1. Introduction
Wasabi, a perennial plant belonging to the Brassicaceae family, has been cultivated in various countries, including Japan, Korea, China, the United States, and New Zealand. The pungent taste and unique aroma of this plant are primarily produced when glucosinolates (GSLs), mainly stored in the rhizomes, react with oxygen in the air to form volatile isothiocyanates (ITCs). These ITCs are the key components that make wasabi a highly valued spice [
1,
2].
Since 2017, the import amount of wasabi in Korea has begun to surge, reaching 1293 tons in 2023, which is a 356% increase compared to the previous year. During this period, the average annual import growth rate of wasabi was recorded at 74% [
3]. Wasabi rhizomes, which trade at 220,000 KRW per kilogram, are a high-income crop, drawing significant interest from many farmers. In its natural state, wasabi grows in cool, shaded, mountainous areas along riverbanks. To meet these specific ecological conditions, it requires maintaining a growth temperature of 8–18 °C for about two years, high levels of dissolved oxygen, and an abundant water supply [
4]. Despite the growing consumer market, replicating these natural environments involves considerable effort and expense, deterring many farmers from attempting cultivation.
Since 1997, the Cheorwon region in Gangwon-do has successfully utilized water cultivation methods, leveraging consistent spring water temperatures and flow rates. However, the limited cultivation areas and dependency on natural water resources constrain the scalability of production. Modern agricultural practices, such as smart farming, use artificial lighting to increase crop yields or enhance quality [
5,
6]. By supplementing natural daylight with artificial light, extending photoperiods, increasing photosynthetic activity, and emphasizing specific wavelengths, they contribute to improving nutritional value and taste by promoting the production of antioxidant substances [
7,
8]. Quantum dots (QDs) are an advanced lighting technology that enables tailored light spectra optimized for plant-specific absorption peaks. Recent studies demonstrate that QD lighting enhances light-use efficiency and promotes crop growth by delivering plant-specific spectral compositions [
9,
10]. This makes QD lighting a promising solution for sustainable agricultural systems, especially for crops like
Wasabia japonica. Additionally, to address the issue of rising operating costs associated with artificial plant lighting, technologies utilizing AI (Artificial Intelligence) and IoT (Internet of Things) are being developed to improve energy efficiency and maintain optimal plant growth [
11]. Similarly, intelligent lighting control technologies are being proposed to supplement DLI (Daily Light Integral) under varying light conditions, such as cloudy or rainy weather [
12].
Furthermore, new research directions are emerging as the mechanisms by which photoreceptors sense light signals and transmit them through intracellular signaling pathways to induce changes in gene expression are detailed [
13]. Photoreceptors signaling photomorphogenesis include phytochromes (PhyA~PhyE) that absorb red/far-red light, cryptochromes (Cry1 and Cry2), phototropins (Phot1 and Phot2), and the ZTL/FKF1/LKP2 complex that absorbs blue and UV-A light and UVR8 that absorbs UV-B light, all of which are well-studied in Arabidopsis [
14,
15]. These photoreceptors interact with the COP1/SPA complex, a central inhibitor of light signals in darkness, and the metabolic processes signaling photomorphogenesis under light exposure, indicating the need for precise control of additional wavelengths along with blue and red wavelengths [
16].
This study aims to determine the optimal spectral compositions for artificial light sources tailored to enhance wasabi growth and rhizome production. In contrast to previous studies focusing on common crops like lettuce and strawberries, this research is specifically tailored to W. japonica, providing essential data to maximize its rhizome production. Additionally, this study contributes to the broader understanding of light’s role in plant photoreception, offering practical applications for wasabi cultivation in smart farming systems. By exploring the potential for scaling wasabi cultivation beyond traditional water sources, this research addresses the industry’s growing demand and paves the way for sustainable wasabi farming practices.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.1.1. Plant Factory Condition
The present experiment was conducted in a fully controlled plant factory located at the Cheorwon Plasma Research Institute (CPRI). Seedlings of W. japonica (cv. ST1), a commercial variety, were sourced from Cheorwon Saemtong Wasabi (Gangwon-do, Republic of Korea). The plants were cultivated in vertical beds with dimensions of 3000 mm × 300 mm × 400 mm, arranged in three rows with two layers per bed. The beds were filled with commercial soil (BM6, Berger, QC, Canada). Each layer accommodated 30 plants with a spacing of 20 cm between each plant. Plant growth was measured 140 days after planting (DAP).
