil microbes and their function are influenced by varying irrigation conditions during distinct phases of crop growth [43,51].
Soil microbial functions are closely related to microbial cell growth and nutrient utilization. The expression of genes associated with amino acid transport and metabolism functions facilitates the absorption of inorganic nitrogen from the environment, thereby enabling the synthesis of proteins and nitrogenous substances, which is crucial for maintaining normal microbial activities [52]. Furthermore, the expression levels of proteins involved in translation, ribosomal structure, and biogenesis functions are positively correlated with microbial cell growth in soil [53]. Microbial anabolism and catabolism contribute significantly to stable organic carbon pools’ formation in soil [54]. The crop yield increases with the increase in soil SOC [55], and active microbial growth accelerates microbial catabolism and SOC utilization, which leads to a loss of soil SOC [56]. This, in turn, may affect the supply of nutrients for crop growth. The occurrence of plant diseases as a result of pathogenic nutritive fungal groups can lead to a reduction in crop yields. This phenomenon may explain the observed negative correlation between certain soil microbial functions and crop yields (Figure 5b).
5. Conclusions
In the present study, we elucidated the effects of various irrigation treatments on soil health and tomato yield from the perspective of soil microorganisms. Our findings revealed that the diversity of bacterial communities increased, while that of fungal communities decreased, under irrigation treatments as compared to control treatments. The abundance of eutrophic microbial communities in the soils of tomato plants that received mulched drip irrigation exhibited a relative increase, while that of oligotrophic microbial communities exhibited a relative decrease, with an increase in irrigation water volume. Under different irrigation levels, the function of bacterial communities remained unaltered, but the structural composition of fungal communities exhibited distinct variations, with a significant augmentation in the proportion of the saprotroph functional group observed. The relative abundance of common tomato soil beneficial bacterial communities was increased by drip irrigation treatments under the membrane, and the abundance of soil pathogenic bacteria was reduced. The W2 irrigation treatment, meanwhile, demonstrated a slight decline in yield, and its yield and water use efficiency tended to decrease as the planting years continued, but its pathogenic nutrient fungi function types significantly increased relative to those under other irrigation treatments, suggesting a potential reduction in its resistance to soil-borne diseases. Under continuous cropping conditions, water deficit regulation during different growth periods should be considered to further optimize the soil microbial composition and function under mulched drip irrigation. The impact of rhizosphere microorganisms and plant endophytes on plant growth and soil health has been demonstrated; however, these factors were not the focus of this study and may be explored in future research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11020204/s1, Figure S1: The daily meteorological data during the experiment period; Table S1: Factors and levels of experiment; Table S2: Agrochemical soil analysis data in different treatments during the experiment period; Table S3: Multi-group analysis of differential soil bacteria and fungi in different treatments at the phylum level; Table S4: Multi-group analysis of differential soil bacteria and fungi among different irrigation treatments at the phylum level; Table S5: Multi-group analysis of differential species of soil bacteria in different treatments; Table S6: Multi-group analysis of differential species of soil fungi in different treatments; Table S7: Soil bacterial microbial biomarkers in different treatments; Table S8: Soil fungal microbial biomarkers in different treatments; Table S9: ANOVA analysis of soil microbial functions in different treatments; Table S10: ANOVA analysis of soil fungal functions in different irrigation treatments.
