Abstract
The aim of this study was to demonstrate the variability of essential oils (EOs) extracted from the culinary plant Plectranthus amboinicus in different locations, in order to determine their optimal utilization. The EO was extracted from fresh leaves by steam distillation at three locations in the south of Ecuador: Paccha (PAC), El Guabo (GUA), and Arenillas (ARE). Qualitative and quantitative analyses were performed by gas chromatography coupled with mass spectrometry (GC-MS) and flame ionization detection (GC-FID). A total of 41 compounds were identified in Paccha (97.37%), 38 compounds in Arenillas (97.43%), and 41 compounds in El Guabo (98.31%). The most abundant compounds identified were germacrene D (17.60%, 14.59%, and 12.85%), alfa humulene (10.88%, 4.65%, and 5.08%), alfa-terpinene (10.66%, 13.85%, and 6.79%), and carvacrol (5.11%, 5.97%, and 9.13%), in PAC, ARE, and GUA, respectively. In addition, the antioxidant activity was evaluated by ABTS and DPPH assays, as well as the total phenol content of the EOs. Finally, a principal compound analysis (PCA) was performed in order to evaluate the variation in compounds using the geographical location of the samples as a variable.
1. Introduction
In recent years, there has been a growing interest in studying the chemistry and effects of medicinal plants, which promises advances in the field of health [1]. Among the enormous diversity of plants, which includes at least 10,000 species, about 3000 belong to the families Asteraceae, Lamiaceae, and Apiaceae, which are known for their ability to produce essential oils [2].
The genus Plectranthus contains more than 300 species, 62 of which are known to have medicinal, antiseptic, repellent, food, flavouring, and other properties [3]. According to the taxonomy of Plectranthus (or Coleus), it was deduced that the species Plectranthus amboinicus originated in tropical Africa, from where the Arabs and Portuguese introduced it to India and Southeast Asia, and then to Europe and from there to the New World where it was christened ‘Broadleaf Oregano’ [4,5].
P. amboinicus is one of the most popular medicinal plants in Latin America. It belongs to the Lamiaceae family, also known as the mint or dead nettle family [6], which is characterized by generally including herbs, shrubs, and trees with a worldwide distribution. It comprises approximately 200 genera and about 3200 species, with important uses in the treatment of diseases and in culinary uses [7]. In Ecuador, approximately 27 genera and 219 species have been recorded, of which 29 are endemic. The species P. amboinicus grows in warm, dry, and sunny areas, tolerating temperatures between 15 °C and 30 °C, or even lower, down to about 5 °C, and suffers from stress that inhibits its growth [8]. In Ecuador, it is found in the provinces of Pichincha, Azuay, Cañar, Loja, and El Oro. It grows to a height of 0.5 to 1 m and has thick, oval, or round leaves with a characteristic aroma that last between 3 and 10 years. The stems are fleshy and can reach 90 cm [9].
P. amboinicus, also known as Coleus aromaticus, Coleus amboinicus Lour., and Plectranthus aromaticus (Benth.) Roxb. [10] Indian borage [11], French oregano [12], is an herbaceous plant commonly used in Chinese folk medicine for the treatment of cough, fever, mumps, skin disorders, mosquito bites, genito-urinary disorders, musculoskeletal disorders, sore throat, and it also shows the ability to treat arthritis [13,14]. The leaves have been studied for their potential antioxidant, antifungal, antimicrobial, antimutagenic, antitumor, and antigenotoxic activities [15].
Previous research has shown that P. amboinicus has the ability to restore glutathione in diabetic rats, more effectively than vitamin E [16]. It has high antioxidant power (98.88%) and anti-inflammatory activity from 83.37% to 88.66%. In addition, it shows analgesic activity between 90.33% and 80.65% compared to indo-methacin, and antibacterial activity on Gram-positive bacteria, especially oral pathogens such as Streptococcus mutans and Lactobacillus acidophilus, making it a potential use in mouthwashes and toothpastes [17]. Studies on the essential oil of the leaves showed high levels of bioactive compounds including carvacrol, thymol, β-caryophyllene, α-humulene, γ-terpinene, p-cymene, α-terpineol, β-selinene, α-cubebene, eudesma-4-11-diene, 4-terpineol, and caryophyllene oxide [18,19,20]. In addition, phytochemical evaluation of P. amboinicus extract revealed the presence of phenolics such as rosmarinic acid, caffeic acid, rutin, gallic acid, quercetin, and p-coumaric acid, as well as flavonoids and condensed tannins [21].
The aim of this study was to perform a comparative analysis of the chemical composition and biological activity of P. amboinicus EOs from three sectors south of Ecuador. This will enable us to assess the variability in the composition of P. amboinicus EOs in places with different soil and climatic conditions. Furthermore, the results will provide useful information for the design of potential pharmaceuticals and will contribute to a broader understanding of its uses in traditional medicine.