A cultivation period of 140 days was chosen in the plant factory to allow for the observation of significant morphological and physiological changes under controlled conditions. This duration was sufficient to evaluate the impact of different light treatments on plant growth without external environmental influences.
A 50 L reservoir was used for each treatment to store and supply the nutrient solution. The nutrient solution, based on a modified Yamazaki tri-leaf formula, contained the following components (in mg·L⁻1): KNO3 1131; Ca(NO3)2·4H2O 377.6; NH4H2PO4 304.0; MgSO4·7H2O 393.6; NaFe-EDTA 32.0; H3BO3 1.92; MnSO4·4H2O 1.15; ZnSO4·7H2O 0.14; CuSO4·5H2O 0.06; Na2MoO4·2H2O 0.02.
For the first week after planting, the seedlings were watered, after which the nutrient solution was provided five times daily. The electrical conductivity (EC) of the solution was maintained between 1.5 and 2.0 dS/m, and the pH was kept within a range of 5.8–6.8 by adjusting with 1 M H2SO4 and 1 M KOH. The fresh nutrient solution was added to the reservoir when the water level neared the pump at the bottom, and the solution was fully renewed every two weeks.
The temperature inside the plant factory was maintained at 18 ± 2 °C, while relative humidity (RH) was controlled at 90 ± 5% under ambient CO2 conditions.
2.1.2. Greenhouse Condition
This experiment was also conducted in a greenhouse located at Cheorwon Saemtong Wasabi (Gangwon-do, Republic of Korea). The wasabi seedlings, prepared similarly to those in the plant factory, were planted in the greenhouse with a spacing of 20 cm between each plant. Growth measurements were taken 520 days after planting (DAP).
The longer cultivation period of 520 days in the greenhouse was selected to ensure that the wasabi rhizomes reached full maturity under semi-natural conditions. This period reflects the time typically required for rhizome development in a less controlled environment. Additionally, under natural conditions without supplemental lighting, W. japonica generally requires approximately two years to fully mature due to its slow growth rate.
The soil was prepared by placing a sufficient amount of saprolite in the lower layer, where rocks were present, ensuring water could percolate effectively. On top of this layer, water cultivation was performed using the continuous irrigation of Saemtong Spring, which flows from a basalt aquifer, maintaining a water depth of approximately 10 cm.
To prevent excessive temperature increases during the summer months, a double shading curtain was installed on the ceiling, blocking incoming radiant energy. Additionally, a 50% shading net was used to provide optimal light conditions for wasabi growth, minimizing the effects of excessive sunlight exposure. A greenhouse-type water sprayer (GZ380A, YunlinLi, Guangzhou, China) was used to cool the interior and maintain RH above 90%. During winter, a multi-layer thermal curtain was employed to ensure the temperature did not fall below 8 °C.
