Effect of Branch-Bagged Shading on the Photosynthetic Physiology of Sweet Cherry Leaves in a Greenhouse Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation and Processing
2.2. Photosynthetic Physiological Indicators
2.3. Chl Concentration
2.4. Antioxidative Enzymes
2.5. Leaf Starch Content
2.6. Leaf-Soluble Sugar Content
2.7. Statistical Analysis
3. Results
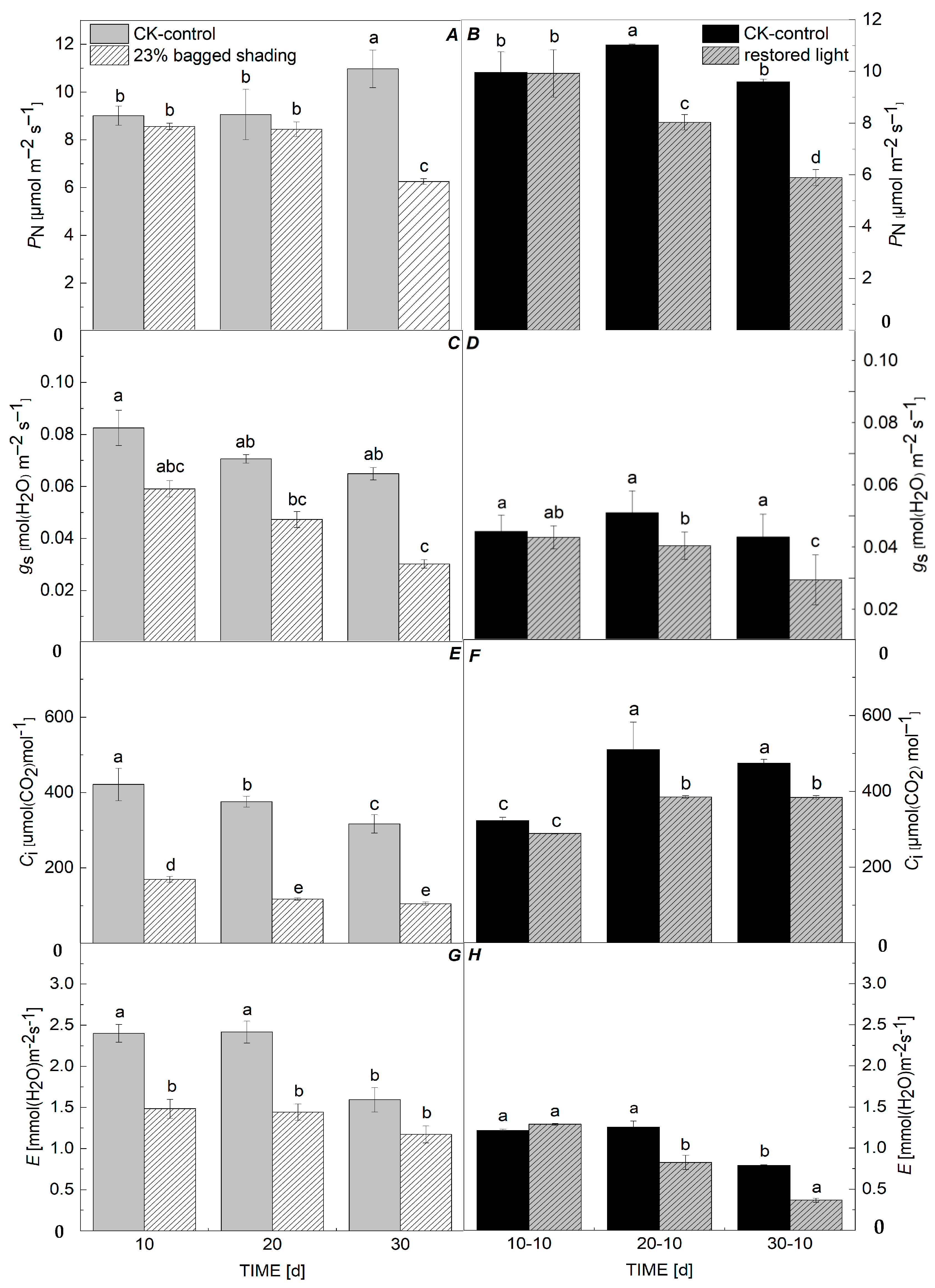
3.1. Photosynthetic Physiological Indicators
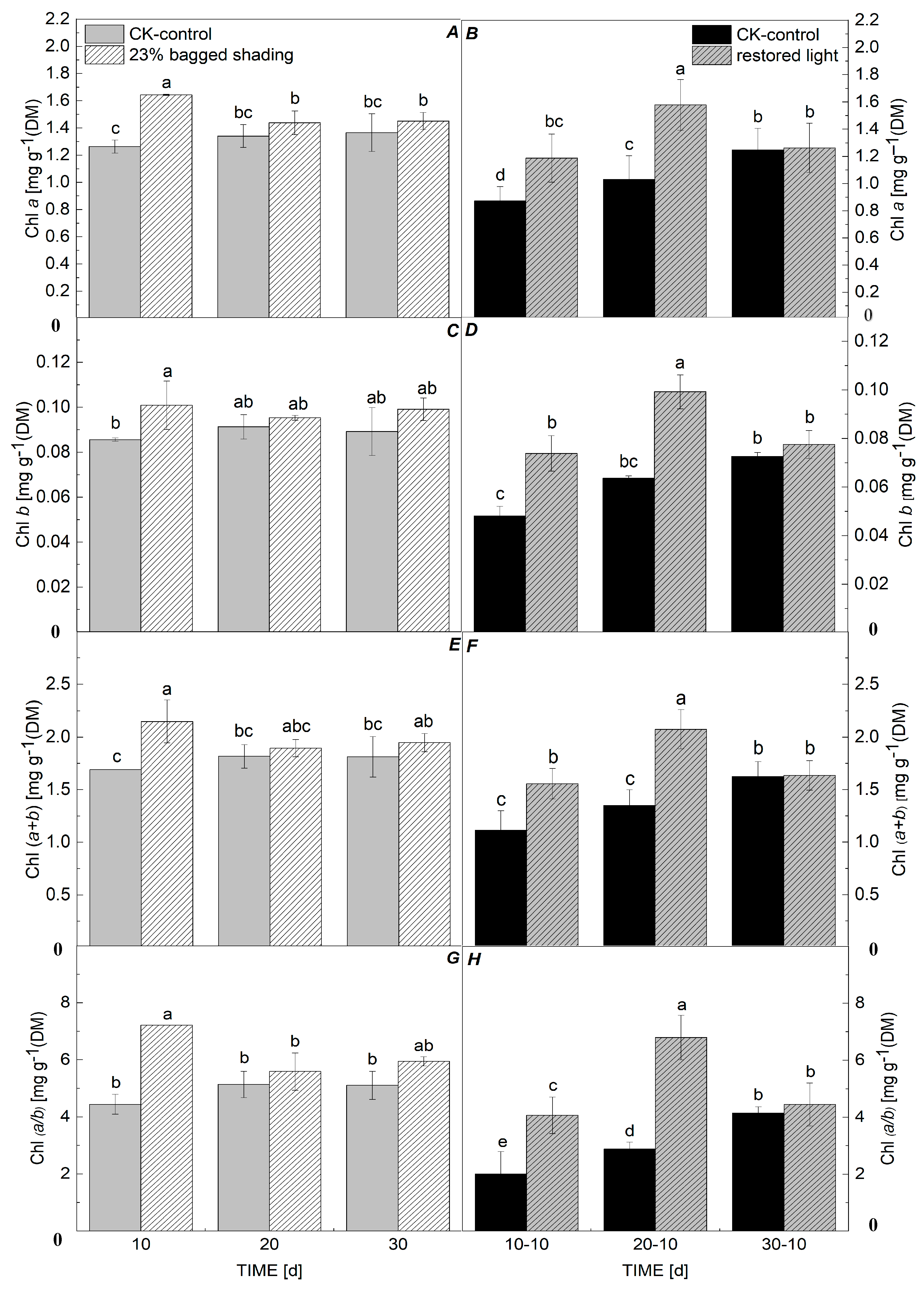
3.2. Chl Concentration
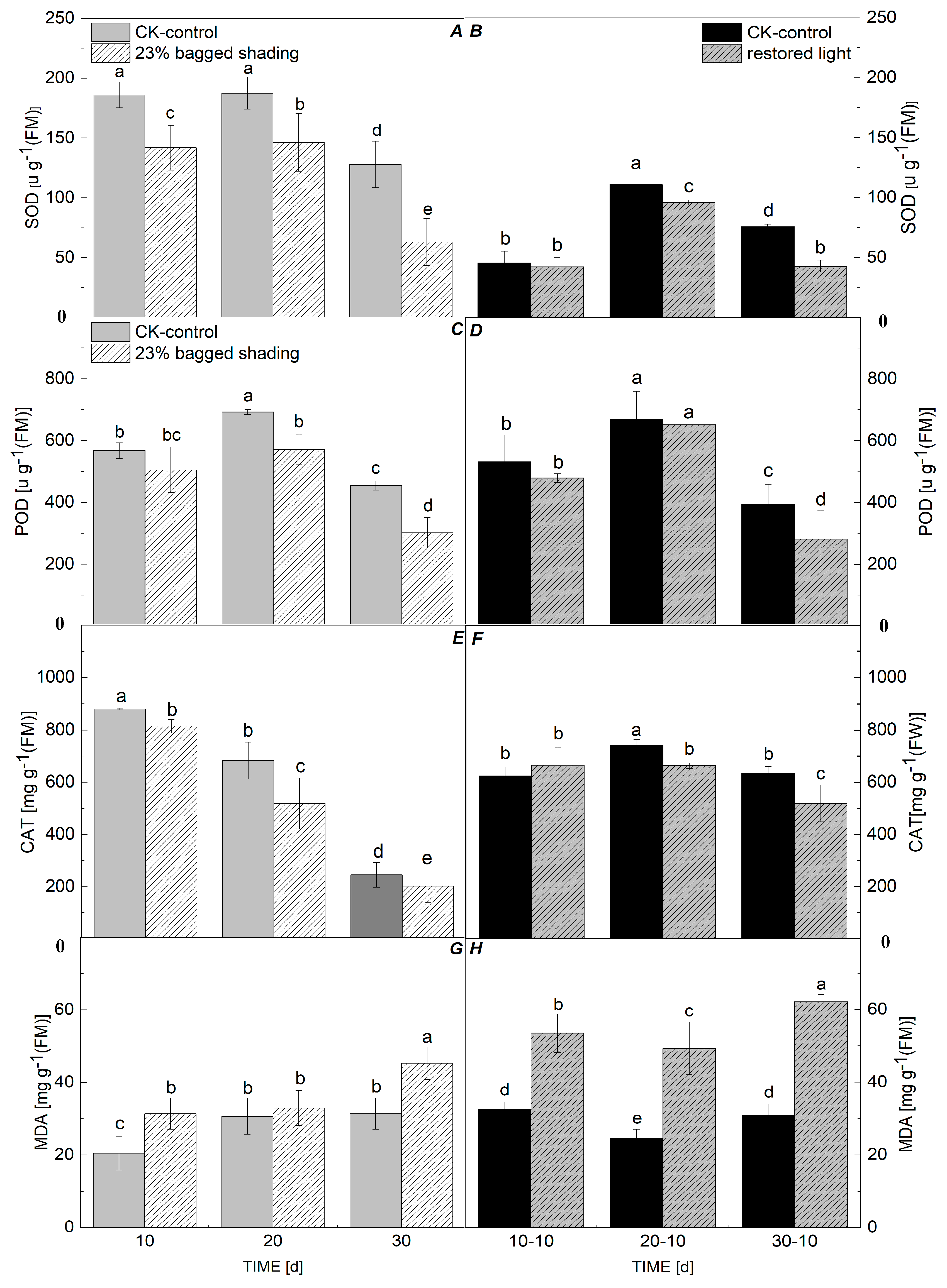
3.3. Enzyme Activity
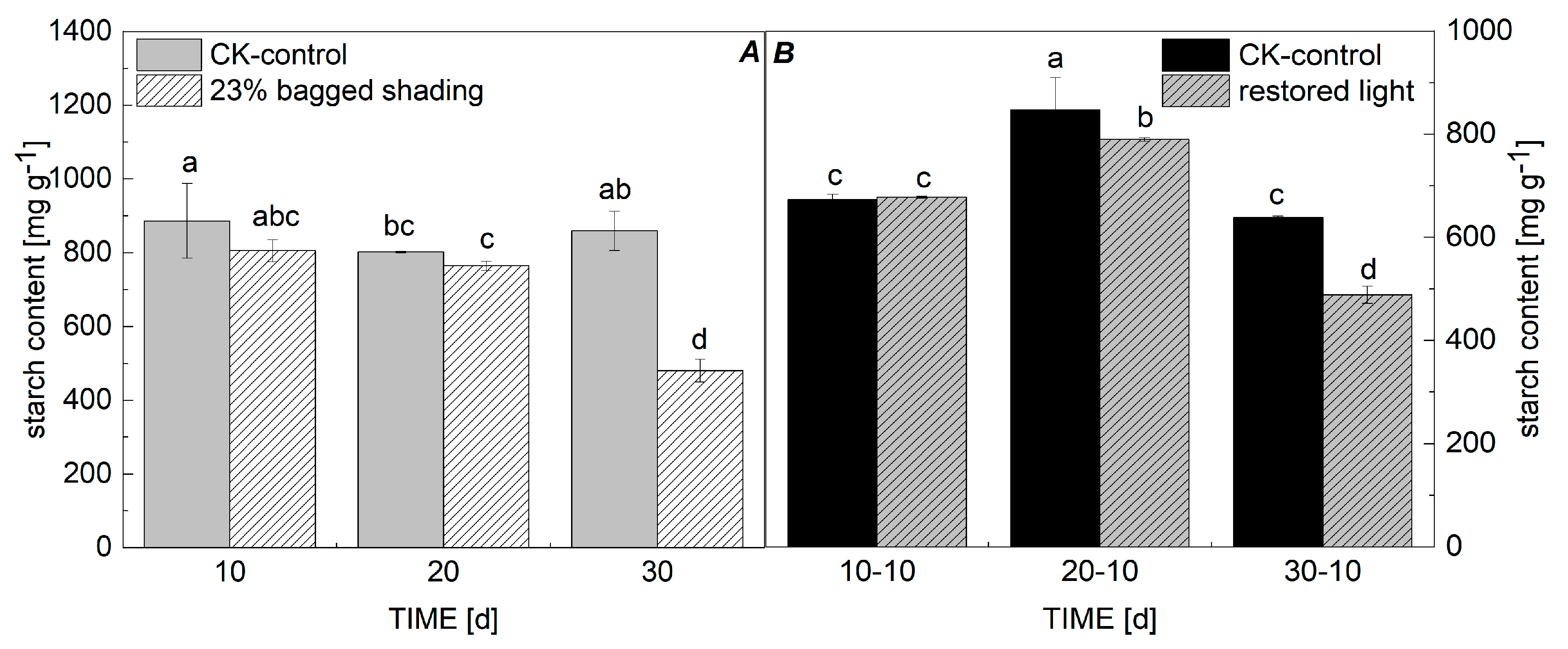
3.4. Starch Content
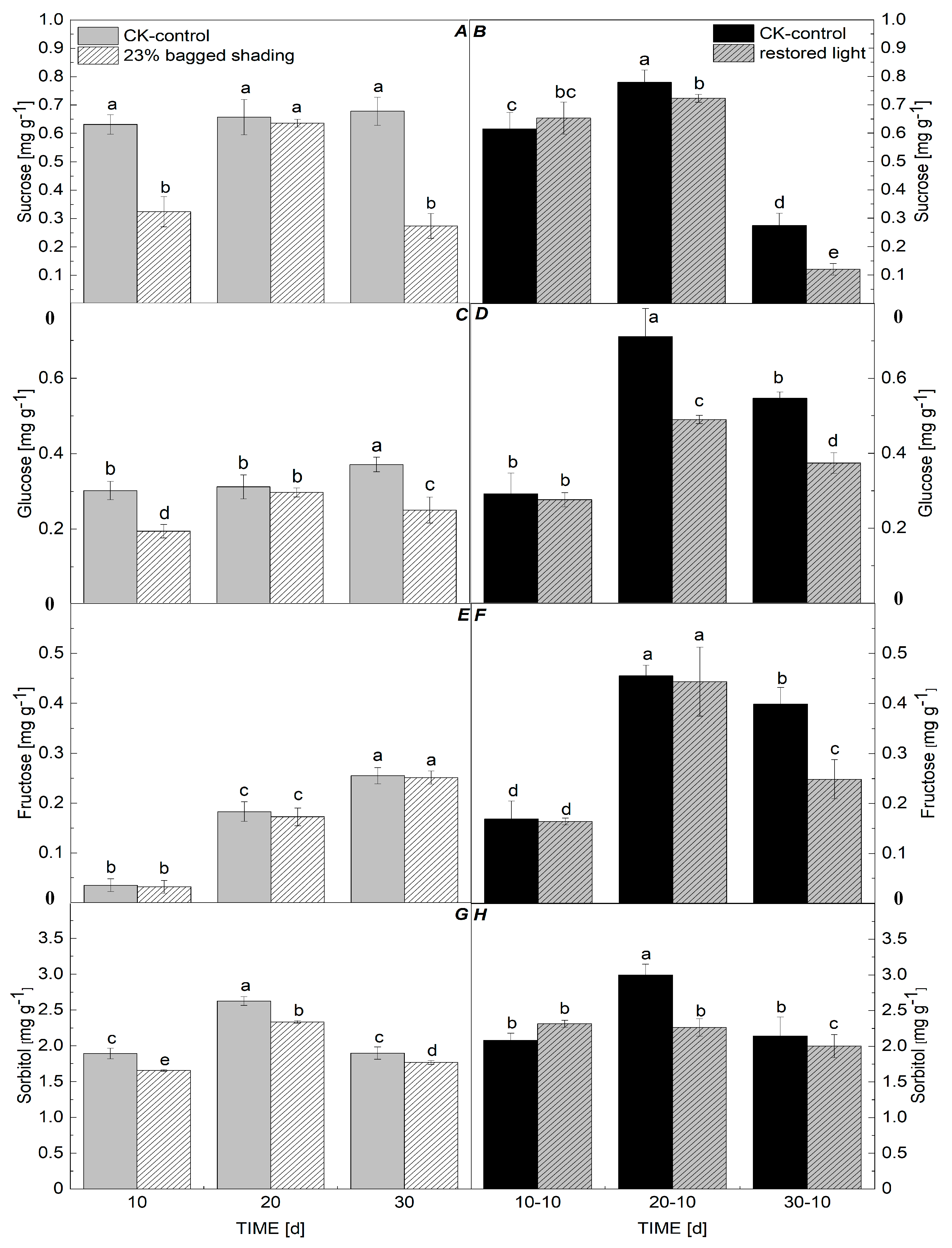
3.5. Sucrose, Glucose, Fructose, and Sorbitol Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| Ci | Intercellular CO2 concentration |
| CAT | Catalase |
| Chl | Chlorophyll |
| Chl a(b) | Chlorophyll a(b) |
| Chl (a + b)(a/b) | Chlorophyll (a + b)(a/b) |
| CK | Control |
| D | Day |
| E | Transpiration rate |
| gs | Stomatal conductance |
| LSD | Least significant difference |
| MDA | Malondialdehyde |
| POD | Peroxidase |
| PN | Net photosynthetic rate |
| SOD | Superoxide dismutase |
| SD | Stomatal density |
References