2. Materials and Methods
2.1. Collection of Plant Material
P. amboinicus leaves were collected in three different areas of southern Ecuador during summer season, see Figure 1. The collection was carried out in the canton Paccha (latitude 3°35′16′ S and longitude 79°40′02′ W) on 18 June 2022, at an altitude of 1583 m.a.s.l, where the average temperature was approximately 18 °C, and a relative humidity of 78. In El Guabo (latitude 3°13′35′ S and longitude 79°49′40′ W), on 4 June 2022, at an altitude of 15 m.a.s.l., the weather was characterized by a higher average temperature of 25 °C, and a relative humidity of 85%, Finally, in Arenillas (latitude 2°57′52′ S and longitude 79°44′29′ W) on 11 July 2022, at an altitude of 55 m.a.s.l, the average temperature was 28 °C, with a relative humidity of 75%. These environmental data, including temperature, humidity, and climatic conditions, were retrieved from the NASA POWER (Prediction of Worldwide Energy Resources) database https://power.larc.nasa.gov/ (accessed on 25 January 2025), providing reliable and site-specific meteorological information that contextualizes the ecosystems where the plant material was collected [22].
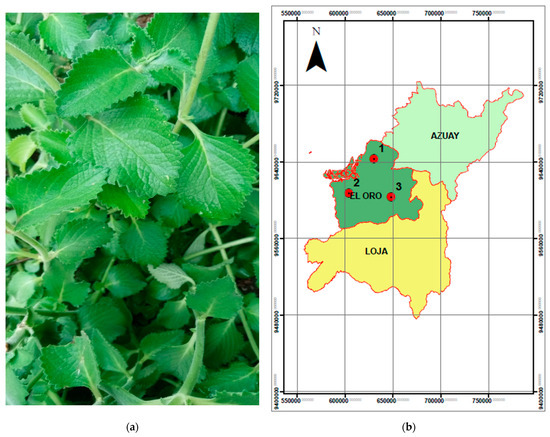
Figure 1.
(a) Plectranthus amboinicus species. (b) Map of the leaf collection of Plectranthus amboinicus in the three sectors from south of Ecuador.
2.2. Extraction of Essential Oils
Leaves of P. amboinicus from the three sectors, were subjected to steam distillation for 2 h in a Clevenger apparatus from 7400 g of the sample from El Guabo 8300 g from Arenillas, and 6850 g from Paccha. The leaves were cut into small pieces to release more volatile compounds and distilled immediately. The EO was dried over Na2SO4 to remove moisture and stored at −7 °C until use.
2.3. Chemical Composition
2.3.1. GC-MS Analysis
The chemical analysis of the EOs was performed by gas chromatography (GC-MS) using a Thermo Scientific gas chromatograph Trace 1310 (Thermo Scientific, Wan-than, MA, USA) with electrical pressure control. The instrument was coupled to an ISQ 7000 single quadrupole mass spectrometry detector. A non-polar 5% phenyl-methylpolysiloxane-based DB-5ms column (30 m × 0.25 mm internal diameter and 0.25 mm layer thickness) was used. The column temperature was programmed starting at 50 °C for 3 min followed by a rate of 3 °C/min to a final temperature of 230 °C. The column temperature was then increased to 350 °C. Helio was used as the carrier gas at a flow rate of 1 μL/min from a nominal initial pressure of 6.49 psi and an average pressure of 35 cm/s. Injection was also performed in triplicate in split mode (50:1) [23].
The identification results were compared with the mass spectra and the calculated linear retention index (LRI) reported in the literature, with a value acceptable of ±20 units. The calculated linear retention indices (LRI) were obtained using the method of Van Den Dool and Kratz [24] and a mixture of n-alkanes (C10-C25) injected under the same chromatographic conditions as the EOs.
2.3.2. GC-FID Analysis
The GC-FID was performed with the same equipment used GC-MS analysis and the chromatographic analytical gas-chromatographic conditions were the same as described previously.
2.4. Antioxidant Analysis
2.4.1. ABTS Radical Method
The ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulphonic acid) assay was performed according to the method described by Thaipong [25] with minor modifications. The working solution was prepared using 40 µL of ABTS per millilitre of methanol. The absorbance was adjusted to 1.1 ± 0.02 and 270 µL working solution and 30 µL methanol were added to a multiwell plate and measured at 734 nm. Each sample was read in triplicate by adding 270 µL of ABTS working solution and 30 µL of each sample to be analyzed at the appropriate doses for the assay. Reaction kinetics were then measured in a BIOTEK Epoch2 microplate reader at 734 nm for 1 h in the dark at room temperature.