2.2. Light Treatments and Selection
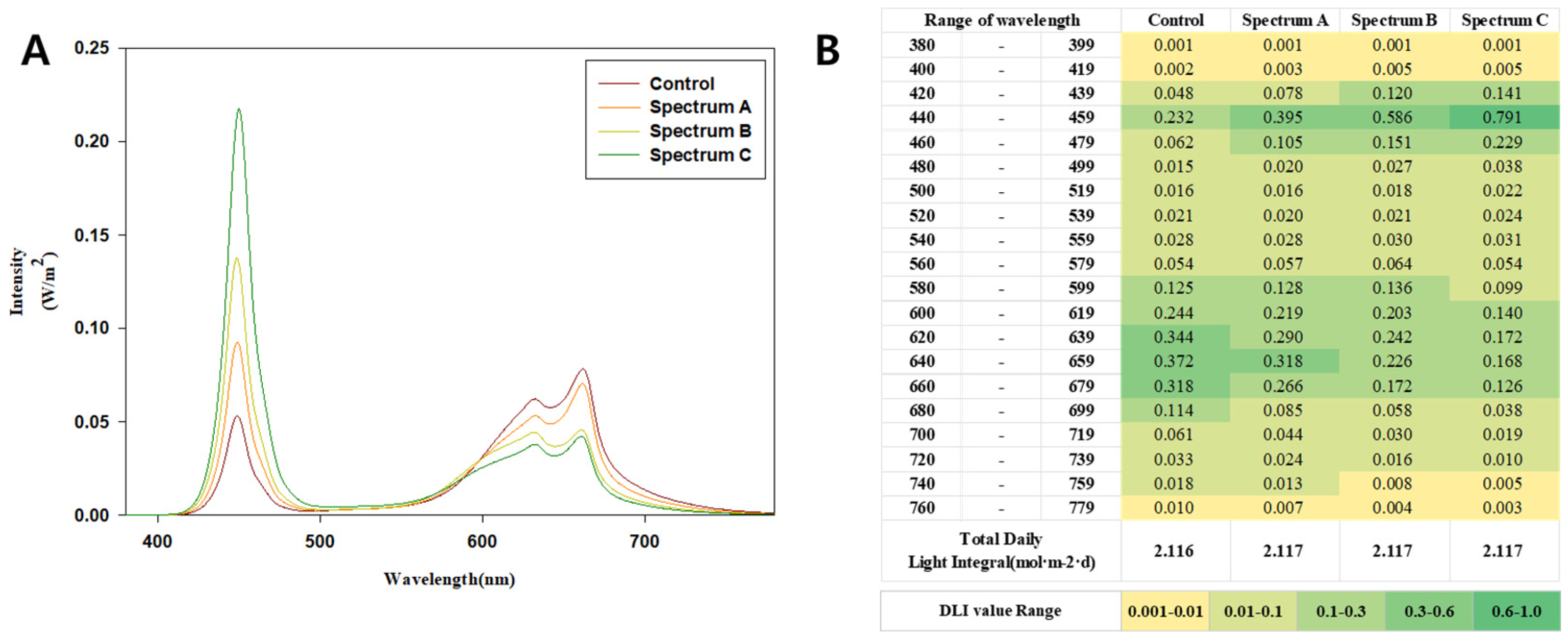
Four distinct spectral compositions were designed to assess their impact on Wasabi growth and photosynthesis. These included blue-to-red ratios corresponding to treatments labeled as Control, Spectrum A, Spectrum B, and Spectrum C. The lighting system utilized blue LED chips (450 nm) with tailored conversion lenses to adjust red light emissions, expanding the spectral range effectively for Wasabi cultivation. Quantum dots were utilized solely as a tool to tailor spectral compositions optimized for Wasabia japonica’s absorption characteristics, ensuring precise adjustments to meet the plant’s specific light requirements.
The detailed spectral properties of the light treatments are shown in
Figure 1A, where the spectral distribution curves illustrate the variations in intensity and wavelength among treatments. Additionally,
Figure 1B presents a heatmap of the daily light integral (DLI) values across the spectral range (400–800 nm) for each treatment. The DLI values indicate that the total light energy was comparable across treatments, while the distribution and balance of blue and red light varied significantly depending on the spectral ratios.
Three LED bars were uniformly mounted on top of each layer of the plant beds. The distance between the lights and the plant canopy was adjusted to maintain a photosynthetic photon flux density (PPFD) of 50 ± 5 μmol·m−2·s⁻1 over the wasabi canopy. The PPFD was measured using a quantum sensor (PG-200N, UPRtek, Miaoli, Taiwan). The photoperiod was controlled using timers, achieving a daily light integral (DLI) of 2.16 ± 0.04 mol·m−2·day⁻1. DLI calculations were performed by multiplying the PPFD (in μmol·m−2·s⁻1) by the total photoperiod (in seconds), and the results were expressed in mol·m2·day⁻1.
2.3. Growth Measurements
2.3.1. Plant Morphological Parameters
Growth measurements were taken from three randomly selected plants per treatment every two weeks from 70 DAP (Days After Planting). To minimize bias from repeated measurements, different plants were selected at each time point. At the end of the experiment, three separate plants were harvested for final growth parameter analyses. Each growth parameter was measured as follows: Plant length was measured from the stem base to the tip of the tallest leaf using a ruler. Petiole length was recorded as the average of the three longest petioles measured with a ruler, while petiole width was calculated as the average of their widest parts measured with a vernier caliper. The number of leaves included all fully formed leaves on the plant, excluding fallen or senescent ones. Leaf length and width were determined by measuring the longest and widest parts of the largest leaf using a ruler. Fresh rhizome weight was measured immediately after harvesting and dry weight was recorded after drying the rhizomes in an oven at 60 °C for 72 h. At the time of harvest, all measurements followed the same protocols to ensure consistency across growth stages and treatments.
