Abstract
The imbalance in soil microcosm systems caused by the long-term monoculture of ginseng is the main cause of continuous cropping disorder in ginseng, an important factor limiting the development of the ginseng industry. The ecological intercropping pattern of medicinal plants is a planting technology that achieves efficient, high-quality and sustainable production of Chinese medicinal materials by increasing the diversity of farmland ecosystems and improving the stability of soil micro-ecosystems, thereby alleviating the continuous cropping disorder of medicinal plants. However, there remains a lack of research on the ecological intercropping cultivation of ginseng. We constructed a Panax ginseng/Arisaema amurense intercropping model to explore the changes in soil nutrients, enzyme activities, soil microbial communities and ginseng quality. The findings of this study demonstrated that intercropping could decelerate the acidification process of soils and effectively increased 37.02% of soil organic matter, 32.39% of total nitrogen, 5.18% of total potassium and 9.03% of available phosphorus contents in ginseng inter-root soil compared with monocropping. The results revealed that intercropping increased the soil urease and soil acid phosphatase activities while reducing the soil sucrase activity in the inter-root soil. Additionally, intercropping elevated the α-diversity of the inter-root soil bacterial community and diminished the composition and abundance of the fungal community. The intercropping exhibited a pronounced inhibitory impact on two common genera of pathogenic fungi, Fusarium and Cylindrocarpon Furthermore, the total ginsenosides and diverse monomer ginsenosides present in the roots of intercropped ginseng exhibited varying degrees of enhancement. The results of the analyses indicated that the observed increase in ginsenoside content under intercropping was attributable to interactions between soil microorganisms, including the Prevotella_7, Penicillium, Humicola and Deconica, and soil factors such as SOM, NH4+–N, AP and S-UE. Thus, implementing P. ginseng/A. amurense ecological intercropping can effectively mitigate soil acidification, enhance soil nutrient effectiveness, optimize soil microbial community composition and augment ginsenoside content.
1. Introduction
Panax ginseng C. A. Meyer, a perennial herb belonging to the Araliaceae family, is a valuable traditional Chinese medicine with high medicinal value [1]. Ginseng cultivation is often hindered by serious continuous cropping disorders, which manifest as stunted growth, low seedling retention and a significant reduction in yield due to soil acidification and an increase in soil-borne diseases [2]. A number of relevant studies have demonstrated an increased prevalence of soil-borne pathogenic bacteria and an imbalance in the soil microcosm system, resulting from the excessive accumulation of chemosensory substances in ginseng root secretions in successive soil layers, represent the primary factors contributing to the disruption of succession in ginseng [3]. Fusarium and Cylindrocarpon are the two main pathogenic fungal genera that cause root and rust rot in ginseng, and they can survive for long periods of time in the soil or diseased ginseng root tissues and have the ability to persist in the infestation [4,5]. Therefore, root rot and rust rot are the main diseases that lead to ginseng continuous cropping disorder. The use of chemosensitizers, including ginsenosides, phenolic acids and amino acids, has exhibited a favorable chemotactic impact on the pathogens associated with diseases prevalent in ginseng such as ginseng rust and root rot [6,7]. A comparison of ginseng with and without continuous cropping disorder revealed significant alterations in the synthesis of phytohormones and other substances, as well as in the transcription levels of genes related to the plant–pathogen interaction pathway. These changes render ginseng more susceptible to infection by pathogenic bacteria, thereby exacerbating the continuous cropping disorder of ginseng [8].
Presently, the succession disturbance in ginseng is mainly controlled by reducing the number of pathogenic bacteria and restoring the balance of the soil microbial system. The ecological intercropping pattern of rotational cropping and intercropping can intensively utilize farmland resources, enhance the stability of farmland ecosystems and play an important role in alleviating continuous cropping disorder of medicinal plants [9]. Ginseng land rotation is an enhanced ecological model that capitalizes on the discrepancies in the utilization of ecological resources by diverse plant species and their interactions, intending to rehabilitate habitats that have been degraded due to prolonged ginseng cultivation and facilitating ginseng replanting [10]. Using celandine [2], Perilla frutescens [11] and Brassica napus [12] for the crop rotation of ginseng has been demonstrated to be an effective method for ameliorating soil acidification and salinization, as well as for promoting soil nutrient cycling and energy metabolism. The root exudates of celandine and P. frutescens were observed to specifically recruit beneficial bacteria, reduce the abundance of pathogenic bacteria such as Ilyonectria and reduce the pathogen load in the soil of ginseng. Changing the planting pattern is an important means and an effective method of preventing and controlling the continuous cropping disorder of medicinal plants. However, owing to the long rotation cycle of medicinal plants, some studies are attempting to construct an ecological intercropping pattern to alleviate the continuous cropping disorder of Chinese medicinal materials while making full use of the land.
Appropriate intercropping can facilitate the optimal utilization of the complementary advantages of plants, influence soil physicochemical properties and the structural composition of soil microbial communities through inter-root interactions between neighboring plants, alleviate continuous cropping disorder, ensure the quality and yield of Chinese medicinal materials while obtaining additional products and enhance the comprehensive benefits of the land [13]. The intercropping of Chinese medicinal crops is one of the main planting modes in Chinese medicinal crop eco-agriculture [14]. The intercropping of Chinese medicinal crops has obvious advantages. It can enhance the activity of relevant enzymes in the soil, thus effectively improving soil quality, soil nutrient effectiveness and the ability of medicinal plants to resist adversity stress [15,16]. In addition, it is a method that improves the stability of soil micro-ecosystems and the structure of inter-root microbial communities and stabilizes the inter-root micro-ecological environment of medicinal plants. This is achieved by the chemosensitization of microorganisms by different species of root exudates from neighboring plants, which is the main factor in this process [17]. An increase in plant diversity in the field results in a greater variety and quantity of root exudates in the soil, and two intercropped plants can interact with each other to change the metabolic activity of the roots. This provides soil flora with a greater abundance of nutrients and a more expansive inter-root ecological niche, so rational intercropping measures are conducive to enhancing the number of beneficial bacteria and reducing the impact of pathogenic microorganisms on the plant [18]. Certain secondary metabolites present in the root exudates of intercropped plants can exert a direct inhibitory effect on pathogenic bacteria [19,20]. The presence of citronellol and camphor in the root exudates of Litsea cubeba demonstrated efficacy in inhibiting pathogenic bacteria, resulting in a notable reduction in the abundance of Fusarium, Paraphaeosphaeria and Xanthomonadales in the soil intercropped with tea [21]. Furthermore, it has been proposed that root exudates from neighboring plants function as signaling molecules, modifying the metabolic expression of the plant and consequently influencing the specific recruitment process to the soil flora [22]. Intercropping has the potential to enhance the yield and active ingredient content of medicinal plants. For instance, the intercropping of Codonopsis pilosula with garlic has been demonstrated to significantly elevate its polysaccharide and kynurenine content, while increasing its yield by 49.92% [23]. The intercropping cultivation of Chinese medicinal crops can enhance their quality, improve soil microcosm systems and alleviate continuous cropping disorder, which has good economic and ecological benefits. Presently, various Chinese medicinal crops have made progress in intercropping practice [24]. However, there remains a lack of research, development and application of intercropping planting patterns for ginseng.
A. amurense Maxim. is a perennial herbaceous plant of the Arisaema family. Its dried tubers are used as medicine for subduing swelling and relieving pain and have anti-inflammatory, anti-bacterial, anti-cancer and anti-tumor properties [25]. The main components of A. amurense are organic acids, flavonoids and alkaloids [26]. Numerous studies have reported that tuber extracts from plants belonging to the Arisaema family exhibit an inhibitory effect on a range of plant pathogens, including Pseudoperonospora cubensis, Magnaporthe oryzae, Xanthomonas campestris, Xanthomonas oryzae and other plant pathogens [27,28]. This indicates the existence of secondary metabolites with bacteriostatic properties in plants belonging to the Arisaema family, suggesting that these substances possess broad-spectrum bacteriostatic functions. In the preliminary work, our preliminary participated in the Fourth National Survey of Chinese Materia Medica Resources. In the investigation of the growing environment of ginseng under the forest, we discovered a variety of ginseng companion medicinal plants, of which Arisaema amurense is one of them. Based on this phenomenon and previous studies on the active constituents of Arisaema family, we hypothesized that in intercropping systems, A. amurense may reduce the abundance of pathogenic fungus in ginseng rhizosphere, thereby alleviating the ginseng continuous cropping disorder to some extent, which would be beneficial to ginseng growth. For this reason, we studied the ecological intercropping pattern of P. ginseng/A. amurense and revealed the ecological adaptation mechanisms of the two intercropping patterns from in terms of: (I) the effects of intercropping ginseng with A. amurense on soil nutrient effectiveness and soil enzyme activity, (II) the effects of intercropping ginseng with A. amurense on the inter-root microbial community, and (III) the effect of intercropping ginseng with A. amurense on the content of ginsenosides.