2.4.2. DPPH Radical Method
The DPPH (2,2-diphenyl-1-picrylhydrazyl) method was developed based on the method of Thaipong [25]. The blank was read in triplicate by adding 300 µL of methanol to each well. The working solution was prepared by adding 250 µL of DPPH (625 M) per millilitre of methanol. The absorbance was adjusted to 1.1 ± 0.02. Then, 270 µL of the working solution and 30 µL of methanol were added to a multiwell plate and measured at 515 nm. Samples were analyzed in triplicate with 270 µL of DPPH working solution and 30 µL of each sample to be analyzed. Reaction kinetics were determined at an absorbance of 515 nm in an Epoch2 microplate reader for 1 h in the dark at 25 °C.
2.4.3. Total Phenolic Content
The determination of total phenols was performed according to the procedure described by Thaipong [25] with modifications for reading in 96-well microplates and a final volume of 300 µL. The blank was read in triplicate. For each well, 255 µL of ultrapure water, 15 µL of Folin’s 0.25 N reagent solution, and 30 µL of 1 N sodium carbonate solution were prepared. The mixture was homogenized, and the reaction was carried out in an Epoch2 microplate reader for 2 h in the dark at 22 °C. Absorbance was read at 756 nm.
3. Results
3.1. Physical Properties of Essential Oils
The essential oil from the three different sectors showed yields ranging from 0.02 to 0.04%, as well as insignificant relative density and refractive index. These data are shown in Table 1. It is important to note that the samples collected had a slightly greenish appearance.

Table 1.
Physical properties of three different samples of Plectranthus amboinicus collected from three locations.
3.2. Chemical Composition of Plectrancthus amboinicus Essential Oils
The chemical analysis of P. amboinicus EOs from the three sectors is presented in Table 2, Figure 2. In total, 56 compounds were identified in the steam distillation of the three essential oils (EO) from the aerial parts of P. amboinicus. In Paccha 41 compounds were identified, representing 97.16% of the total, the main components (>3%) were as follows: Germacrene D (17.60%), γ-terpinene (11.25%), α-terpinene (10.66%), o-cymene (6.00%), carvacrol (5.11%), and (E)-caryophyllene (3.73%). In El Guabo, 39 compounds were identified, representing 97.43% of the total, the majority compounds (>5%) were as follows: germacrene D (14.59%), α-terpinene (13.85%), γ-terpinene (13.46%), carvacrol (5.97%), (E)-β-ocimene (5.88%), and ο-cymene (5.72%). Finally, in Arenillas 41 compounds were identified, representing 98.31% of the total, the main compounds (>6%) were as follows: γ-terpinene (15.52%), germacrene D (12.85%), carvacrol (9.13%), ο-cymene (8.17%), α-terpinene (6.79%), and (E)-caryophyllene (6.72%).

Table 2.
Chemical composition of Plectranthus amboinicus essential oil from aerial parts collected at South of Ecuador.
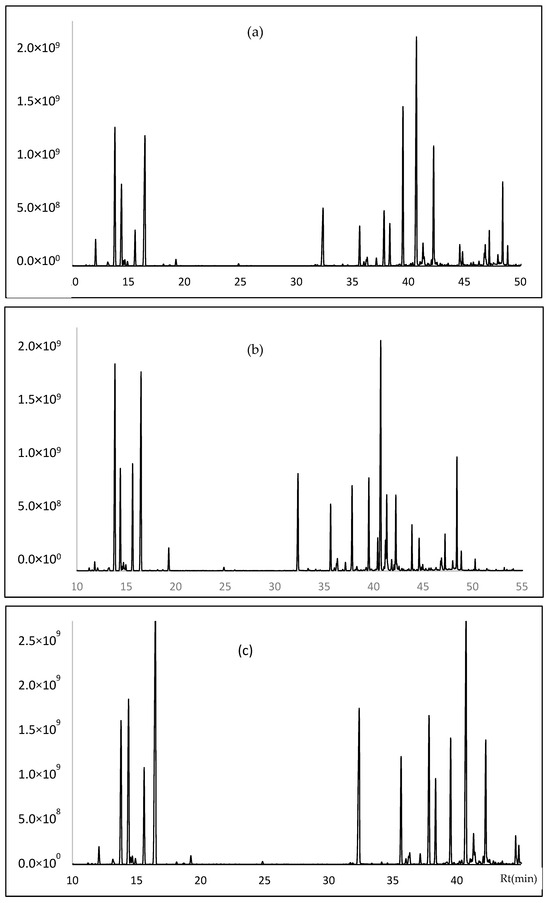
Figure 2.
Gas chromatogram of Plectranthus amboinicus leaves EOs from (a) Paccha, (b) El Guabo, and (c) Arenillas using DB-5ms column.
3.3. Principal Component Analysis
Principal Component Analysis (PCA) was used to determine the correlation between the main compounds and the concentration of each sample of EOs from the different areas from south of Ecuador. The rotated component plot shows the similarity of the metabolites between the three samples of P. amboinicus EOs (Figure 3).