2.3.2. Leaf Gas Exchange Analysis
A portable photosynthesis system (GFS-3000, WALZ Inc., Effeltrich, Germany) was used to measure light response curves and gas exchange characteristics, such as photosynthetic rate (A, μmol·m⁻2·s⁻1), stomatal conductance (GH2O, mmol·m⁻2·s⁻1), transpiration rate (E, mmol·m⁻2·s⁻1), and intercellular CO2 concentration (Ci, ppm). Water use efficiency (WUE) was calculated as the ratio of the net photosynthetic rate (A) to the transpiration rate (E), providing a measure of how efficiently plants utilize water during photosynthesis. The average vapor pressure deficit (VPD) in the leaf chamber was maintained at 2.8 Pa/kPa, and measurements were taken when Ci reached a stable value.
2.4. Chlorophyll Absorption Spectrum Analysis
Chloroplasts were extracted from wasabi leaves using the MinuteTM Chloroplast Isolation Kit (Invent Biotechnologies, Inc., Plymouth, MN, USA). Two grams of fresh leaves were processed according to the manufacturer’s instructions to isolate chloroplasts. The absorption spectrum of the extracted chloroplasts was analyzed using a UV–vis spectrophotometer (Mega-800, Scinco Inc., Seoul, Republic of Korea), measuring absorbance in the range of 380 to 780 nm. The obtained data were used to analyze the absorption characteristics of chloroplast pigments.
2.5. Experimental Design and Statistical Analysis
The experiment employed two distinct cultivation systems: a controlled plant factory and a greenhouse. The plant factory provided a highly controlled environment where temperature, relative humidity, and light conditions could be precisely regulated. This setup allowed for an accurate assessment of the effects of tailored spectral lighting on the growth of W. japonica without interference from external environmental factors. Conversely, the greenhouse experiment was designed to evaluate how the optimized light conditions from the plant factory would influence plant growth and rhizome production in a more natural environment.
In the greenhouse, supplemental lighting was provided for 4 h daily (6:00–8:00 AM and 6:00–8:00 PM) to complement natural light. In contrast, lighting conditions in the plant factory were strictly controlled throughout the cultivation period. The use of these two environments was intended to assess both the theoretical benefits of tailored spectral lighting in a fully controlled system and its practical applications in semi-natural agricultural conditions.
The experiment was designed following a Randomized Complete Block Design (RCBD) with three replications. Growth parameters were measured at regular intervals from three plants in each treatment group. Before conducting statistical tests, the data were checked for normality using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. The Shapiro–Wilk test was used to assess whether the data followed a normal distribution, with p-values greater than 0.05 indicating that the data did not deviate significantly from normality. The Levene’s test was applied to test for equal variances between groups, with p-values greater than 0.05 indicating homogeneity of variance.
For data that satisfied both normality and equal variance assumptions, a t-test was used to compare treatment groups. In cases where normality was satisfied but variances were unequal, Welch’s t-test was applied. If normality was not satisfied, a Mann–Whitney U test was performed to analyze the differences between treatments. Statistical significance was determined at p < 0.05, and all analyses were performed using SPSS software (version 23.0; IBM, Chicago, IL, USA).
3. Results
3.1. Impacts of Spectral Lighting on Morphological Characteristics of Indoor Wasabi
The morphological characteristics of Wasabi plants varied significantly among different lighting conditions, as shown in
Table 1.
Spectrum C resulted in the greatest plant length and petiole length, both of which were significantly higher than those of the other treatments. Spectrum A followed, with plant length and petiole length values of 31.6 cm and 25.1 cm, respectively, which were significantly greater than those of the control. On the other hand, Spectrum B showed the lowest plant length and petiole length, significantly lower than all other treatments.