Author Contributions
Edited the original draft, J.A.; reviewed and edited the draft, J.M., L.M. and L.Z.; collected and analyzed the original data, X.M.; funding acquisition, J.M. and L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (52079085, 52109061) and the Natural Science Research Project of Shanxi Province (202403021211047).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Wang, C.; Kuzyakov, Y. Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Corti, C.; Facundo, R.; Arcerito, M.; Landa, G.F.D.; Mazzei, M.P.; Landa, M.F.D.; Maggi, M.; Galetto, L. Tomato production under greenhouse conditions: Bumblebees or hormones? Sci. Hortic. 2024, 326, 112747. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Ma, J. Biochar Improves Greenhouse Tomato Yield: Source-Sink Relations under Deficit Irrigation. Agronomy 2023, 13, 2336. [Google Scholar] [CrossRef]
- Xue, R.; Shen, Y.; Marschner, P. Soil water content during and after plant growth influence nutrient availability and microbial biomass. J. Soil Sci. Plant Nutr. 2017, 17, 702–715. [Google Scholar] [CrossRef]
- Guo, T.; Huang, X.; Feng, K.; Mao, X. Impact of Deficit Drip Irrigation with Brackish Water on Soil Water–Salt Dynamics and Maize Yield in Film-Mulched Fields. Agronomy 2025, 15, 379. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Matthias, C.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Somefun, O.T.; Masasi, B.; Adelabu, A.O. Irrigation and Water Management of Tomatoes-A Review. J. Sustain. Agric. Environ. 2024, 3, e70020. [Google Scholar] [CrossRef]
- Burato, A.; Fusco, G.M.; Pentangelo, A.; Nicastro, R.; Modugno, A.F.; Scotto di Covella, F.; Ronga, D.; Carillo, P.; Campi, P.; Parisi, M. Regulated Deficit Irrigation to Boost Processing Tomato Sustainability and Fruit Quality. Sustainability 2024, 16, 3798. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Huang, G.; Huang, G.; Xu, X.; Huang, Q. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the Hetao Irrigation District. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Han, Z.; Yang, Q.; Jiang, Z. Effects of deficit irrigation on soil microorganisms and growth of Arabica coffee (Coffea arabica L.) under different shading cultivation modes. Int. J. Agric. Biol. Eng. 2021, 14, 99–108. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Wen, Y.; Li, W.; Zhu, Y.; Song, L.; Li, Y.; Liang, Y.; Wang, Z. Effects of Different Film Types on Cotton Growth and Yield under Drip Irrigation. Sustainability 2024, 16, 4173. [Google Scholar] [CrossRef]
- Zong, R.; Wang, Z.; Li, W.; Li, H.; Ayantobo, O.O. Effects of practicing long-term mulched drip irrigation on soil quality in Northwest China. Sci. Total Environ. 2023, 878, 163247. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Author Correction: Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, T.; Friman, V.P.; Xu, Y.; Shen, Q.; Jousset, A. Trophic network architecture of root-associated bacterial communities determines pathogen invasion and plant health. Nat. Commun. 2015, 6, 8413. [Google Scholar] [CrossRef]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.; Chu, H. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Ritz, K.; Black, H.I.J.; Campbell, C.D.; Harris, J.A.; Wood, C. Selecting biological indicators for monitoring soils: A framework for balancing scientific and technical opinion to assist policy development. Ecol. Indic. 2009, 9, 1212–1221. [Google Scholar] [CrossRef]
- Evans, S.E.; Wallenstein, M.D. Climate change alters ecological strategies of soil bacteria. Ecol. Lett. 2014, 17, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Annelein, M.; Samuel, J.; Snoek, B.L.; Ten Hooven, F.C.; Van der Putten, W.H. Drought legacy effects on the composition of soil fungal and prokaryote communities. Front. Microbiol. 2018, 9, 294. [Google Scholar] [CrossRef]