2. Materials and Methods
2.1. Materials
The experiment used uniformly sized, disease-free, 2-year-old ginseng seedlings and well-budded 2-year-old A. amurense seedlings of a consistent quality. Ginseng seedlings and A. amurense seedlings were supplied by the Medicinal Plants Resource Nursery of the Zuojia Township Medicinal Plants Cultivation Base of the Institute of Special Animal and Plant Science, Chinese Academy of Agricultural Sciences (CAAS).
2.2. Field Experiment Design
The experimental site was located in the cultivation base of medicinal plants in Zuojia Township, Institute of Special Animal and Plant Science, CAAS (44°03′08.0″ N, 126°05′24.9″ E, Altitude 230 m). The experiment was conducted using a randomized block design with two treatment groups. Ginseng and A. amurense were used in a 1:1 intercropping as an experimental group, with ginseng monocropping as a control group, and each treatment was triplicated, with each plot being 2.4 m2. In October 2022, ginseng seedlings and A. amurense seedlings were manually transplanted in ginseng beds. The ginseng beds were 1.2 m wide and 0.3 m high, with a plant spacing of 0.1 m and a row spacing of 0.25 m. In May 2023, the participant beds were trellised and covered with shade netting for shade (30% light transmission), and regular manual weeding was performed.
2.3. Sample Collection and Preprocessing
In August 2023, five ginseng plants were randomly selected from each plot within each treatment group and uprooted. The loose soil surrounding the ginseng root system was shaken off, and the soil attached to the root system was removed using a sterilized bristle brush and thoroughly mixed to obtain an inter-root soil sample. Five points were randomly selected in the central region of the two rows of each treatment group, and the soil was excavated at a depth of 5–15 cm and thoroughly mixed to obtain inter-row soil samples. The residual fine roots of plants in each soil sample were manually removed, and a portion of the soil samples was transferred into sterile freezing tubes and stored at −80 °C. The remaining soil samples were stored in a natural air-drying process. Ginseng samples were cleaned with distilled water, dried on the surface and left to dry naturally in a ventilated environment at room temperature until constant weight for subsequent experiments.
2.4. Measurement Items and Methods
2.4.1. Determination of Soil Physical and Chemical Properties
The determination of soil physical and chemical properties is conducted using soil samples that have been ground and crushed through a sieve with a pore size of 0.85 mm. Soil pH was determined using a METTLER TOLEDO SK220 pH meter with a soil–water ratio of 1:2.5. Soil organic matter (SOM) and total nitrogen (TN) were determined using an elemental analyzer Vario EL III (EA300, Eurovector, Reggio Emilia, Italy). Nitrate nitrogen (NO3−–N) and ammonium nitrogen (NH4+–N) were extracted using a KCl solution and determined using an automatic flow analyzer (Auto analyzer 3-AA3, SEAL analytical, Hamburg, Germany). Total phosphorous (TP) was determined using the sodium hydroxide fusion–molybdenum antimony colorimetric method. Total potassium (TK) was determined using sodium hydroxide fusion–flame photometry. Available phosphorous (AP) was determined using sodium bicarbonate leaching and molybdenum antimony resist colorimetric method. Available potassium (AK) was determined using the ammonium acetate leaching–flame photometric method. For specific methods, refer to the third edition of Bao Shidan’s ‘Soil Agrochemical Analysis’ [29].
2.4.2. Determination of Soil Enzyme Activity
Fresh soil samples were sieved through a pore size of 0.25 mm. Soil urease (S-UE), soil sucrase (S-SC), soil acid phosphatase (S-ACP) and soil catalase (S-CAT) activities were determined using kits (Beijing Box Biotechnology Co., Ltd., Beijing, China) and the SpectraMax iD3 multifunctional enzyme marker (Molecular Devices, San Jose, CA, USA) [30].
2.4.3. High-Throughput Sequencing of Soil Microorganisms
The high-throughput sequencing of soil bacteria and fungi was conducted using 16s rDNA and ITS sequences. Soil total DNA extraction, amplification, library construction and sequencing were performed by Beijing Baimike Biotechnology Co. The primers for the soil bacterial 16s rRNA (V3 + V4) region were F: 5′-ACTCCTACGGGAGGCAGCA-3′; R: 5′-GGACTACHVGGGTWTCTAAT-3′, and the primers for the fungal ITS region were F: 5′-CTTGGTCATTTAGAGGAAGTAA-3′; R: 5′-GCTGCGTTCTTCATCGATGC-3′. Qualified libraries obtained via PCR amplification and purification were subjected to high-throughput sequencing using Illumina NovaSeq 6000 (Illumina, San Diego, CA, USA) [31]. The raw reads obtained from sequencing were filtered using Trimmomatic v0.33 software, and the primer sequences were identified and removed using cutadapt 1.9.1 software. This resulted in clean reads without primer sequences, which were then denoised using the dada2 method in QIIME2 2020.6 to obtain ASVs and OTUs. The results were then analyzed [32]. The high-throughput sequencing data for bacteria and fungi from each soil sample have been uploaded to the National Center for Biotechnology Information (NCBI). The sequence accession number of the soil bacterial sequencing data is PRJNA1217121 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1217121 (accessed on 2 February 2025)). All bacteria grouping sequence names and the accession numbers: IN (SRX27542618; SRX27542619; SRX27542622); IR (SRX27542623; SRX27542624; SRX27542625); MN (SRX27542626; SRX27542627; SRX27542628); MR (SRX27542620; SRX27542621; SRX27542629). The sequence accession number of the soil fungi sequencing data is PRJNA1217123 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1217123 (accessed on 2 February 2025)). All fungi grouping sequence names and the accession numbers: IN (SRX27542638; SRX27542639; SRX27542642); IR (SRX27542643; SRX27542644; SRX27542645); MN (SRX27542646; SRX27542647; SRX27542648); MR (SRX27542649; SRX27542640; SRX27542641).
2.4.4. Determination of Ginsenoside Content
The dried ginseng root samples were crushed and passed through a sieve with a pore size of 0.25 mm. Total ginsenoside determination was based on The Chinese Pharmacopoeia (2020 Edition) [33]. The content of monomer ginsenoside was determined by methanol extraction and liquid chromatography–tandem mass spectrometry (LC–MS/MS) [34]. Chromatographic conditions: electrospray anion source temperature 450 °C, voltage 2.5 kV, desolvent nitrogen flow rate 1000 L/h; the chromatographic column was Waters BEH-C18 (1.7 µm, 2.1 mm × 100 mm), the column temperature was 45 °C, flow rate 0.5 mL/min; mobile phase A was acetonitrile–0.01% formic acid solution, and B was 0.01% formic acid solution; the elution program was 19% A (0–4.4 min), 19–21% A (4.4–6.4 min), 21–28%A (6.4–8.8 min), 28–31%A (8.8–13.6 min), 31–46% A (13.6–21.6min), 46–54% A (21.6–23.2 min), 54% A (23.2–25.44 min), 54–90% A (25.44–25.84 min), 90% A (25.84–26.4 min), 90–19% A (26.4–26.5 min), and 19% A (26.5–30 min).
2.5. Data Analysis
Excel was used to organize experimental data, correlation analysis of soil microbial sequencing data was performed using the Bemac Cloud Analytics Platform (https://international.biocloud.net/zh/dashboard, accessed on 22 March 2024), data processing and significance analysis between different treatment groups was conducted using IBM SPSS Statistics 26 and GraphPad Prism 10.1.2, plotting was conducted using GraphPad Prism 10.1.2 and Origin 2022 and Adobe Illustrator 2023 was used for image retouching.
3. Results
3.1. Effect of Intercropping on Soil pH and Nutrient Content
The pH and nutrient content of monocropped ginseng inter-root (MR) and inter-row (MN) soils, and intercropped ginseng inter-root (IR) and inter-row (IN) soils are shown in Table 1. It can be observed that following the planting of ginseng, there was a notable alteration in the inter-root soil pH in comparison to the inter-row soil. Compared with the inter-row soils of the two treatment groups, the pH of monocropped and intercropped inter-root soil decreased to 0.11 and 0.05, respectively, indicating an acidification trend. However, the decline in inter-root soil pH resulting from the mono-crop of ginseng was more pronounced. In terms of nutrient changes, intercropping mainly affected the content of organic matter and total nutrients in the inter-root soil. SOM, TN and TK contents in IR were markedly higher than that in MR, exhibiting a 37.02%, 32.39% and 5.18% increase, respectively. Conversely, the TP content in MR was 1.02 g/kg, which was significantly higher than that in IR (0.84 g/kg). Among the four available nutrient contents determined, the NH4+–N content in IR was significantly higher than that in MR, but the NO3−–N content in MR was significantly higher than that in IR, which showed a certain correlation with the changes in inter-root soil pH of ginseng in the two treatment groups. AP content was significantly higher in IR than in MR, but AK content did not show a significant difference between MR and IR. It can be reasonably deduced that P. ginseng/A. amurense intercropping has the potential to markedly augment SOM, TN, TK, NH4+–N and AP contents in the soil. This could prove an effective method for alleviating soil acidification, a common consequence of ginseng monoculture.