The number of leaves was significantly higher under Spectrum B, which was not significantly different from Spectrum C or Spectrum A but significantly greater than the control. Leaf dimensions (length and width) were significantly larger under Spectrum C compared to all other treatments. Spectrum A also produced larger leaves (13.8 cm in length and 13.3 cm in width) compared to the control and Spectrum B.
3.2. Impacts of Spectral Lighting on Leaf Gas Exchange Analysis of Indoor Wasabi
The effects of different spectral lighting treatments on the photosynthetic characteristics and water/CO
2 use efficiency of Wasabi are summarized in
Table 2.
The photosynthetic rate (Pn) was highest under Spectrum C, which was significantly higher than all other treatments. Spectrum A followed (2.07 μmol·m⁻2·s⁻1), which was significantly higher than both the control and Spectrum B (1.99 μmol·m⁻2·s⁻1).
Stomatal conductance was highest under Spectrum A, which was significantly greater than all other treatments. Spectrum C (79.6 mmol·m⁻2·s⁻1) and Spectrum B (80.9 mmol·m⁻2·s⁻1) also showed higher stomatal conductance compared to the control.
The transpiration rate was significantly higher under Spectrum A, while Spectrum C and Spectrum B showed moderate transpiration rates (0.31 and 0.29 μmol H2O·m⁻2·s⁻1, respectively). The control group had the lowest transpiration rate.
Water use efficiency (WUE) was highest in the control, followed by Spectrum C (7.21), both of which were significantly greater than Spectrum A (5.50) and Spectrum B (6.87). The intercellular to ambient CO2 concentration ratio (Ci/Ca) was highest in Spectrum A and Spectrum B, followed by Spectrum C (0.85), while the control had the lowest ratio.
3.3. Effect of Supplemental Lighting on Morphological Characteristics of Greenhouse-Grown Wasabi
Growth characteristics observed after 520 days of planting indicated that supplemental lighting significantly affected petiole width but did not influence other above-ground parts. The petiole width under supplemental lighting was significantly greater compared to the control, averaging 25.9 mm compared to 22.3 mm in control plants (
Table 3).
During the early growth period (100–300 DAP), plants receiving supplemental lighting exhibited larger leaves, with significantly greater leaf length and width compared to the control group. This effect is evident in
Figure 2, suggesting that supplemental lighting positively influenced vegetative development in the early stages. However, as the plants matured, these differences diminished. Afterward, due to seasonal factors (monsoon and high temperatures), it decreased in both the control and treatment groups and then stabilized at a certain level.
The number of leaves and leaf length showed no significant differences between treatments, aligning with the table results that also demonstrated non-significant differences in leaf length between the two groups. The impact of supplemental lighting became particularly evident in the rhizome characteristics. The optimized spectral composition of supplemental lighting increased the average size of rhizomes, with a statistically significant increase in rhizome width. (
Table 3). Despite the high variability in this trait, supplemental lighting profoundly enhanced rhizome biomass accumulation. In fact, the rhizomes of plants receiving supplemental lighting had 152% and 206% greater fresh and dry weights, respectively, than those of the control group. These results underscore the positive impact of supplemental lighting on rhizome development, as also visually depicted in
Figure 3.
3.4. Absorption Spectrum of Wasabi Chloroplast
The absorption spectrum of chloroplasts extracted from wasabi was measured using a spectrophotometer, as shown in
Figure 4.
The chloroplasts of wasabi exhibited the highest absorption rate in the blue light region (381–480 nm), accounting for approximately 57.3% of the total absorption. There were three peaks in the blue light region, with the highest absorption peak at 433 nm, followed by peaks at 414 nm and 455 nm. After 455 nm, the absorption decreased sharply, and in the green light region (481–580 nm), the average absorption was about 25% of that at 433 nm, with an absorption ratio of approximately 19.4%. In the red light region (581–680 nm), the absorption increased again, peaking at 664 nm and reaching about 50% of the level at 433 nm. The absorption ratio in the red light region was about 20%. Meanwhile, the absorption ratio in the infrared region was 3.3%.
4. Discussion
The results of this study clearly indicate that the tailored spectral composition significantly influences the growth and rhizome production of W. japonica. Several key findings emerged from the comparative analysis between different spectral lighting treatments, both in controlled plant factory environments and greenhouses.