- Ai, J.Y.; Zhou, C.H.; He, M.L.; Zhang, Q.J. Research progress in sweet cherry photosynthesis. J. Fruit Sci. 2023, 40, 1235–1244. [Google Scholar] [CrossRef]
- Tang, W.J.; Chen, C.Q.; Zhang, Y.; Chu, Y.Q.; Yang, W.L.; Cui, Y.L.; Kou, G.Q.; Chen, H.X.; Song, H.Y.; Gong, R.G. Effect of low-light stress on sugar and acid accumulation during Fruit Development and Ripening of Sweet Cherry. Hortic 2023, 9, 654. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.Q.; Luvisi, A.; De Bellis, L.G.; Aprile, A.; Chen, F.X. Influence of Bagging on the Development and Quality of Fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Salvadores, Y.; Bastías, R.M. Environmental factors and physiological responses of sweet cherry production under protective cover systems: A review. Chil. J. Agric Res. 2023, 83, 484–498. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, W.; Wang, Z.B.; Ma, L.; Guo, Y.P.; Ren, X.L.; Mei, L.X. Influence of shading on photosynthesis and antioxidative activities of enzymes in apple trees. Photosynthetica 2019, 57, 6. [Google Scholar] [CrossRef]
- Xiang, X.W.; Song, K.K.; Li, Y.Y.; Zhang, C.Y.; Zhou, R.Q.; Feng, Y.; You, J.G.; Wu, J.D.; Zhang, Y.H.; Jiang, C.C.; et al. Screening and Expression Analysis of POD Gene in POD-H2O2 Pathway on Bud Dormancy of Pear (Pyrus pyrifolia). Forests 2024, 15, 434. [Google Scholar] [CrossRef]