Table 1.
Changes in pH and nutrient content in ginseng inter-root and inter-row soil under different treatments.
3.2. Effect of Intercropping on Soil Enzyme Activities
The results of the S-UE, S-SC, S-ACP and S-CAT activities of the inter-root and inter-row soils of the two treatment groups are presented in Table 2. The data presented in the table demonstrate that the planting of ginseng has resulted in alterations to soil enzyme activities. Specifically, the S-UE, S-SC and S-CAT activities of MR increased by 11.59%, 22.90% and 13.27%, respectively, compared to MN. These increases were reached significant levels. However, the activity of S-ACP in MR was 22.143 μmol/g·d−1 significantly lower than that of MN. Only IR had a significantly higher S-CAT activity (9.76%) than IN. In contrast to IN, S-SC activity was significantly lower in IR. Additionally, S-ACP activity was observed to be lower in IR than in IN, although this did not reach a significant level. A comparison of the enzyme activities in the inter-root soil of ginseng in the two treatment groups revealed that the S-UE activity in IR was significantly higher than that in MR, with an increase of 19.45% in S-UE activity. However, the S-ACP activity in IR was higher than that in MR, although this did not reach a significant level (p > 0.05). In contrast, the S-SC activity in IR was significantly lower than that in MR, with a decrease of approximately 21.80%. In contrast, the intercropping treatments had a relatively limited impact on S-CAT activity in ginseng inter-root soil, with no significant difference observed compared with the activity in MR soil. It can be observed that P. ginseng/A. amurense intercropping can effectively increase S-UE activity and promote S-ACP. However, this practice also reduces S-SC activity.

Table 2.
Changes in enzyme activity in ginseng inter-root soil and inter-row soil under different treatments.
3.3. Effect of Intercropping on Soil Microbial Communities
3.3.1. Changes in Relative the Abundance of Species
Regarding the alterations in the relative abundance of bacterial communities at the phylum level, the predominant bacterial phyla identified in the inter-root region of ginseng in the two treatment groups were Firmicutes, Bacteroidota and Fusobacteriota. The mean relative abundance of the three predominant bacterial phyla in the inter-root soils of MG and IG was 68.82% and 73.10%, respectively (Figure 1a). In comparison to monocropping, the relative abundance of the Ascomycetes phylum in ginseng inter-root soil was reduced under intercropping conditions. As a whole, intercropping treatments did not significantly affect the relative abundance of each bacterial phylum in the inter-root of ginseng but caused significant changes in the relative abundance of dominant bacterial genera (Figure 1b). The relative abundance of specific bacterial species, including Treponema, Leptotrichia and Prevotella_7, was found to be significantly elevated in ginseng inter-root soils subjected to intercropping treatments. Conversely, the relative abundance of dominant bacterial genera such as Fusobacterium, Neisseria, Veillonella, Actinomyces and Streptococcus in MR soils was significantly lower than in soils under intercropping treatments.
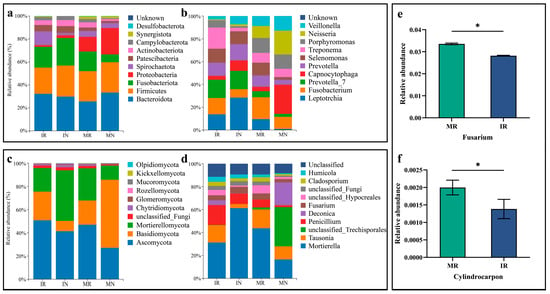
Figure 1.
Changes in the relative abundance of bacteria at the phylum (a) and genus levels (b), and of fungi at the phylum (c) and genus levels (d) in ginseng inter-root and inter-row soils, and differences in the relative abundance of main pathogenic fungal genera, Fusarium (e), and Cylindrocarpon (f) in ginseng inter-root soil after intercropping and monocropping treatments. ‘*’ indicates that the difference reaches a significant level (p < 0.05) after the independent-samples t-test.
Regarding the alterations in the relative abundance of fungal communities at the phylum level, the predominant fungal phyla identified in the inter-root region of ginseng in the two treatment groups were Ascomycota, Mortierellomycota and Basidiomycota. Furthermore, the mean relative abundance of the three predominant fungal phyla in the inter-root soils of MG and IG was 95.46% and 95.67%, respectively (Figure 1c). At the genus level, the relative abundance of the dominant fungal genera exhibited a declining trend in comparison to monocropping, except for five fungal genera: Penicillium, Humicola, Deconica, Tetracladium and Alternaria These genera were observed to be enriched in intercropping ginseng inter-roots (Figure 1d). The intercropping treatments had a lesser impact on the relative abundance of dominant fungal phyla in the ginseng inter-root than on the relative abundance of dominant fungal species. Except for individual fungal genera, intercropping reduced the relative abundance of the majority of fungal genera in the inter-root soil of ginseng. This may be attributed to the presence of fungicidal activity of root secretions of A. amurense on fungi under intercropping conditions, with this action potentially affecting soil fungi on a widespread basis.
3.3.2. Changes in the Relative Abundance of Pathogenic Fungi of Ginseng
Ginseng root rot and rust rot are two prevalent diseases affecting ginseng roots. The pathogens responsible for these diseases are Fusarium and Cylindrocarpon. The presence of a significant quantity of pathogenic fungi within the ginseng inter-root soil has been identified as a primary contributing factor in the development of ginseng diseases. Intercropping resulted in a notable alteration in the abundance of ginseng inter-root soil fungi. This may potentially contribute to reducing the accumulation of pathogenic fungi within the ginseng inter-root soil. The relative abundance of both Fusarium and Cylindrocarpon fungi in the inter-root soil of ginseng following intercropping was significantly lower than that observed in monocropping. The relative abundance of both fungi in the inter-root soil was reduced by 16.01% and 30.73%, respectively (Figure 1e,f). The findings indicate that intercropping A. amurense with ginseng may mitigate the accumulation of pathogenic fungi, including those responsible for root rot and rust rot, in the soil. This could potentially contribute to reducing the prevalence of root rot and rust rot in ginseng.
3.3.3. Change in α-Diversity Index
The Shannon and Chao1 indices of bacteria and fungi for each soil sample are shown in Figure 2a,b. The α-diversity results within the two treatment groups revealed that both fungal and bacterial communities in the inter-root soil exhibited higher Shannon indices than those in the inter-row soil, this suggests that soil microbial diversity is richer among ginseng roots, which may be caused by the recruitment of microorganisms by the metabolic activities of the ginseng root system. Notably, the Shannon indices of the fungal communities demonstrated significant changes, whereas those of the bacterial communities exhibited non-significant changes (Figure 2a). This may be due to the fact that fungi are able to better utilize the secreted substances of the monocropped ginseng root system and are in an advantageous position in colonization and competition. The Chao1 indices of the fungal community were found to be significantly higher than those of the inter-row soil in the monoculture inter-root soil, whereas those of the bacterial community were observed to be lower, although the difference was not found to be significant. The Chao1 indices of fungal communities were observed to be lower than those of IN soils, whereas those of bacterial communities were found to be significantly higher in IR soils (Figure 2b). A comparative analysis of the α-diversity results between the two treatment groups revealed that the Shannon index of the fungal community in the MR soil exhibited a significantly higher value than that observed in the IR soil. Conversely, the Shannon index of the bacterial community demonstrated a lower value in the MR soil than in the IR soil. The Chao1 index of the fungal community in the MR soil was found to be higher than that of the IR soil, although the difference was not statistically significant. In contrast, the Chao1 index of the bacterial community was observed to be significantly higher than that of the MR soil. The findings indicate that the intercropping treatment of ginseng and A. amurense can effectively enhance the diversity of ginseng inter-root microbial communities.
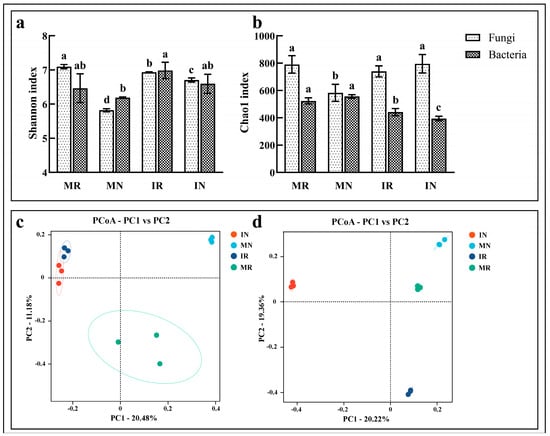
Figure 2.