- Haugwitz, M.S.; Bergmark, L.; Priemé, A.; Christensen, S.; Beier, C.; Michelsen, A. Soil microorganisms respond to five years of climate change manipulations and elevated atmospheric CO2 in a temperate heath ecosystem. Plant Soil 2014, 374, 211–222. [Google Scholar] [CrossRef]
- Hartmann, M.; Brunner, I.; Hagedorn, F.; Bardgett, R.D.; Stierli, B.; Herzog, C.; Chen, X.; Zingg, A.; Graf-Pannatier, E.; Rigling, A.; et al. A decade of irrigation transforms the soil microbiome of a semi-arid pine forest. Mol. Ecol. 2017, 26, 1190–1206. [Google Scholar] [CrossRef] [PubMed]
- Gordon, H.; Haygarth, P.M.; Richard, D.B. Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem. 2008, 40, 302–311. [Google Scholar] [CrossRef]
- Jiao, S.; Chu, H.; Zhang, B.; Wei, X.; Chen, W.; Wei, G. Linking Soil Fungi to Bacterial Community Assembly in Arid Ecosystems. iMeta 2022, 1, e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lei, S.; Wu, H.; Liao, L.; Wang, X.; Zhang, L.; Liu, G.; Wang, G.; Fang, L.; Song, Z. Simplified microbial network reduced microbial structure stability and soil functionality in alpine grassland along a natural aridity gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Sarula; Yang, H.; Zhang, R.; Li, Y. Shallow-buried drip irrigation promoted the enrichment of beneficial microorganisms in surface soil. Rhizosphere 2023, 28, 100776. [Google Scholar] [CrossRef]
- Jin, X.; Jia, H.; Ran, L.; Wu, F.; Liu, J.; Schlaeppi, K.; Dini-Andreote, F.; Wei, Z.; Zhou, X. Fusaric acid mediates the assembly of disease-suppressive rhizosphere microbiota via induced shifts in plant root exudates. Nat. Commun. 2024, 15, 5125. [Google Scholar] [CrossRef]
- Ma, H.; Bai, G.; Sun, Y.; Kostenko, O.; Zhu, X.; Lin, S.; Ruan, W.; Zhao, N.; Martijn Bezemer, T. Opposing effects of nitrogen and water addition on soil bacterial and fungal communities in the Inner Mongolia steppe: A field experiment. Appl. Soil Ecol. 2016, 108, 128–135. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Wei, Y.; Yang, S. Different rhizosphere soil microbes are recruited by tomatoes with different fruit color phenotypes. BMC Microbiol. 2022, 22, 210. [Google Scholar] [CrossRef]
- Zhang, R.; Vivanco, J.M.; Shen, Q. The unseen rhizosphere root–soil–microbe interactions for crop production. Curr. Opin. Microbiol. 2017, 37, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, S.; Semenov, M.V.; Yao, F.; Ye, J.; Bu, R.; Ma, R.; Lin, J.; Kurganova, I.; Wang, X.; et al. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Change Biol. 2021, 27, 2763–2779. [Google Scholar] [CrossRef]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S.X. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2023, 2, e66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, H.; Wang, J.; Gao, W.; Sun, X.; Xiong, Q.; Shu, X.; Miao, Y.; Shen, S.; Xun, W.; et al. Nonpathogenic Pseudomonas syringae derivatives and its metabolites trigger the plant “cry for help” response to assemble disease suppressing and growth promoting rhizomicrobiome. Nonpathogenic Pseudomonas syringae derivatives and its metabolites trigger the plant “cry for help” response to assemble disease suppressing and growth promoting rhizomicrobiome. Nat. Commun. 2024, 15, 1907. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Valdés, M. Micromonospora: An important microbe for biomedicine and potentially for biocontrol and biofuels. Soil Biol. Biochem. 2010, 42, 536–542. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, H.S.; Sang, M.K.; Song, J.; Weon, H.Y. Effect of Bacillus mesonae H20-5 Treatment on Rhizospheric Bacterial Community of Tomato Plants under Salinity Stress. Plant Pathol. J. 2021, 37, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Sungthong, R.; Nakaew, N. The genus Nonomuraea: A review of a rare actinomycete taxon for novel metabolites. J. Basic Microbiol. 2015, 55, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef] [PubMed]