- Yao, Y.C.; Wang, S.H.; Kong, Y. Changes in the structure and photosynthetic characteristics of peach leaves and chloroplast ultrastructure under low light conditions. Chin. Agric. Sci. 2007, 40, 9. (In Chinese) [Google Scholar] [CrossRef]
- Tian, T.; Qiao, G.; Wen, Z.; Deng, B.; Qiu, Z.L.; Hong, Y.; Wen, X.P. Comparative transcriptome analysis reveals the molecular regulation underlying the adaptive mechanism of cherry (Cerasus pseudocerasus Lindl.) to shelter covering. BMC Plant Biol. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.D.; Wu, L.K.; Zhan, J.C. Effect of weak light on the peroxidation of membrane-lipid of cherry leaves. J. Integr. Plant Biol. 2002, 44, 920. [Google Scholar] [CrossRef]
- Huang, W.D.; Wu, L.K.; Zhan, J.C. Adaptive regulation of leaf growth and photosynthesis of Chinese dwarf cherry to low light environment. Chin. Agric. Sci. 2004, 12, 1981–1985. [Google Scholar] [CrossRef]
- Xu, C.; Wang, M.T.; Yang, Z.Q.; Zheng, Q.T. Low temperature and low irradiation induced irreversible damage of strawberry seedlings. Photosynthetica 2020, 58. [Google Scholar] [CrossRef]
- Thorne, J.H.; Koller, H.R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974, 54, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, S.; Li, X.L.; Luo, X.; Deng, Q.X. Shading inhibits sugar accumulation in leaf and fruit of jujube (Ziziphus jujuba Mill.). Hortic 2022, 8, 592. [Google Scholar] [CrossRef]