Changes in Shannon index (a) and Chao1 index (b) of bacteria and fungi, and the principal coordinate analysis of changes in bacterial (c) and fungal (d) community structure in ginseng inter-root and inter-row soil after intercropping and monocropping treatments. Different letters indicate significant differences at p < 0.05.
3.3.4. Changes in Microbial Community Structure
The similarity of microbial community composition in each soil sample was analyzed using principal coordinates analysis based on the Bray–Curtis algorithm. A total of 30.54% (Figure 2c) and 42.70% (Figure 2d) of the sample information is explained by the two main coordinates, PC1 and PC2 axes, for bacteria and fungi, respectively, in the graph. The ADONIS test showed that there was a significant difference in the composition of the soil microbial community structure between the ginseng monoculture and ginseng—A. amurense intercropping treatments (Bacteria: R2 = 0.411, p = 0.001; Fungi: R2 = 0.585, p = 0.001). The results presented in the figure demonstrate that the fungal samples from MR and MN are situated nearby, while the bacterial samples from IR and IN exhibit a similar pattern. However, the fungal and bacterial samples from IR and IN are notably separated from the MR and MN. The results indicate that the fungal and bacterial communities in the inter-root soil of ginseng plants were significantly altered by the practice of intercropping with A. amurense, resulting in a notable change in the structural composition of the microorganisms present in the inter-root region of the ginseng plants.
3.4. Effect of Intercropping on the Content of Ginsenosides
The results of the determination of total saponins (TS) and the content of 10 monomer ginsenosides, including Rb1, Rg1 and Re, in the roots of ginseng from monocrops and intercrops are shown in Table 3. The TS content in intercropped ginseng (IG) was found to be significantly higher (p < 0.05) than that in monocropped ginseng (MG), with values of 4.93% and 4.32%, respectively. Furthermore, the TS content of IG was observed to increase by 0.61%. Ginsenosides Rb1, Re and Rg1 are the main index components of ginseng, and the results show that the Rb1, Rg1 and Re contents in IG were 2.185, 2.261 and 4.181 g/kg, respectively; likewise, in MG, the contents were 2.147, 1.941 and 4.087 g/kg, respectively. Therefore, the contents of Rg1 and Re ginsenosides in IG were significantly higher than those in MG (p < 0.05), and the Rg1 and Re increased by 16.49% and 2.30%, respectively. In addition, the contents of the monomer ginsenosides, Rb2, Rc, Rd, Rf, Rg2 and Ro, in IG were significantly higher than those in MG (0.185, 0.022, 0.251, 0.406, 0.101, 0.043 and 0.256 g/kg, respectively; p < 0.05). After the independent-samples t-test, the differences in the contents of Rg1, Re, Rb2, Rc, Rd, Rf, Rg2 and Ro ginsenosides reached a significant level (p < 0.05), and the differences between the contents of Rb1 and Rb3 were not significant in the two cultivation modes (p > 0.05).

Table 3.
Changes in the contents of total saponins and 10 monomer ginsenosides under different treatments.
3.5. Correlation Between Soil pH, Nutrient and Soil Microbiology
The correlation analysis of soil pH, soil nutrient content, soil enzyme activity and soil microbial α-diversity are shown in Figure 3a,b. The α-diversity index of bacteria Abundance-based Coverage Estimator (ACE) was found to be significantly and positively correlated with TK, AK and S-UE (p < 0.01), and also demonstrated a significant positive correlation with TN. The Chao1 index demonstrated a highly significant positive correlation with AK and S-UE, as well as a significant positive correlation with TN and TK. The Simpson’s index exhibited a highly significant positive correlation with S-UE and a significant positive correlation with TK, NH4+–N and AP. The Shannon index displayed a highly significant positive correlation with S-UE and a significant positive correlation with TK, NH4+–N and AP (Figure 3a). The ACE and Chao1 indices of fungi exhibited a significant and positive correlation with TP and S-UE. The Simpson index demonstrated a highly significant and positive correlation with NO3−–N and S-CAT, as well as a significant and positive correlation with S-UE. The Shannon index also exhibited a significant and positive correlation with NO3−–N, S-UE and S-CAT (Figure 3b). S-UE activity and the contents of TN, TK, NH4+–N and AP exerted a significant influence on the abundance and diversity of bacteria. Furthermore, S-UE was identified as the primary factor influencing the diversity of fungi. Additionally, the abundance and diversity of fungi were found to be influenced by S-CAT activity and TP content.
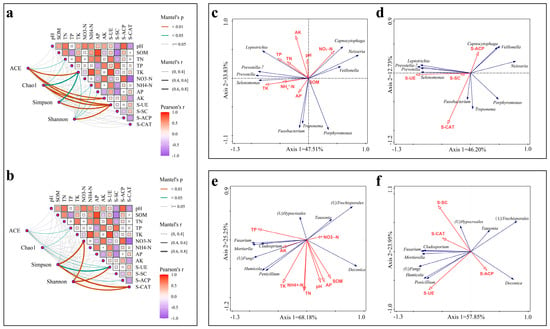
Figure 3.
Network plots of the α-diversity indices of bacteria (a) and fungi (b) against environmental factors and Pearson’s correlation heatmap between environmental factors. Line color indicates the significance level and line thickness indicates the size of the correlation coefficient. (c,d) indicate redundancy analysis between dominant bacterial genera and soil nutrients and enzyme activities. (e,f) indicate the redundancy analysis between dominant fungal genera and soil nutrients and enzyme activities. The blue arrows in the figure represent microorganisms at the genus level and the red arrows represent soil elements and soil enzymes.
The top 10 relatively abundant bacteria and fungi genera were analyzed for redundancy with soil nutrients and enzyme activities, respectively (Figure 3c–f). The impact of soil factors on fungi and bacteria varies considerably, with the influence of soil environmental factors on species abundance being particularly noteworthy (p < 0.05). The cumulative explanation of bacterial species abundance by soil pH and nutrients was 91.39% (Figure 3c). Capnocytophaga, Neisseria and Veillonella demonstrated a notable positive correlation with NO3−–N, while exhibiting a significant negative correlation with TK, NH4+–N and TP. Leptotrichia, Prevotella_7, Prevotella and Selenomonas displayed a substantial positive correlation with TN, TP and TK, as well as a notable negative correlation with NH4+–N. Fusobacterium, Porphyromonas and Treponema showed a significant positive correlation with AK. The cumulative explanation of bacterial species abundance by soil enzyme was 64.05% (Figure 3d). Capnocytophaga, Veillonella and Neisseria were significantly and positively correlated with S-ACP. Leptotrichia, Prevotella_7, Prevotella and Selenomonas showed a significant positive correlation with S-UE and S-SC, and some negative correlation with S-ACP. Fusobacterium and Treponema showed a significant positive correlation with S-CAT and a significant negative correlation with S-ACP. Porphyromonas had a negative correlation with S-ACP and S-UE.
A total of 97.84% of the cumulative abundance of fungal species was explained by soil pH and nutrients (Figure 3e). Tausonia demonstrated a notable positive correlation with NO3−–N and a pronounced negative correlation with TN, TP, TK, NH4+–N and AK. Deconica exhibited a substantial positive correlation with pH, AP and SOM and a notable negative correlation with TP and AK. The results revealed that Fusarium, Mortierella, Cladosporium, Humicola and Penicillium exhibited a significant positive correlation with TP, TK and AK while displaying a significant negative correlation with NH4+–N. The cumulative explanation of fungal species abundance by soil enzyme was 85.55% (Figure 3f). Deconica demonstrated a notable positive correlation with S-ACP and a pronounced negative correlation with S-SC and S-CAT. Fusarium, Cladosporium and Mortierella demonstrated a notable positive correlation with S-CAT and a pronounced negative correlation with S-ACP. Humicola and Penicillium exhibited a substantial positive correlation with S-UE. Tausonia displayed a significant correlation with S-UE.