- Halo, B.A.; Al-Yahyai, R.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M. Talaromyces variabilis interferes with Pythium aphanidermatum growth and suppresses Pythium-induced damping-off of cucumbers and tomatoes. Sci. Rep. 2019, 9, 11255. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, R.; Wang, D.; Qiu, M.; Feng, H.; Zhang, N.; Shen, Q. Enhanced Control of Cucumber Wilt Disease by Bacillus amyloliquefaciens SQR9 by Altering the Regulation of Its DegU Phosphorylation. Appl. Environ. Microbiol. 2014, 80, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Karačić, V.; Miljaković, D.; Marinković, J.; Ignjatov, M.; Milošević, D.; Tamindžić, G.; Ivanović, M. Bacillus Species: Excellent Biocontrol Agents against Tomato Diseases. Microorganisms 2024, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.P.; Bastos, M.S.; Xavier, V.B.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Plant growth and resistance promoted by Streptomyces spp. in tomato. Plant Physiol. Biochem. 2017, 118, 479–493. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Tao, C.; Wang, Z.; Liu, S.; Lv, N.; Deng, X.; Xiong, W.; Shen, Z.; Zhang, N.; Geisen, S.; Li, R.; et al. Additive fungal interactions drive biocontrol of Fusarium wilt disease. New Phytol. 2023, 238, 1198–1214. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wang, T. Effects of increasing drip irrigation at different maize growth stages on soil microorganisms. Front. Microbiol. 2024, 15, 1343302. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- John, E.; Jacques, S.; Phan, H.T.T.; Liu, L.; Pereira, D.; Croll, D.; Singh, K.B.; Oliver, R.P.; Tan, K.C. Variability in an effector gene promoter of a necrotrophic fungal pathogen dictates epistasis and effector-triggered susceptibility in wheat. PLoS Pathog. 2022, 18, e1010149. [Google Scholar] [CrossRef]
- Du, Y.; Cao, H.; Liu, S.; Gu, X.; Cao, Y. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in Northwestern China. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Ning, H.; Zhang, X.; Li, S.; Pang, J.; Wang, G.; Sun, J. Optimizing irrigation frequency and amount to balance yield, fruit quality and water use efficiency of greenhouse tomato. Agric. Water Manag. 2019, 226, 105787. [Google Scholar] [CrossRef]
- Cantore, V.; Lechkar, O.; Karabulut, E.; Sellami, M.H.; Albrizio, R.; Boari, F.; Stellacci, A.M.; Todorovic, M. Combined effect of deficit irrigation and strobilurin application on yield, fruit quality and water use efficiency of “cherry” tomato (Solanum lycopersicum L.). Agric. Water Manage. 2016, 167, 53–61. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar] [CrossRef]
- Dangi, S.R.; Zhang, H.; Wang, D.; Gerik, J.; Hanson, B.D. Soil Microbial Community Composition in a Peach Orchard Under Different Irrigation Methods and Postharvest Deficit Irrigation. Soil Sci. 2016, 181, 208–215. [Google Scholar] [CrossRef]
- Huang, D.; Xu, R.; Sun, X.; Li, Y.; Xiao, E.; Xu, Z.; Wang, Q.; Gao, P.; Yang, Z.; Lin, H.; et al. Effects of perfluorooctanoic acid (PFOA) on activated sludge microbial community under aerobic and anaerobic conditions. Environ. Sci. Pollut. Res. 2022, 29, 63379–63392. [Google Scholar] [CrossRef]
- Liu, D.; Keiblinger, K.M.; Leitner, S.; Wegner, U.; Zimmermann, M.; Fuchs, S.; Lassek, C.; Riedel, K.; Zechmeister-Boltenstern, S. Response of Microbial Communities and Their Metabolic Functions to Drying–Rewetting Stress in a Temperate Forest Soil. Microorganisms 2019, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Woolf, D.; Fan, M.; Qiao, L.; Li, R.; Lehmann, J. Global crop production increase by soil organic carbon. Nat. Geosci. 2023, 16, 1159–1165. [Google Scholar] [CrossRef]
- Jin, J.; Wood, J.; Franks, A.; Armstrong, R.; Tang, C. Long-term CO2 enrichment alters the diversity and function of the microbial community in soils with high organic carbon. Soil Biol. Biochem. 2020, 144, 107780. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).