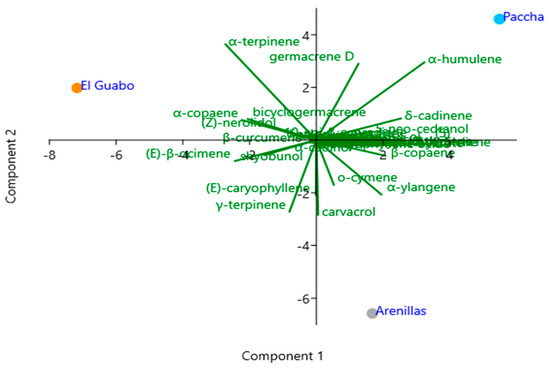
Figure 3.
PCA of (GC−MS) P. amboinicus essential oil from different sectors from south of Ecuador.
The PCA of the chemical composition of aerial parts showed different compounds found in the different collection sites. After the PCA, it was possible to determine the dispersion of the chemical composition obtained after chromatographic analysis on the DB-5ms column. The first component accounted for 56.7% with carvacrol, o-cymene, β-copaene, α-terpinene, among others, while the second component accounted for 43.3% of the variance, characterized by the compounds α-humulene, δ-cadinene, germacrene D, among others. In terms of similarity, the sectors of El Guabo and Arenillas showed greater variability than Paccha.
3.4. Antioxidant Activity
The EOs were tested for antioxidant activity using ABTS and DPPH radicals. Trolox was used as a positive control. The results obtained are shown below in Figure 4.
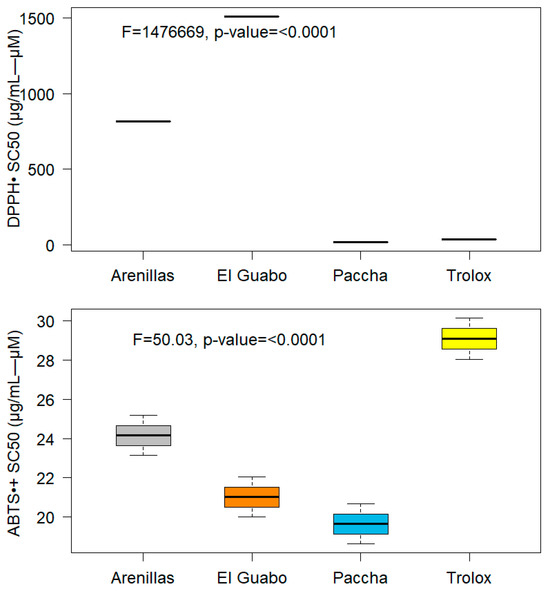
Figure 4.
Antioxidant activity of Plectrancthus amboinicus essential oils from three different locations. SC50; Clearance capacity of half expressed as µg/mL-µM.
3.4.1. ABTS Radical Uptake
From the Trolox calibration curve with a correlation coefficient of R2 = 0.9995, an SC50 value of 29.09 µM was obtained, as shown in Figure 5. The graphs obtained for each sample analyzed by the ABTS test, where the concentration of the samples tested is plotted as a logarithmic function on the X-axis and the standardized response obtained from the absorbances recorded at the end of the monitoring is plotted on the Y-axis.

Figure 5.
Calibration curve of ABTS analysis of P. amboinicus essential oils obtained by steam distillation from (a) Paccha, (b) El Guabo, and (c) Arenillas.
3.4.2. DPPH Radical Uptake
Results are expressed as the mean ± standard deviation of three replicates with three experiments (n = 9) and the Coefficient of Variation (CV) is expressed as a percentage, see Figure 6.
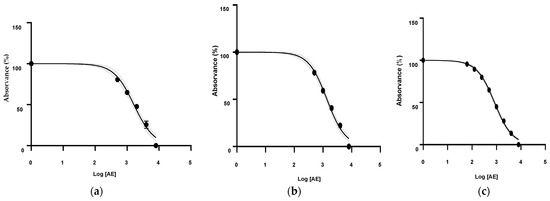
Figure 6.
Calibration curve of the DPPH analysis of P. amboinicus essential oils obtained by steam distillation from (a) Paccha, (b) El Guabo, and (c) Arenillas.
Following an analysis of the calibration curve using the Trolox correlation coefficient (R2 = 0.9971), an SC50 of 35.54 µM was determined.
3.4.3. Determination of Total Phenolics
Total Phenolic content was performed, and the results are shown in Figure 7. The absorbances obtained for each dilution of the essential oils in the presence of Folin’s reagent 0.25 N, and the results are expressed in mg/L.