- Dayer, S.; Murcia, G.; Prieto, J.A.; Durán, M.; Martínez, L.; Píccoli, P.; Peña, J.P. Non-structural carbohydrates and sugar export in grapevine leaves exposed to different light regimes. Physiol. Plant. 2020, 171, 728–738. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Chen, M.X.; Chan, W.L.; Yang, F.; Tian, Y.; Song, T.; Xie, L.J.; Zhou, Y.; Xiao, S.; Zhang, J.H.; et al. SWATH-MS quantitative proteomic investigation of nitrogen starvation in Arabidopsis reveals new aspects of plant nitrogen stress responses. J. Proteom. 2018, 187, 161–170. [Google Scholar] [CrossRef]
- Wang, Y.N.; Liang, C.Z.; Meng, Z.G.; Li, Y.Y.; AliAbid, M.H.; Askari, M.; Wang, P.L.; Wang, Y.; Sun, G.Q.; Cai, Y.P.; et al. Leveraging Atriplex hortensis choline monooxygenase to improve chilling tolerance in cotton. Environ. Exp. Bot. 2019, 162, 364–373. [Google Scholar] [CrossRef]
- Li, B.B.; Ding, Y.; Tang, X.L.; Wang, G.Y.; Wu, S.J.; Li, X.X.; Qu, T.T.; Chen, J.F.; Tang, X.M. Effect of L-Arginine on Maintaining Storage Quality of the White Button Mushroom (Agaricus bisporus). Food Bioprocess Technol. 2019, 12, 563–574. [Google Scholar] [CrossRef]