3.6. Correlation Between Soil Factors and Ginsenosides
3.6.1. Correlation of Soil Nutrients, Enzyme Activities and Ginsenosides
A correlation was observed between soil pH, nutrients, enzyme activities and the total ginsenoside and 10 monomer ginsenoside contents for both the treatment groups (Figure 4). The results of Pearson’s correlation heat map demonstrated a positive correlation between ginsenoside content and SOM, TN, TK, NH4+–N, AP, S-UE and S-ACP, as well as pH changes in the inter-root soil. Conversely, there was a negative correlation between ginsenoside and TP, NO3−–N, AK, S-SC and S-CAT contents. The TS content exhibited a markedly positive correlation with AP, SOM, S-UE and NH4+-N, while displaying a markedly negative correlation with TP, NO3−–N and S-SC. Eight monomer ginsenosides, including Re and Rg1, demonstrated significant correlations with a range of soil parameters, including pH, SOM, TN, TP, NO3−–N, NH4+–N, AP, S-UE and S-SC. By contrast, the correlations between TK and AK, as well as the activities of the S-ACP and S-CAT and the content of ginsenosides were comparatively weaker. Ginsenoside Rb1 was significantly negatively correlated with S-CAT activity only in the inter-root soil, and none of the correlations between Rb3 and any of the soil factors were significant. Overall, alterations in soil potassium levels, S-ACP and S-CAT exerted a relatively minor impact on ginsenoside accumulation. Conversely, the enhancement of SOM, NH4+–N and AP as well as the improvement of S-UE under intercropping treatments could facilitate the development of superior ginseng quality.
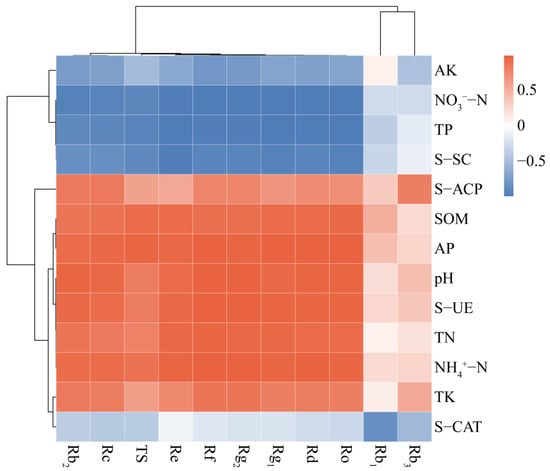
Figure 4.
Heat map of Pearson’s correlation between soil pH, nutrients, enzyme activities and ginsenosides content.
3.6.2. Correlation of Soil Microorganisms with Ginsenosides
The 15 most dominant genera in terms of relative abundance of species were significantly correlated with TS and 10 monomer ginsenoside contents in both the soil sample sets. The Pearson’s correlation heat map between fungal genera and ginsenosides demonstrated that alterations in TS and monomer ginsenosides were positively correlated with changes in the relative abundance of five fungal genera, including Deconica, Humicola, Penicillium and Tetracladium Conversely, the remaining fungal genera exhibited a negative correlation (Figure 5a). Furthermore, no significant correlation was observed between Rb1 or Rb3 and changes in the relative abundance of fungal genera. Additionally, there was a negative but non-significant correlation between Re and the relative abundance of Tausonia Neither Rb2 nor Rc exhibited a significant positive correlation with the relative abundance of Chactomium.
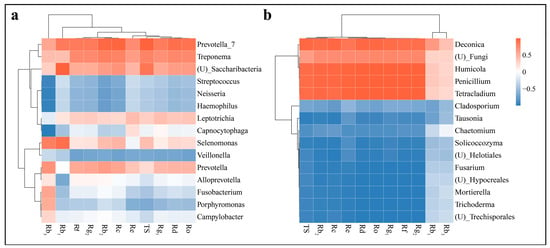
Figure 5.
Heat map of Pearson’s correlation between the relative abundance of soil bacteria (a) and fungi (b) at the genus level and ginsenosides content.
The relative abundance of bacterial species varied in their influence on changes in ginsenosides (Figure 5b). Prevotella_7 and Treponema were positively correlated with changes in ginsenosides, whereas Veillonella, Streptococcus, Neisseria and Haemophilus were negatively correlated with changes in ginsenosides. The relative abundance of Prevotella_7 demonstrated a statistically significant positive correlation with TS alterations and Rb2, Rc, Rd and Ro ginsenosides. This finding suggests that an increase in the relative abundance of Prevotella_7 in the inter-root soil of ginseng facilitates the accumulation of ginsenosides under intercropping conditions. A significant positive correlation was observed between Rb1 and the relative abundance of Selenomonas Conversely, a significant negative correlation was evident between Rb3 and the relative abundance of Haemophilus, Streptococcus, Neisseria and Capnocytophaga The reduction in the relative abundance of certain dominant fungal genera following intercropping may facilitate the accumulation of ginsenosides compared with ginseng monocropping.
4. Discussion
4.1. Effect of Intercropping on Soil Nutrients and Enzymes
pH is a significant determinant of soil physicochemical properties and serves as a crucial indicator of soil fertility. The soil acidification problem caused by long-term monoculture is one of the important factors triggering continuous cropping disorder in medicinal plants [35]. This study revealed a decrease in pH across all ginseng planted soils, with a notable reduction observed in the monocrops relative to the intercrops. This suggests that intercropping is effective in mitigating soil acidification due to long-term monoculture planting, which is consistent with the results of Xing study on intercropping legumes with tea trees [36]. Soil acidification has been demonstrated to affect the efficacy of soil nutrients between plant roots. Furthermore, it enhances the activity of deleterious elements (such as Al) within the soil, modify the composition and functionality of the soil microbial community, and increase the likelihood of continuous cropping disorders, which ultimately impedes the long-term growth of medicinal plants [37]. Therefore, we suggest that P. ginseng/A. amurense intercropping can affect the acidification process of ginseng land soils to some extent, which may be beneficial in slowing down the occurrence of continuous cropping disorder in ginseng.
Compared with soil nutrients in ginseng monoculture, SOM, TN, TK, NH4+–N and AP contents were significantly higher following intercropping with A. amurense, indicating that the intercropping system has the potential to enhance soil fertility. Incorporating SOM enhances soil quality, physicochemical properties and biodiversity. Furthermore, an increase in the organic matter content of cultivated soils positively impacts agronomic productivity [38]. Many studies have indicated that cropping patterns exert a degree of influence on SOM content [39]. The results of the experiment demonstrated that the SOM of IR soils was significantly higher than that of MR soils and also exhibited a significantly higher level compared with IN soils. Conversely, intercropping can potentially result in a relative increase in plant roots and biomass in field soils [40]. Conversely, in intercropping systems, the root secretions of different plants can alter the composition and function of inter-root microbial communities. The accumulation of flavonoids in soil has been observed to increase the abundance of specific microorganisms, including Pseudomonas, Bacillus and Rhizobia, which have been shown to effectively promote carbon and nitrogen nutrient cycling in cultivated soils [41]. This may be one of the reasons for the higher TN and NH4+–N contents in intercropped soils than in monocropped soils.
Soil enzymes are biologically active substances that originate from plant roots and soil microorganisms. They play an important role in regulating the interaction of soil nutrients, plants and microorganisms [42]. The level of soil enzyme activity is a crucial indicator for the assessment of soil quality and ecological functions within the context of intercropping systems. The interaction between the root exudates of ginseng and A. amurense in intercropping systems has the potential to influence soil enzyme activity [43]. This study revealed that S-UE and S-ACP activities in the inter-root soils of intercrops were significantly elevated compared with those of monocrops, with the difference in S-UE activity reaching a notable level. S-UE can facilitate the conversion of organic nitrogen to inorganic nitrogen, thereby enhancing the effective nitrogen content within the soil. This, in turn, elevates the nitrogen nutrition within the intercropping system. The alterations in S-UE activity and soil nitrogen content observed in this study follow the findings reported by Wu et al. [44]. Therefore, changes in soil N content after intercropping may also be the result of S-UE action. Soil phosphatases are a class of enzymes that catalyze the mineralization of soil organic phosphorous. It is generally observed that soil quick-acting phosphorous content is positively correlated with phosphatase activity. The primary sources of soil phosphatases are plant root secretions and soil bacteria. Wang et al. discovered that the relative abundance of Saccharibacteria in maize inter-root soil could be modified by the introduction of exogenous plant root-secreted substances, resulting in an enhancement of soil phosphatase activity [45]. It was therefore hypothesized that the increase in AP in intercropped soils might be due to the enhanced activity of S-ACP caused by changes in the content of root secretion substances in intercropped soils, which promoted the turnover of organic phosphorous and thus enhanced the phosphorus supply capacity of the IR soils [46].
4.2. Effect of Intercropping on Rhizosphere Microorganisms
Rhizosphere microbes are called the ‘second genome’ of plants [47]. Rhizosphere microorganisms are integral components of plant rhizosphere micro-ecosystems, exerting significant influence on the regulation of plant access to soil nutrients, modulation of plant growth and immune response [48]. This study revealed that Penicillium, Humicola and Deconica interacted with SOM and S-UE. These findings suggest that these two fungi may play a role in nutrient cycling processes within the soil [49]. Following intercropping, there was a notable increase in the abundance of Penicillium, Humicola and Deconica in the rhizospheres of ginseng. This shift in microbial community structure enhanced soil nutrient uptake by ginseng, thereby improving the efficiency of soil nutrient utilization under intercropping conditions.