4.1. Enhanced Morphological Growth with Tailored Spectral Lighting
Morphological measurements showed that supplemental lighting with optimized spectral composition, particularly treatment Spectrum C, significantly improved plant length, petiole length, and leaf dimensions compared to the control. The observed growth enhancement likely results from the tailored spectral composition, which aligns with wasabi’s photoreceptor absorption peaks. While past studies have primarily focused on photosynthetic pigments such as chlorophyll a and b and carotenoids, this study aimed to explore the potential influence of newly identified photoreceptive pigments. However, the lack of quantitative data on these photoreceptors necessitates further investigation.
Optimizing the red and blue wavelength ratios is critical for maximizing photosynthetic efficiency in wasabi, as shown in previous research [
9,
17]. The expanded red spectrum in the tailored spectral composition promotes greater biomass accumulation and rhizome production, aligning well with wasabi’s absorption needs. Unlike traditional lighting systems, the tailored spectral composition provides a broader spectral range (630 to 680 nm), closely matching wasabi’s absorption spectrum (see
Figure 4). This broader spectrum likely enhances light utilization, supporting improved growth outcomes.
Consistent with prior findings, the importance of red and blue wavelengths for chlorophyll absorption and photosynthetic efficiency is well-documented [
7,
14]. These results highlight the potential of tailored spectral lighting as an effective solution for controlled environment agriculture, providing optimal growth conditions for wasabi while enhancing sustainability and productivity
4.2. Photosynthetic Efficiency and Gas Exchange Parameters
The photosynthetic rate and other gas exchange parameters exhibited significant variations under different lighting treatments. The control group showed relatively low stomatal conductance, intercellular CO
2 concentration, and transpiration rate but exhibited higher water use efficiency compared to Spectrum A, B, and C treatments. However, the control group also demonstrated lower values across all morphological traits, including the number of leaves and leaf length (
Table 1). This suggests that while the control achieved relatively high water use efficiency, the utilization of assimilates produced by photosynthesis may have been less effective. Therefore, optimizing the light spectrum to balance photosynthetic efficiency and assimilate utilization is critical.
Spectrum A showed the highest stomatal conductance and intercellular CO
2 concentration, but its high transpiration rate resulted in low water use efficiency. The tailored spectral composition of Spectrum A likely induced guard cell activity through specific photoreceptor pathways, optimizing stomatal opening [
18].
Spectrum C exhibited the highest photosynthetic rate and relatively high water use efficiency, indicating its potential to enhance photosynthetic efficiency while maintaining effective stomatal regulation. Additionally, supplemental lighting with Spectrum C significantly influenced key morphological traits of leaves, such as leaf area and petiole length (see
Figure 2). These changes may be attributed to the physiological roles of photoreceptors, including phytochromes and cryptochromes, which are known to regulate leaf expansion and stomatal responses under specific light wavelengths [
14]. By promoting better stomatal function and morphological development, Spectrum C optimizes the overall photosynthetic capacity of wasabi.
Considering the photosynthetic efficiency and gas exchange parameters, Spectrum C appears to provide the most optimal conditions among the tested treatments. This tailored spectral lighting not only enhances photosynthesis but also supports balanced water use efficiency and morphological development.
4.3. Rhizome Production and Biomass Accumulation
One of the most significant outcomes of this study was the substantial increase in rhizome fresh and dry weights under Spectrum C lighting in the greenhouse. The fresh weight of rhizomes under Spectrum C reached 75.6 g, significantly higher than the 30.0 g observed under natural light conditions (
Table 3). This improvement can be attributed to the tailored spectral composition, which likely enhanced photoassimilate production and allocation to rhizomes.
As shown in
Figure 2, earlier attainment of optimal leaf traits under supplemental lighting treatments likely influenced the dynamics of photoassimilate allocation. Earlier leaf development enhanced light interception and photosynthetic efficiency during critical early growth stages, establishing a stable vegetative structure earlier. This stabilization allows the plant to redirect energy and carbon resources to rhizome growth for a longer period, maximizing biomass accumulation in the storage organ.