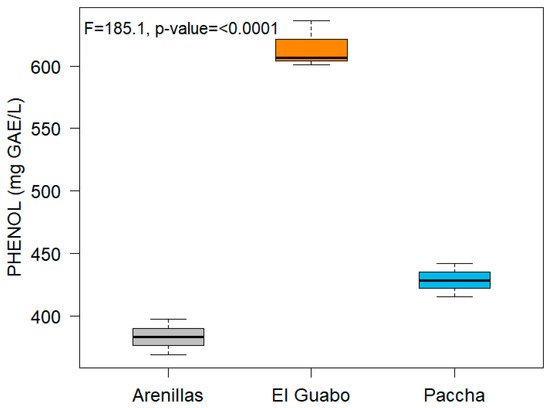
Figure 7.
Total phenol content from Plectrancthus amboinicus essential oils from three locations.
4. Discussion
EO was obtained by steam distillation of fresh samples of P. amboinicuos collected at three different sites in southern Ecuador. The average (w/w) yields were 0.036%, 0.049%, and 0.025% in Paccha, El Guabo, and Arenillas, respectively. In a study by Pino [27], P. amboinicus showed a yield of 0.55% (w/w) of EOs by steam distillation, while other publications reported yields of 0.05 and 0.078% by hydrodistillation and microwave-assisted hydrodistillation, respectively [28].
Chemical analysis by GC-MS and GC-FID identified a total of in Paccha 41 components representing 97.37% of the total of Eos, 39 compounds in El Guabo corresponding at 97.43%, and 41 compounds in Arenillas corresponding to 98.31%. The results indicated that the EOs consisted mainly by sesquiterpenes and monoterpenes hydrocarbons and the majority of chemical compounds included γ-terpinene, followed by germacrene D, α-terpinene, carvacrol, and ο-cymene. Slightly different chemical composition was reported in previous research on the same species in Magdalena, Colombia with thymol (49.7%) and p-cymene (13.2%) as the main compounds [12]. Other similar study in Colombia and India, where carvacrol (64.55% and 28.65%) and thymol (10.11% and 21.66%) followed by p-cymene (13.2% and 6.46%) and γ-terpinene (12.8% and 7.76%) [28,29]. A similar composition was determined in the Comaras Islands reported at carvacrol (23.0%), camphor (22.2%), δ-3-carene (15.0%), λ-terpinene (8.4%), o-cymene (7.7%), and α-terpinene (4.8%) as majority components [30].
Although the composition of the genus Plectranthus is not well defined, most species are composed of mono- and sesquiterpenes. For example, the EO of the species P.rugosus [31] showed a lower amount of monoterpenes among which p-cymene (3.2%), γ-terpinene (2.0%), α-terpineol (1.2%), limonene (1.0%), sesquiterpenes such as germacrene-D (9.7%), β-caryophyllene (7.6%), ar-curcumene (5.0%), (E)-β-farnesene (1.0%), humulene (0.2%), β-elemene (0.1%), and oxygenated sesquiterpenes such as α-bisabolol (41.9%), α-cadinol (3.2%), T-muurolol (2.5%), germacrene D-4-ol (0.6%), and spathulenol (0.5%) [32,33]. In contrast, compounds such as thymol (68.5%), terpinolene (5.3%), β-selinene (4.7%), β-caryophyllene (4.0%), δ-cadinol (2.1%), and ar-curcumene (1.7%) were identified in P. cylindraceus EO [34].
EO from Paccha showed poor antioxidant activity for DPPH and ABTS, with a DPPH SC50 of 1688.67 ± 1.03 µg/mL and an ABTS SC50 activity of 19.65 ± 1.02 µM. Similarly, the El Guabo EO showed zero DPPH removal with an SC50 of 1508.33 ± 1.02 µg/mL and a moderate ABTS activity SC50 of 21.03 ± 1.01 µM. Finally, the antioxidant activity reported for Arenillas EO was higher, with a DPPH SC50 of 816.57 ± 1.02 µg/mL and a positive result for ABTS activity SC50 24.15 ± 1.02 µM. Although P. amboinicus is known for its strong antioxidant activity, a study on P. amboinicus leaf extract in ethanol determined a considerably good DPPH (IC50 271.82 ± 29.23 µg/mL) [35]. In contrast, another study determined an IC50 of 500 µg/mL and a value of ABTS of IC50 750 µg/mL [36]. Furthermore, according to studies, the highest antioxidant activity was obtained in ethyl acetate (3085.8 ± 2.53 µM AAE/g extract) and acetone (3020.55 ± 0.18 µM AAE/g extract) [11]. Vijayanand et al. (2023) compared the antioxidant potential of stem and leaf extracts of P. amboinicus using methanolic extracts. They found that the methanolic stem extract showed higher antioxidant activity compared to the fresh and dried leaf extracts [37]. In another study, the antioxidant activity in EO at different doses and found a significant and stable antioxidant activity with values between 5.25% to 8.25% [38]. Our result showed that the antioxidant and free radical scavenging activities of P. amboinicus vary significantly across different locations and altitudes, which could be attributed to changes in the active components present in the plant [39,40].