The release of chemosensory substances into the rhizospheres can result in the recruitment of pathogenic fungi, which in turn leads to the deterioration of the rhizosphere microbial community structure of ginseng. This process is directly responsible for the occurrence of continuous cropping disorder in ginseng [50,51]. Ma et al. demonstrated that intercropping could alter the diversity of the structural composition of the rhizosphere bacterial community, thereby increasing the complexity and stability of the soil microbial network, as evidenced by intercropping experiments between maize and other crops [52]. Similarly, our analyses of the α-diversity of soil microbial communities demonstrated that the diversity of ginseng rhizosphere bacterial communities was significantly higher following intercropping compared with that in the monocropping treatment. The diversity and abundance of fungal communities were significantly lower than that in the monocropping treatment, and the abundance of fungi in IR was significantly lower following intercropping compared with IN. The findings indicate that intercropping with A. amurense is an effective method for preventing the accumulation of fungi in the rhizosphere of ginseng and for slowing down the transformation of ginseng soils into ‘fungal’ soils. Tausonia may be a potential source of carbohydrate-active enzymes and can secrete auxin-like compounds to inhibit the growth of pathogens [53,54]. Cladosporium also enhances plant immunity, and Tausonia and Cladosporium may work synergistically to enhance ginseng’s resistance to disease [55]. Wu et al. posited that the altered rhizosphere microbial diversity observed following intercropping can be attributed to differential changes in root exudates [56]. This is the primary mechanism through which intercropping can reduce pathogen abundance and mitigate continuous cropping disorder in medicinal plants. The results of this study indicate a reduction in the prevalence of two pathogenic fungi, Fusarium and Cylindrocarpon, in the rhizosphere soil by 16.01% and 30.73%, respectively, which reached statistical significance. These findings may help mitigate root rot and rust rot in ginseng. Secondary metabolites produced by neighboring plants can act as signaling molecules, inducing changes in plant root metabolism. This can result in the recruitment of biocontrol bacteria or the inhibition of pathogen tropism [22]. Additionally, plant root exudates contain secondary metabolites with bacteriostatic activity, which inhibit or inactivate pathogenic bacteria [57]. In previous studies, the alcoholic extract of the subterranean parts of Asparagus demonstrated bacteriostatic activity against a diverse range of microorganisms [28]. It is therefore hypothesized that specific secondary metabolites present in the root exudates of A. amurense exert direct inhibitory effects on certain soil fungi, including Fusarium and Cylindrocarpon. However, further investigation is required to determine whether the root exudates influence the metabolic activities of ginseng roots in intercropping environments and alter the chemosensory effects of ginseng on pathogenic fungi.
4.3. Intercropping Promotes the Accumulation of Ginsenosides
The alteration of soil physicochemical characteristics after intercropping represents a pivotal element influencing the condition of the soil and the development of ginseng quality. The suitability of pH is a crucial factor influencing plant growth. Ginseng predominantly flourishes in acidic soil. However, an increase in soil acidity over time will not only impede the release of nutrients but also foster the proliferation of pathogenic microorganisms within the inter-root space, which is detrimental to the quality formation of ginseng [58,59]. P. ginseng/A. amurense intercropping retards the process of soil acidification and enhances the prevention and control of ginseng diseases, while simultaneously promoting the accumulation of ginsenosides in ginseng. SOM represents a direct source of carbon and nitrogen for soil microorganisms, enabling them to carry out essential life activities. Furthermore, it exerts a direct influence on the availability of rapidly utilized nutrients within the soil [60,61]. The results of the Pearson correlation analysis showed that the increase in SOM after intercropping could promote the use of S-UE by Penicillium, Humicola and Deconica to decompose soil nutrients and promote the accumulation of Rg1 and Re ginsenosides. Available nutrients are essential for secondary metabolic activities in ginseng. Significant interactions were observed between NO3−–N, AP and the accumulation of total ginsenosides and monomer ginsenosides. Previous research has indicated that N and P are not only essential for the synthesis of ginsenosides but also constitute key components of pivotal enzymes involved in the ginsenoside synthesis pathway. Moreover, alterations in the N and P levels have been shown to exert a direct influence on the metabolic process of ginsenosides [62,63]. Soil enzymes are the primary catalyst for biochemical reactions in soil. The decomposition and transformation of organic matter and the subsequent release of nutrients in the soil are inextricably linked to the influence of soil enzyme activity [64]. Yang et al. found that high sucrase and phosphatase activities in field soils inhibit the accumulation of ginsenosides [65]. This study revealed that S-ACP activity exhibited a slight increase following intercropping. Conversely, S-SC activity demonstrated a notable decline and S-SC exhibited a significant negative correlation with ginsenosides, including Rg1 and Re. These findings align with those of previous studies. Our findings indicate that increased SOM, N and P contents, along with enhanced S-UE activity, Penicillium, Humicola and Deconica in P. ginseng/A. amurense intercropped soils represent the primary influencing factors in promoting ginsenoside accumulation and quality formation.
5. Conclusions
In conclusion, the ecological intercropping pattern of P. ginseng/A. amurense significantly enhanced the content of total ginsenosides as well as monomer ginsenosides in the roots of ginseng. The soil physicochemical properties, enzyme activities and soil microbiota exhibited notable alterations which compared between the intercropping system and the ginseng monoculture. Following the implementation of intercropping, the decline in pH was arrested, whereas the concentrations of SOM, TN, TK, NH4+–N and AP exhibited a notable increase. Additionally, S-UE activity was markedly elevated, and the diversity of the ginseng rhizosphere microbial community exhibited a significant enhancement. The relative abundance of microorganisms associated with soil nutrient cycling and ginsenoside accumulation, such as Prevotella_7, Penicillium, Humicola and Deconica, was significantly increased, whereas the relative abundance of pathogenic microorganisms, such as Fusarium and Cylindrocarpon was significantly decreased in the ginseng rhizosphere after intercropping. The establishment of an ecological intercropping pattern of P. ginseng/A. amurense in the northeast has a positive effect on alleviating the soil acidification problem of perennial ginseng cultivation, alleviating the continuous cropping disorder of ginseng and promoting ginsenoside accumulation and ginseng quality enhancement. It is worth noting that the fungal diversity and abundance in the rhizosphere of ginseng were significantly reduced after intercropping, which may be due to the existence of the chemosensory effect of the root exudates of A. amurense on soil fungi. The mechanism of its chemosensory effect needs to be further studied.
Author Contributions
Conceptualization, B.L., C.S. and Y.Z.; methodology, B.L., J.Z. and H.S.; software, W.C.; data curation, H.S.; formal analysis, H.L. (Hao Liang) and W.C.; investigation, H.L. (Hongjie Long) and Y.C.; validation, B.L., H.L. (Hongjie Long) and Y.C.; writing—original draft preparation, B.L.; writing—review and editing, C.S.; visualization, J.Z.; supervision, Y.Z.; project administration, C.S. and Y.Z.; funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of MOF and MARA, grant number CARS-21, and the National Key R&D Program of China, grant number 2021YFD1600902, and the CAAS Agricultural Science and Technology Innovation Program, grant number CAAS-ASTIP-2021-ISAPS.