- Chen, G.L.; Jia, Z.M.; Wang, L.P.; Hu, T.Z. Effect of acute exposure of saxitoxin on development of zebrafish embryos (Danio rerio). Environ. R 2020, 185, 109432. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Mei, H.; Chen, M.; Qin, S.; Li, L.; Zhang, W.; Chen, T. Protective effect of agmatine against hyperoxia-induced acute lung injury via regulating lncRNA gadd7. Biochem. Biophys. Res. Commun. 2019, 516, 68–74. [Google Scholar] [CrossRef]
- Chen, M.X.; Zhu, F.Y.; Wang, F.Z.; Ye, N.H.; Gao, B.; Chen, X.; Zhao, S.S.; Fan, T.; Cao, Y.Y.; Liu, T.Y.; et al. Alternative splicing and translation play important roles in hypoxic germination in rice. J. Exp. Bot. 2019, 70, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Li, T.H.; Li, S.H. Leaf responses of micropropagated apple plants to water stress: Nonstructural carbohydrate composition and regulatory role of metabolic enzymes. Tree Physiol. 2005, 25, 495. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.S.O.; Junior, L.F.G.O.; Gonzaga, M.I.S.; Sena, E.D.O.A.; dos Santos Maciel, L.B.; Matheus, P.F.; Mattos, E.C.D.; Carnelossi, M.A.G. Effects of calcium particle films and natural shading on ecophysiological parameters of conilon coffee. Sci. Hortic. 2019, 245, 171–177. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Zhao, B.; Liu, P.; Zhang, J.W. Physiological and comparative proteomic analysis provides new insights into theeffects of shade stress in maize (Zea mays L.). BMC Plant Biol. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.Z.; Cui, H.Y.; Camberato, J.J.; Dong, S.; Liu, P.; Zhao, B.; Zhang, J.W. Effects of shading on the photosyntheticcharacteristics and mesophyll cell ultrastructure of summer maize. Sci.Nat. 2016, 103, 67. [Google Scholar] [CrossRef] [PubMed]
- Borel, C.; Frey, A.; Marion-Poll, A.; Tardieu, F.; Simonneau, T. Does engineering abscisic acid biosynthesis in Nicotiana plumbaginifolia modify stomatal response to drought? Plant Cell Environ. 2010, 24, 477–489. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.J.; Anand, A.; Mandal, P.K.; Bhatt, R.K. Irradiance influences contents of photosynthetic pigments and proteins in tropical grasses and legumes. Photosynthetica 2005, 43, 47–53. [Google Scholar] [CrossRef]
- Wang, Y.B.; Huang, R.D.; Zhou, Y.F. Effects of shading stress during the reproductive stages on photosynthetic physiology andyield characteristics of peanut (Arachis hypogaea Linn). Integr. Agric. 2021, 20, 1250–1265. [Google Scholar] [CrossRef]