Finally, the total phenolic content of the EOs was evaluated, the following values were obtained: 428.76 ± 13.27 mg GAE/L in El Guabo, 601.25 ± 14.88 mg GAE/L in Paccha, and 383.36 ± 14.10 mg GAE/L in Arenillas. Previous studies have determined a maximum concentration of 11.6 mg GAE/g dry matter using P. amboinicus leaves in different solvents such as petroleum ether, ethanol, and chloroform by cold maceration [41]. However, research by Koztowska [42] reported an average concentration of 146.77 mg GAE/g using leaves in 70% ethanol, probably this activity may be related to the compound carvacrol, one of the main compounds of our EO.
Principal Component Analysis (PCA) was used to understand the similarity between the essential oils in terms of the content of their chemical constituents [43]. The Principal Components Analysis (PCA) showed a better similarity in the composition of the EOs from El Guabo and Arenillas, which was slightly different from those from Paccha. These data may be related to volatile contributors during the flowering (pollination) and vegetative phases, the response of the species to pathogens or herbivores, as well as environmental variations [44].
This study examined the chemical composition, antioxidant capacity, and phenol content of P. amboinicus in three different sectors was reported, it provides valuable insight into how variations in temperature, harvesting time, and damage to the physical structure affect the chemical composition of the plant. This information is essential for evaluating the therapeutic properties and identifying potential alternatives that ensure a reliable supply chain, while minimizing health and environmental risks.
5. Conclusions
The comparative study of the essential oils obtained from the fresh leaves of Plectranthus amboinicus by steam distillation, showed that the chemical composition of the different EOs was slightly different depending on the sector of collection. The majority of components included germacrene D (17.60%, 14.59%, and 12.85%), α-humulene (10.88%, 4.65%, and 5.08%), α-terpinene (10.66%, 13.85%, and 6.79%) and carvacrol (5.11%, 5.97%, and 9.13%), in Pacha, Arenillas, and El Guabo, respectively. In addition, antioxidant activity analysis revealed moderate to strong ABTS+ radical scavenging activity and high DPPH values. A high content of total phenolics (>300 mg/L) was also found. The differences in the chemotype and biological activity of EOs can be attributed to environmental factors such as altitude, soil type, temperature, precipitation amount and sun exposure, its known that this factors directly influence the secondary metabolism of plants. The PCA and the subsequent analyses of the Clusters, based on the different chemical classes, represent a useful tool towards a complete taxonomic investigation, also leading to an understanding of the diversification of the genus Plectranthus.
Author Contributions
Conceptualization, S.B. and J.C.; methodology, G.A. and J.M.; formal analysis, J.C.; investigation, K.G., M.P. and W.L.; writing—original draft preparation, S.B.; writing—review and editing, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Universidad Técnica de Machala, El Oro, Ecuador.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Authors would like to thank the Universidad Técnica de Machala for funding this open access publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ashaari, N.S.; Mohamad, N.E.; Afzinizam, A.H.; Ab Rahim, M.H.; Lai, K.S.; Ong Abdullah, J. Chemical composition of hexane-extracted Plectranthus amboinicus leaf essential oil: Maximizing contents on harvested plant materials. Appl. Sci. 2021, 11, 10838. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J.; Nikabadi, S.; Varma, A. Importance of medicinal and aromatic plants in human life. In Medicinal Plants and Environmental Challenges; Springer: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Bandeira, J.; Barbosa, F.; Barbosa, L.M.; Rodrigues, I.C.; Bacarin, M.; Peters, J.; Braga, E.J. Composição do óleo essencial de quatro espécies do gênero Plectranthus. Rev. Bras. Plantas Med. 2011, 13, 157–164. [Google Scholar] [CrossRef]
- Rice, L.J.; Brits, G.J.; Potgieter, C.J.; Van Staden, J. Plectranthus: A plant for the future? S. Afr. J. Bot. 2011, 77, 947–959. [Google Scholar] [CrossRef]