Data Availability Statement
Soil nutrient content and soil enzyme activity data are contained within this article. The high-throughput sequencing results of soil microorganisms were submitted to NCBI. The sequence accession number of the soil bacterial sequencing data is PRJNA1217121. The sequence accession number of the soil fungi sequencing data is PRJNA1217123.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, B.L.; Sun, X.B.; Zhao, X.J. Panax Ginseng, 1st ed.; World Publishing Corporation: Beijing, China, 2023; pp. 3–12. [Google Scholar]
- Zhang, J.X.; Zhou, D.P.; Yuan, X.Q.; Xu, Y.H.; Chen, C.B.; Zhao, L. Soil Microbiome and Metabolome Analysis Reveals Beneficial Effects of Ginseng–celandine Rotation on the Rhizosphere Soil of Ginseng-used Fields. Rhizosphere 2022, 23, 100559. [Google Scholar] [CrossRef]
- Xiao, C.P.; Yang, L.M.; Zhang, L.X.; Liu, C.J.; Han, M. Effects of Cultivation Ages and Modes on Microbial Diversity in the Rhizosphere Soil of Panax ginseng. J. Ginseng Res. 2016, 40, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, S.; Lu, B.A.; Yang, L.N.; Wang, X.; Zhang, Y.J.; Gao, J. Fusarium oxysporum f. sp. ginseng, A New forma specialis Causing Fusarium Root Rot of Panax ginseng. Phytopathol. Mediterr. 2022, 61, 417–429. [Google Scholar] [CrossRef]
- Li, Z.B.; Sun, C.L.; Liu, X.R.; Zhou, R.J. Molecular Identification and Analysis on Differential Pathogenicity of Cylindrocarpon Species Associated with Ginseng Root Rust Rot in Northeastern China. Front. Plant Sci. 2022, 13, 894104. [Google Scholar] [CrossRef] [PubMed]
- Li, Q. Effect of Ginsenoside on Chemotaxis Aggravation of Soil Rust Rot in Continuous Cropping Soil and Its Mechanism. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2020. [Google Scholar]
- Xu, Z.Y. Study on Effects of Ginseng Root Exudates to Chemotaxis of Its Pathogens and Allelopathy to Seeds and Seedlings of Panax. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2021. [Google Scholar]
- Shen, Y.L.; Zhang, H.; Zhan, Y.; Zhang, T.; Li, Q.; Wang, E.P.; Chen, C.B. Transcriptomics and Metabolomics of the Molecular Mechanisms of Ginseng’s Response to the Continuous Cropping Obstacle. Physiol. Mol. Plant Pathol. 2024, 133, 102329. [Google Scholar] [CrossRef]
- Wang, X.Y.; Shen, B.Y.; Sun, L.F.; Song, G.Z.; Jia, H.Q.; Sun, W.S. Development and Advantages of Ecological Planting Patterns for Dictamnus dasycarpus. Hortic. Seed 2023, 43, 22–32. [Google Scholar]
- Yang, L.M. Theory and Technology Frontiers of Ecological Planting of Chinese Medicinal Materials. J. Jilin Agric. Univ. 2020, 42, 355–363. [Google Scholar]
- Bian, X.B.; Yang, X.H.; Zhang, K.X.; Zhai, Y.R.; Li, Q.; Zhang, L.X.; Sun, X. Potential of Medicago sativa and Perilla frutescens for Overcoming the Soil Sickness Caused by Ginseng Cultivation. Front. Microbiol. 2023, 14, 1134331. [Google Scholar] [CrossRef]
- Bian, X.B.; Yang, X.H.; Li, Q.; Sun, X. Effects of Planting of Two Common Crops, Allium fistulosum and Brassica napus, on Soil Properties and Microbial Communities of Ginseng Cultivation in Northeast China. BMC Microbiol. 2022, 22, 182. [Google Scholar] [CrossRef]
- Gao, S.H.; Lv, C.J. Advances in the Mechanism of Intercropping to Alleviate Continuous Cropping Obstacles in Plants. Hortic. Seed 2023, 43, 98–100. [Google Scholar]
- Huang, L.Q.; Guo, L.P. Ecological Agriculture of Chinese Materia Medica, 2nd ed.; Shanghai Scientific & Technical Publishers: Shanghai, China, 2022; pp. 81–87. [Google Scholar]
- Hang, Y.; Luo, F.L.; Zhao, Z.; Tang, C.L.; Lin, J.; Li, Q.W.; Deng, C. Effect of Pinellia ternata with Different Intercropping Crops on Soil Microorganism, Nutrient and Enzyme Activity. J. Chin. Med. Mater. 2018, 41, 1522–1528. [Google Scholar]
- Zhang, X.H. Studies on Mechanism of Continuous Angelica sinensis Cropping Obstacle and Its Preparatory Bioremediation. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2009. [Google Scholar]
- Lyu, B.C.; Sun, H.; Qian, J.Q.; Liang, H.; Zhu, J.P.; Zhang, Q.S.; Shao, C.; Zhang, Y.Y. Interaction between Root Exudates of Medicinal Plants and Rhizosphere Microorganisms and Its Application in Ecological Planting of Chinese Medicinal Materials. China J. Chin. Mater. Med. 2024, 49, 2128–2137. [Google Scholar]
- Zhou, Y.F.; Feng, R.; Tang, L. Research Progress on the Root Exudate-mediated Mechanism of Intercropping for Prevention and Control of Soil-borne Diseases. J. Yunnan Agric. Univ. Nat. Sci. 2023, 38, 353–360. [Google Scholar]
- Iwamoto, K.; Kawamoto, H.; Takeshita, F.; Matsumura, S.; Ayaki, I.; Moriyama, T.; Zaima, N. Mixing Ginkgo biloba Extract with Sesame Extract and Turmeric Oil Increases Bioavailability of Ginkgolide A in Mice Brain. J. Oleo Sci. 2019, 68, 923–930. [Google Scholar] [CrossRef]
- Zeng, J.R.; Liu, J.Z.; Lu, C.H.; Ou, X.H.; Luo, K.K.; Li, C.M.; He, M.L.; Zhang, H.Y.; Yan, H.J. Intercropping with Turmeric or Ginger Reduce the Continuous Cropping Obstacles That Affect Pogostemon cablin (Patchouli). Front. Microbiol. 2020, 11, 579719. [Google Scholar] [CrossRef]
- Hao, H.P.; Bai, H.T.; Xia, F.; Hao, Y.P.; Li, H.; Cui, H.X.; Xie, X.M.; Shi, L. Effects of Tea-Litsea cubeba Intercropping on Soil Microbial Community Structure in Tea Plantation. Sci. Agric. Sin. 2021, 54, 3959–3969. [Google Scholar]
- Zhou, X.G.; Zhang, J.Y.; Khashi u Rahman, M.; Gao, D.M.; Wei, Z.; Wu, F.Z.; Dini-Andreote, F. Interspecific Plant Interaction Via Root Exudates Structures the Disease Suppressiveness of Rhizosphere Microbiomes. Mol. Plant 2023, 16, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Du, M.X.; Qiu, D.Y.; Ren, F.Y.; Wang, S.J.; Hu, F.D. Effects of Intercropping Crops and Stubbles on Growth, Yield and Quality of Codonopsis pilosula. Acta Bot. Boreali-Occident. Sin. 2021, 41, 1884–1892. [Google Scholar]
- Duan, W.Y.; Chen, X.L.; Ran, Z.F.; Fang, L.; Song, Z.J.; Guo, L.P.; Zhou, J. Intercropping History, Pattern, and Case Analysis of Traditional Chinese Materials. China J. Chin. Mater. Med. 2024, 58, 104. [Google Scholar]
- Chinese Pharmacopoeia Commission. Herbs and tablets. In Chinese Pharmacopeia, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 58–59. [Google Scholar]
- Wang, M. Comparative Analysis of Effective Ingredients and Antioxidant Activity of Arisaema amurense Maxim. Master’s Thesis, Yanbian University, Yanji, China, 2021. [Google Scholar]
- Wang, L.W.; Xu, B.G.; Wang, J.Y.; Su, Z.Z.; Lin, F.C.; Zhang, C.L.; Kubicek, C.P. Bioactive Metabolites from Phoma Species, an Endophytic Fungus from the Chinese Medicinal Plant Arisaema erubescens. Appl. Microbiol. Biotechnol. 2012, 93, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gong, M.F.; Wu, S.L.; Fu, Q.C.; Yan, J.J.; Xu, G.Y. Analysis of Bacteriostatic Activity of Ethanol Extract from Plants in Araceae on Plant Pathogenic Fungi. Genom. Appl. Biol. 2016, 35, 152–157. [Google Scholar]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2015; pp. 70–114. [Google Scholar]
- Li, Y.J.; Xu, G.H.; Yu, Y. Freeze-thaw Aged Polyethylene and Polypropylene Microplastics Alter Enzyme Activity and Microbial Community Composition in Soil. J. Hazard. Mater. 2024, 470, 134249. [Google Scholar] [CrossRef] [PubMed]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between Phyllosphere Bacterial Communities and Plant Functional Traits in a Neotropical Forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Vegetable oils and extracts. In Chinese Pharmacopeia, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2020; pp. 409–410. [Google Scholar]