- Field, K.J.; George, R.; Feam, B.; Quick, W.P.; Davey, M.P. Best of both worlds:simultaneous high-light and shade tolerance adaptations within individual leaves of the living stone Lithops acuampiae. PLoS ONE 2013, 8, e75671. [Google Scholar] [CrossRef]
- Cornah, J.E.; Terry, M.J.; Smith, A.G. Green or red: What stops the traffic in the tetrapyrole pathway. Trends Plant Sci. 2003, 8, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ. Exp. Botany 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Yao, X.D.; Zhou, H.L.; Zhu, Q.; Li, C.H.; Zhang, H.J.; Wu, J.J.; Xie, F.T. Photosynthetic response of soybean leaf to wide light-fluctuation in maize-soybean intercropping system. Front. Plant Sci. 2017, 8, 1695. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Wu, Y.Y.; Shen, Z.L. Effects of shading on leaf physiology and morphology in the ‘Yinhong’ grape plants. Rev. Bras. De Frutic. 2018, 40, 37. [Google Scholar] [CrossRef]
- Wolske, E.T.; Branham, B.E. Growth and productivity of ‘consort’ black currant grown under varying levels of artificial shade. Hortsci 2021, 56, 3–7. [Google Scholar] [CrossRef]
- Pan, J.W.; Sharif, R.; Xu, X.W.; Chen, X.H. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Wang, X.L.; Shang, Q.M. Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci. Hortic. Amst. 2011, 129, 629–636. [Google Scholar] [CrossRef]