- Punet Kumar, S.; Nitin, K. Plectranthus amboinicus: A review on its pharmacological and pharmacognosticalStudies. Am. J. Physiol. Biochem. Pharmacol. 2020, 10, 55–62. [Google Scholar] [CrossRef]
- Xu, Z.; Chang, L. Lamiaceae. In Identification and Control of Common Weeds; Springer: Singapore, 2017; Volume 3, pp. 181–265. [Google Scholar] [CrossRef]
- Satheesh, V.; Kaur, J.; Jarial, S.; Ghosh, P.; Sharma, K.; Patni, M.; Singh, J.; Bhadariya, V. Indian borage: A comprehensive review on the nutritional profile and diverse pharmacological significance. J. Pharm. Innov. 2022, 11, 42–51. [Google Scholar]
- Christaki, S.; Moschakis, T.; Hatzikamari, M.; Mourtzinos, I. Nanoemulsions of Oregano Essential Oil and Green Extracts: Characterization and Application in Whey Cheese. Food Control 2022, 141, 109190. [Google Scholar] [CrossRef]
- Arumugam, G.; Swamy, M.; Sinniah, U. Plectranthus amboinicus (Lour.) Spreng: Importancia botánica, fitoquímica, farmacológica y nutricional. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Murthy, P.S.; Ramalakshmi, K.; Srinivas, P. Fungitoxic activity of Indian borage (Plectranthus amboinicus) volatiles. Food Chem. 2009, 114, 1014–1018. [Google Scholar] [CrossRef]
- Bhatt, P.; Negi, P.S. Antioxidant and antibacterial activities in the leaf extracts of Indian borage (Plectranthus amboinicus). Food Sci. Nutr. 2012, 3, 146–152. [Google Scholar] [CrossRef]
- Cardona, L.F.V.; Díaz, J.C.Q. Extracción y caracterización de aceite esencial de orégano, especie Plectranthus amboinicus, a partir de cultivos orgánicos del Magdalena Medio en Colombia. Braz. J. Anim. Environ. Res. 2022, 5, 550–563. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Huang, T.-H.; Chiu, C.-S.; Lu, T.-C.; Chen, Y.-W.; Peng, W.-H.; Chen, C.-Y. Analgesic and antiinflammatory activities of the aqueous extract from Plectranthus amboinicus (Lour.) Spreng. both in vitro and in vivo. eCAM Evid.-Based Complement. Altern. Med. 2012, 2012, 508137. [Google Scholar] [CrossRef]
- Erny Sabrina, M.N.; Razali, M.; Mirfat, A.H.S.; Mohd Shukri, M.A. Antimicrobial activity and bioactive evaluation of Plectranthus amboinicus essential oil. Am. J. Res. Commun. 2014, 2, 121–127. [Google Scholar]
- Acosta, D.R.; Ramos, G.V. Obtaining a fluid extract of Plectranthus amboinicus (oregano) by the mechanical stirring method. J. Theor. Appl. Chem. 2021, 78, 41–47. [Google Scholar]
- Solfaine, R.; Muniroh, L.; Sadarman, A.; Irawan, A. Anti-inflammatory effect of Coleus amboinicus leaves extract on uric acid-induced nephrotoxicity rats. Adv. Anim. Vet. Sci. 2021, 9, 1553–1558. [Google Scholar] [CrossRef]
- El-hawary, S.S.; El-sofany, R.H.; Abdel-Monem, A.R.; Ashour, R.S.; Sleem, A.A. Polyphenol content and biological activity of the plant of Plectranthus amboinicus (Lour.) growing in Egypt (Lamiaceae). Pharmacogn. J. 2012, 4, 45–54. [Google Scholar] [CrossRef]
- Chen, Y.S.; Yu, H.M.; Shie, J.J.; Cheng, T.J.R.; Wu, C.Y.; Fang, J.M.; Wong, C.H. Chemical constituents of Plectranthus amboinicus and the synthetic analogs possessing anti-inflammatory activity. Bioorg. Med. Chem. 2014, 22, 1766–1772. [Google Scholar] [CrossRef]
- Ruan, T.Z.; Kao, C.L.; Hsieh, Y.L. Constituyentes químicos de las hojas de Plectranthus amboinicus. Chem. Nat. Comp. 2019, 55, 124–126. [Google Scholar] [CrossRef]
- Da Costa, J.G.; Pereira, C.K.; Rodrigues, F.F.; de Lima, S.G. Chemical composition, antibacterial and fungicidal activities of leaf oil of Plectranthus amboinicus (Lour.) Spreng. J. Essent. Oil Res. 2010, 22, 183–185. [Google Scholar] [CrossRef]
- Bhatt, P.; Joseph, G.S.; Negi, P.S.; Varadaraj, M.C. Chemical composition and nutraceutical potential of Indian borage (Plectranthus amboinicus) stem extract. J. Chem. 2013, 2013, 320329. [Google Scholar] [CrossRef]
- Mansour, S.A. Data Analytics Approach to Evaluate the Suitability of NASA-POWER Weather Data to Explain Changes in Crop Yields for Major Crops in Alabama. Doctoral Dissertation, Tuskegee University, Tuskegee, AL, USA, 2022. [Google Scholar]