This observation aligns with studies showing that optimized source-sink dynamics facilitate carbon allocation to storage organs such as rhizomes [
19,
20]. Supplemental lighting with tailored red and blue wavelengths, as provided by Spectrum C, is known to enhance photosynthetic efficiency [
21] and drive higher photoassimilate production [
22]. These photoassimilates are then preferentially allocated to rhizomes during later growth stages, as indicated by the significant increases in rhizome biomass under Spectrum C lighting in this study. These results highlight the importance of tailored light spectra in enhancing both vegetative and reproductive growth, reducing reliance on natural environmental conditions, and providing a sustainable approach to wasabi cultivation in controlled environments.
4.4. Implications for Smart Farming and Controlled Environment Agriculture
The findings of this study highlight the potential of tailored spectral lighting as an integral component of smart farming systems and controlled environment agriculture. By precisely adjusting the spectral composition to meet the specific needs of crops like W. japonica, tailored lighting can enhance growth, optimize resource use efficiency, and reduce environmental impacts.
For example, the results presented in
Table 1 and
Table 2 demonstrate that tailored spectral lighting significantly improved morphological traits, photosynthetic efficiency, and water use efficiency compared to natural light or general control conditions. These findings suggest that integrating tailored spectral lighting with AI and IoT technologies could enable dynamic adjustment of lighting conditions based on real-time environmental and crop physiological data. Such systems can provide precise control over light intensity, spectral composition, and photoperiod to optimize growth conditions for different developmental stages.
Moreover, the ability to design specific light spectra, such as Spectrum C in this study, aligns well with the absorption properties of crop photoreceptors. This approach not only maximizes photosynthetic efficiency but also supports sustainable cultivation practices by reducing energy consumption and water use.
Future implementations of smart farming systems could incorporate tailored spectral lighting as part of a comprehensive cultivation strategy. By leveraging AI algorithms to monitor and analyze plant responses, farmers can automate decision-making processes, ensuring optimal lighting conditions while minimizing resource inputs. This combination of tailored spectral lighting and advanced technologies offers a pathway to achieving both high productivity and sustainability in controlled environment agriculture.
4.5. Future Research Directions
While this study highlights the benefits of tailored spectral lighting for W. japonica cultivation, several areas warrant further investigation to maximize its potential applications.
First, integrating tailored spectral lighting with advanced technologies, such as AI and IoT, requires further exploration. Developing real-time monitoring systems and AI algorithms to predict optimal light conditions based on environmental and physiological data will be critical for implementing dynamic lighting strategies in controlled environments.
Second, the economic feasibility and environmental impact of tailored spectral lighting systems should be evaluated. Long-term studies assessing energy consumption, cost-effectiveness, and reductions in water use and greenhouse gas emissions will provide valuable insights for sustainable adoption in commercial settings.
Third, the long-term physiological and molecular effects of tailored spectral lighting on crops remain to be explored. Future research should investigate its impact on the quality and composition of secondary metabolites, nutritional profiles, and flavor-related compounds. Additionally, understanding how specific wavelengths interact with molecular pathways to regulate plant growth and development can help refine spectral designs for optimal outcomes.
Finally, further studies on the scalability and adaptability of tailored spectral lighting systems across different crop species and cultivation conditions will expand its utility in controlled environment agriculture. These efforts will pave the way for next-generation farming systems that combine precision, efficiency, and sustainability.
5. Conclusions
This study highlights the significant impact of tailored spectral lighting on the growth, photosynthetic efficiency, and rhizome production of W. japonica under controlled environment conditions. The results demonstrated that Spectrum C lighting, with its optimized spectral composition, enhanced morphological traits, improved photosynthetic efficiency, and significantly increased rhizome biomass compared to other treatments. These findings underline the importance of designing light spectra that align with the specific photoreceptor absorption characteristics of crops.
Tailored spectral lighting has shown potential as a transformative tool for smart farming and controlled environment agriculture. By optimizing light composition, it is possible to improve resource use efficiency, reduce environmental impacts, and achieve consistent crop yields. Integrating this technology with AI and IoT systems can further enhance precision and adaptability, offering a sustainable approach to modern agriculture.
Future research should focus on scaling tailored spectral lighting systems for commercial use, evaluating their economic feasibility, and exploring their long-term physiological effects on crops. These efforts will contribute to developing next-generation cultivation systems that combine productivity, sustainability, and precision.