- Zhang, H.; Yang, H.; Wang, Y.P.; Gao, Y.G.; Zhang, L.X. The Response of Ginseng Grown on Farmland to Foliar-applied Iron, Zinc, Manganese and Copper. Ind. Crops Prod. 2013, 45, 388–394. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, H.M.; Zhu, Q.; Chen, J.; Wang, J.Y.; Wu, Y.H.; Lin, H.; Lin, W.X. Effects of Different Amendments on Contents of Phenolic Acids and Specific Microbes in Rhizosphere of Pseudostellaria heterophylla. Chin. J. Appl. Ecol. 2016, 27, 3623–3630. [Google Scholar]
- Xing, H.Q. The Effects of Intercropping Legume Green Manure in Tea Plantation on Soil Properties and Tea Quality. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Zhang, J.G.; Fan, S.H.; Qin, J.; Dai, J.C.; Zhao, F.J.; Gao, L.Q.; Lian, X.H.; Shang, W.J.; Xu, X.M.; Hu, X.P. Changes in the Microbiome in the Soil of an American Ginseng Continuous Plantation. Front. Plant Sci. 2020, 11, 572199. [Google Scholar] [CrossRef] [PubMed]
- Yu, X. Study on the Variation Characteristics of Crop Yield, Carbon and Nitrogen Accumulation and Soil Nutrients under Sweet Corn || Soybean Intercropping System in Upland Red Soil. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2021. [Google Scholar]
- Chen, X.; Tian, Y.; Guo, X.F.; Chen, G.K.; He, H.Z.; Li, H.S. The Effect of Monoculture Peanut and Cassava/Peanut Intercropping on Physical and Chemical Properties in Peanut Rhizosphere Soil under the Biochar Application and Straw Mulching. IOP Conf. Ser. Earth Environ. Sci. 2017, 59, 012021. [Google Scholar] [CrossRef]
- Cong, W.F.; Hoffland, E.; Li, L.; Six, J.; Sun, J.X.; Bao, X.G.; Zhang, F.S.; Van Der Werf, W. Intercropping Enhances Soil Carbon and Nitrogen. Glob. Chang. Biol. 2015, 21, 1715–1726. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne Flavonoids Released into the Rhizosphere: Impact on Soil Bio-activities Related to Plant Nutrition. A Review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Jing, C.L.; Xu, Z.C.; Zou, P.; Tang, Q.; Li, Y.Q.; You, X.W.; Zhang, C.S. Coastal Halophytes Alter Properties and Microbial Community Structure of the Saline Soils in the Yellow River Delta, China. Appl. Soil Ecol. 2019, 134, 1–7. [Google Scholar] [CrossRef]
- Li, Q.S.; Wu, L.K.; Chen, J.; A Khan, M.; Luo, X.M.; Lin, W.X. Biochemical and Microbial Properties of Rhizospheres under Maize/Peanut Intercropping. J. Integr. Agric. 2016, 15, 101–110. [Google Scholar] [CrossRef]
- Wu, X.; Hu, Y.C.; Yang, F.J.; Su, C.J.; Wang, X.Z.; Wu, F.Z. Effects of Intercropping Tomato with Potato Onion on Nitrogen Absorption and Rhizosphere Microbial Diversity of Tomato. J. Plant Nutr. Fert. 2022, 28, 1478–1493. [Google Scholar]
- Wang, G.W.; Jin, Z.X.; Xinxin Wang, X.X.; George, T.S.; Feng, G.; Zhang, L. Simulated Root Exudates Stimulate the Abundance of Saccharimonadales to Improve the Alkaline Phosphatase Activity in Maize Rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Yang, F.; Liao, D.P.; Wu, X.L.; Gao, R.C.; Fan, Y.F.; Ali Raza, M.; Wang, X.C.; Yong, T.W.; Liu, W.G.; Liu, J.; et al. Effect of Aboveground and Belowground Interactions on the Intercrop Yields in Maize-soybean Relay Intercropping Systems. Field Crops Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Chialva, M.; Lanfranco, L.; Bonfante, P. The Plant Microbiota: Composition, Functions, and Engineering. Curr. Opin. Biotechnol. 2022, 73, 135–142. [Google Scholar] [CrossRef]
- Zhao, G.X.; Yang, H.T.; Shao, X.B.; Cui, Z.H.; Liu, H.G.; Zhang, J. Phosphate-solubilizing Properties and Optimization of Cultivation Conditions of Penicillium rubens: A Highly Efficient Phosphate Solubilizer. Biotechnol. Bull. 2023, 39, 71–83. [Google Scholar]
- Li, H.R.; Lin, W.; Wang, T.Y.; Lei, J.; Chen, P. Research Progress on Allelopathic Effects of Ginseng. Mol. Plant Breed. 2024, 22, 1254002. [Google Scholar]
- Qu, Y.T.; Zhang, Q.Q.; Yu, Y.F.; Sayikal, D.; Cai, L.L.; Zhang, S.J.; Li, Y.F.; Li, Y.C. Research Advances on Mechanisms and Preventions of the Medicinal Plants’ Continuous Cropping Obstacles from the Perspective of Rhizosphere Microecology. J. Zhejiang Univ. Agric. Life Sci. 2022, 48, 403–414. [Google Scholar]
- Ma, H.Y.; Surigaoge, S.; Xu, Y.; Li, Y.C.; Christie, P.; Zhang, W.P.; Li, L. Responses of soil microbial community diversity and co-occurrence networks to interspecific interactions in soybean/maize and peanut/maize intercropping systems. Appl. Soil Ecol. 2024, 203, 105613. [Google Scholar] [CrossRef]
- Trochine, A.; Bellora, N.; Nizovoy, P.; Duran, R.; Greif, G.; de García, V.; Batthyany, C.; Robello, C.; Libkind, D. Genomic and Proteomic Analysis of Tausonia pullulans Reveals a Key Role for a GH15 Glucoamylase in Starch Hydrolysis. Appl. Microbiol. Biotechnol. 2022, 106, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Ogundeji, A.O.; Li, Y.; Liu, X.J.; Meng, L.B.; Sang, P.; Mu, Y.; Wu, H.L.; Ma, Z.N.; Hou, J.; Li, S.M. Eggplant by Grafting Enhanced with Biochar Recruits Specific Microbes for Disease Suppression of Verticillium Wilt. Appl. Soil Ecol. 2021, 163, 103912. [Google Scholar] [CrossRef]
- Paulus, J.K.; Kourelis, J.; Ramasubramanian, S.; Homma, F.; Godson, A.; Hörger, A.C.; Hong, T.N.; Krahn, D.; Carballo, L.O.; Wang, S.S.; et al. Extracellular Proteolytic Cascade in Tomato Activates Immune Protease Rcr3. Proc. Natl. Acad. Sci. USA 2020, 117, 17409–17417. [Google Scholar] [CrossRef]
- Wu, H.M.; Lin, M.H.; Rensing, C.; Qin, X.J.; Zhang, S.K.; Chen, J.; Wu, L.K.; Zhao, Y.L.; Lin, S.; Lin, W.X. Plant-mediated Rhizospheric Interactions in Intraspecific Intercropping Alleviate the Replanting Disease of Radix pseudostellariae. Plant Soil 2020, 454, 411–430. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, L.X.; Chen, X.X.; Feng, H.; Sun, W.S.; Qin, N. Effects of Platycodon grandiflorum and Allium fistulosum Intercropping on Soil Nutrients, Microorganism and Enzyme Activity. J. Plant Nutr. Fert. 2018, 24, 668–675. [Google Scholar]
- Yan, L.; Riaz, M.; Liu, J.Y.; Yu, M.; Jiang, C.C. The Aluminum Tolerance and Detoxification Mechanisms in Plants; Recent Advances and Prospects. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1491–1527. [Google Scholar] [CrossRef]
- Bian, X.B.; Xiao, S.Y.; Zhao, Y.; Xu, Y.H.; Yang, H.; Zhang, L.X. Comparative Analysis of Rhizosphere Soil Physiochemical Characteristics and Microbial Communities between Rusty and Healthy Ginseng Root. Sci. Rep. 2020, 10, 15756. [Google Scholar] [CrossRef]
- Accoe, F.; Boeckx, P.; Busschaert, J.; Hofman, G.; Van Cleemput, O. Gross N Transformation Rates and Net N Mineralisation Rates Related to the C and N Contents of Soil Organic Matter Fractions in Grassland Soils of Different Age. Soil Biol. Biochem. 2004, 36, 2075–2087. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.Y.; Sun, C.W.; Zheng, P.H.; Gao, M.; Zhang, L.N. Relationship between Soil Nutrient Status and Ginsenosides Content in Different Growth Patterns of Panax ginseng. Acta Agric. Boreali-Occident. Sin. 2012, 21, 146–152. [Google Scholar]
- Ming, Q.L.; Han, T.; Huang, F.; Qin, L.P. Advances in Studies on Ginsenoside Biosynthesis and Its Related Enzymes. Chin. Tradit. Herb. Drugs 2010, 41, 1913–1917. [Google Scholar]
- Chen, W.; Gao, W.Y.; Jia, W.; Duan, H.Q.; Xiao, P.G. Advances in Studies on Tissue and Cell Culture in Medicinal Plants of Panax L. Chin. Tradit. Herb. Drugs 2005, 36, 616–620. [Google Scholar]
- Ma, W.Q.; Wang, H.Y.; Wang, S.; Wan, X.F.; Kang, C.Z.; Guo, L.P. Effects of Ecological Factors on Shape and Ginsenoside of Panax ginseng. China J. Chin. Mater. Med. 2021, 46, 1920–1926. [Google Scholar]
- Yang, Y.W.; Jiang, Y.T. Study on Relationship between Effective Components and Soil Enzyme Activity in Different Growth Patterns of Panax ginseng. China J. Chin. Mater. Med. 2016, 41, 2987–2992. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).