- Calva, J.; Ludeña, C.; Bec, N.; Larroque, C.; Salinas, M.; Vidari, G.; Armijos, C. Constituents and Selective BuChE Inhibitory Activity of the Essential Oil from Hypericum aciculare Kunth. Plants 2023, 12, 2621. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORACassays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas, Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Pino, J.A.; Garcia, J.; Martinez, M.A. Comparative chemical composition of the volatiles of Coleus aromaticus produced by steam distillation, solvent extraction and supercritical carbon dioxide extraction. J. Essent. Oil Res. 1996, 8, 373–375. [Google Scholar] [CrossRef]
- León Méndez, G.; Osorio Fortich, M.D.R.; Torrenegra, M.E.; Gil González, J. Evidence-based complementary and antioxidant extraction, characterisation and activity of essential oil from Plectranthus amboinicus Lalternative medicine. Cuban J. Pharm. 2015, 49, 708–718. [Google Scholar]
- Senthilkumar, A.; Venkatesalu, V. Chemical composition and larvicidal activity of the essential oil of Plectranthus amboinicus (Lour.) Spreng against Anopheles stephensi: A malarial vector mosquito. Parasitol. Res. 2010, 107, 1275–1278. [Google Scholar] [CrossRef]
- Hassani, M.S.; Zainati, I.; Zrira, S.; Mahdi, S.; Oukessou, M. Chemical Composition and Antimicrobial Activity of Plectranthus amboinicus(Lour) Spring. Essential Oil from Archipelago of Comoros. J. Essent. Oil Bear. Plants 2012, 15, 637–644. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Kaul, V.K.; Meier, N.; Weirauch, M.; Marschall, H. Volatile constituents of Plectranthus rugosus leaf oil. Planta Med. 1983, 48, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Padalia, R.C.; Mathela, C.S. Sesquiterpene rich essential oil from Plectranthus rugosus wall. J. Essent. Oil Bear. Plants 2008, 11, 58–61. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, R.; Prakash, O.; Pant, A.K.; Kumar, M.; Isidorov, V.A.; Szczepaniak, L. Chemical composition, anti-inflammatory, analgesic, antipyretic, myorelaxant, antibacterial and antifungal activity of Rabdosia rugosus Wall. (Syn. Plectranthus rugosus Wall.). J. Med. Herbs Ethnomed. 2019, 5, 8–15. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Wurster, M.; Denkert, A.; Arnold, N.; Fadail, I.; Al-Didamony, G.; Setzer, W.N. Chemical composition, antimicrobial, antioxidant and cytotoxic activity of essential oils of Plectranthus cylindraceus and Meriandra benghalensis from Yemen. Nat. Prod. Commun. 2012, 7, 1099–1102. [Google Scholar] [CrossRef]
- Yii, I.C.Y.; Rosdi, R.A.; Wan Ishak, W.R. Proximate analysis and antioxidant activities of four popular Chinese Lamiaceae herbs in Malaysia. Food Res. 2024, 8, 218–224. [Google Scholar] [CrossRef]
- Mendonça, S.C.; Aazza, S.; Carvalho, A.A.D.; Silva, D.M.D.; Oliveira, N.D.M.S.; Pinto, J.E.B.P.; Bertolucci, S.K.V. Biological screening of herbal extracts and essential oil from Plectranthus species: α-Amylase and 5-lipoxygenase inhibition and antioxidant and anti-Candida potentials. Braz. J. Pharm. Sci. 2023, 59, e21117. [Google Scholar] [CrossRef]
- Vijayanand, S.; Sri Ram, T.; Malathi, R. Comparative study on free radical ameliorating potential of stem and the leaf extracts of Plectranthus amboinicus. J. Stress Physiol. Biochem. 2023, 19, 111–119. [Google Scholar]
- Vijayan, A.; Rachana, C.R.; Divya, M.P.; Lijimol, J.; Indu, B. A comparative study on the effectiveness of Plectranthus amboinicus dried leaf powder and essential oil extract on controlling oxidation in Vechur ghee. Pharm. Innov. J. 2023, 12, 1460–1463. [Google Scholar]
- Khalil, N.; El-Jalel, L.; Yousif, M.; Gonaid, M. Altitude impact on the chemical profile and biological activities of Satureja thymbra L. essential oil. BMC Complement. Med. Ther. 2020, 20, 186. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Patel, R.D.; Mahobia, N.K.; Singh, M.P.; Singh, A.; Sheikh, N.W.; Alam, G.; Singh, S.K. Potencial antioxidante de las hojas de Plectranthus amboinicus (Lour) Spreng. Pharm. Lett. 2010, 2, 240–245. [Google Scholar]
- Kozłowska, M.; Ścibisz, I.; Przybył, J.; Ziarno, M.; Żbikowska, A.; Majewska, E. Phenolic content and antioxidant activity of extracts from selected fresh and dried herbal materials. Pol. J. Food Nutr. Sci. 2021, 71, 269–278. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Silva, S.T.; Bertolucci, S.K.V.; da Cunha, S.H.B.; Lazzarini, L.E.S.; Tavares, M.C.; Pinto, J.E.B.P. Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 129, 501–510. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).