- Yu, X.B.; An, J.G.; Liang, J.Q.; Yang, W.Y.; Zeng, Z.Q.; Feng, J.W.; Hai, Y.; Zhang, M.R. Effects of shading and re illumination on photosynthetic performance and carbon metabolism of soybean leaves. Southwest Agric. J. 2024, 37, 1471–1479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, J.; Wu, M.; Cai, F.; He, M.; Chen, Y.; Zhang, Q. Effect of Branch-Bagged Shading on the Photosynthetic Physiology of Sweet Cherry Leaves in a Greenhouse Environment. Horticulturae 2025, 11, 136. https://doi.org/10.3390/horticulturae11020136
Ai J, Wu M, Cai F, He M, Chen Y, Zhang Q. Effect of Branch-Bagged Shading on the Photosynthetic Physiology of Sweet Cherry Leaves in a Greenhouse Environment. Horticulturae. 2025; 11(2):136. https://doi.org/10.3390/horticulturae11020136
Chicago/Turabian StyleAi, Jiayin, Min Wu, Feng Cai, Mingli He, Yao Chen, and Qijing Zhang. 2025. "Effect of Branch-Bagged Shading on the Photosynthetic Physiology of Sweet Cherry Leaves in a Greenhouse Environment" Horticulturae 11, no. 2: 136. https://doi.org/10.3390/horticulturae11020136
APA StyleAi, J., Wu, M., Cai, F., He, M., Chen, Y., & Zhang, Q. (2025). Effect of Branch-Bagged Shading on the Photosynthetic Physiology of Sweet Cherry Leaves in a Greenhouse Environment. Horticulturae, 11(2), 136. https://doi.org/10.3390/horticulturae11020136



