Plant Probiotic Potential of Native Rhizobia to Enhance Growth and Sugar Content in Agave tequilana Weber var. Blue
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Functional Analysis and Gene Annotation of PPB Traits in Native Rhizobial Strains
2.3. Phenotypic Characterization of Rhizobial Strains
2.4. Assessment of Plant Probiotic Traits in Rhizobial Strains
2.4.1. Phosphate Solubilization
2.4.2. Siderophore Production
2.4.3. Indole Acetic Acid (IAA) Production
2.4.4. Cellulose Production
2.4.5. Cellulase Activity
2.5. Assessment of Biofilm Formation Ability
2.6. Root Colonization Capacity Assay
2.7. Plant Inoculation Assay in Agave tequilana
3. Results
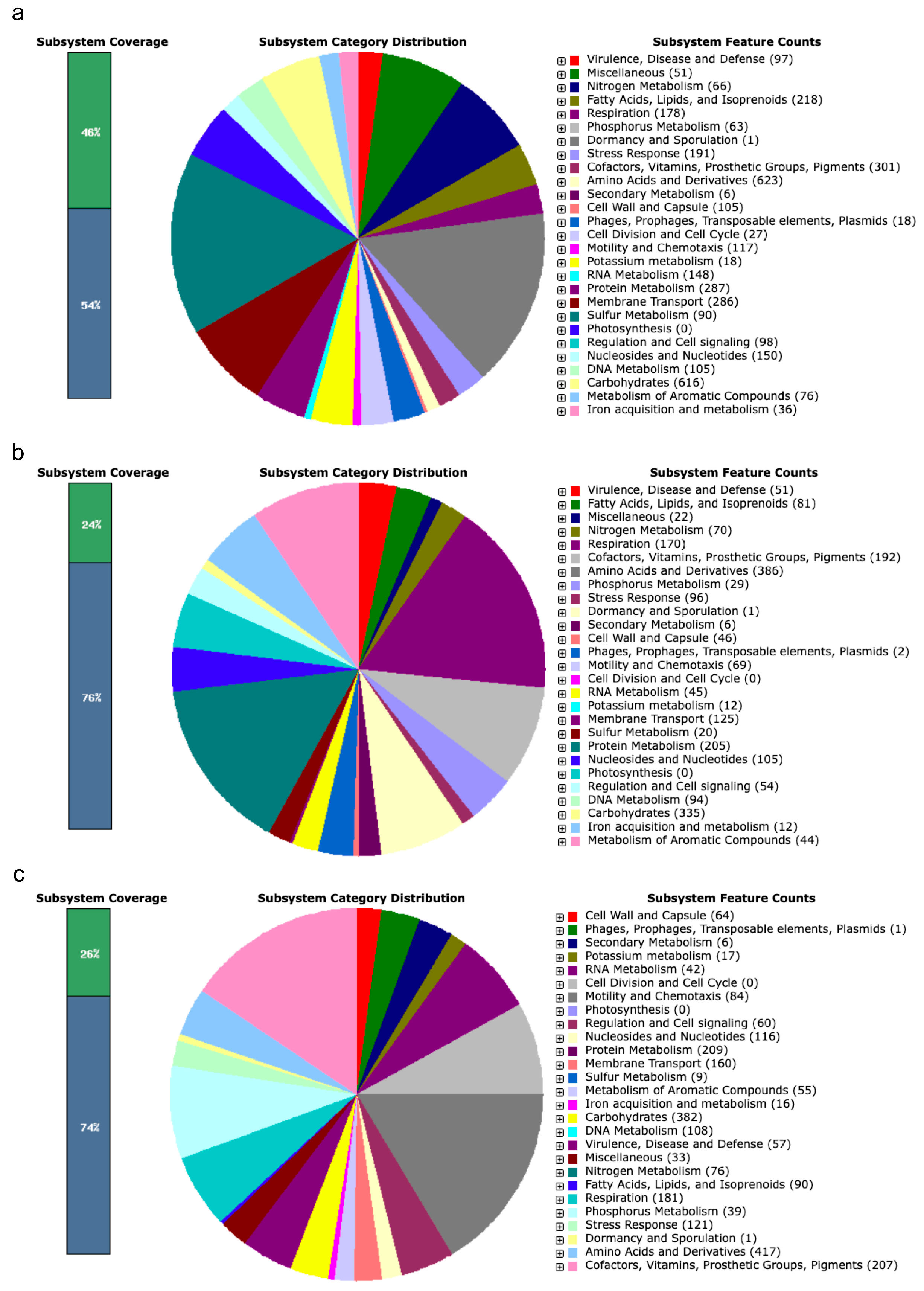
3.1. Biosynthetic Gene Clusters in Native Rhizobial Strains with PGP Potential
3.2. Phenotypic, Genomic, and Tolerance Characteristics of Rhizobial Strains
3.3. Plant Probiotic (PPB) Potential of Native Rhizobial Strains
3.4. Biofilm Production by Native Rhizobial Strains
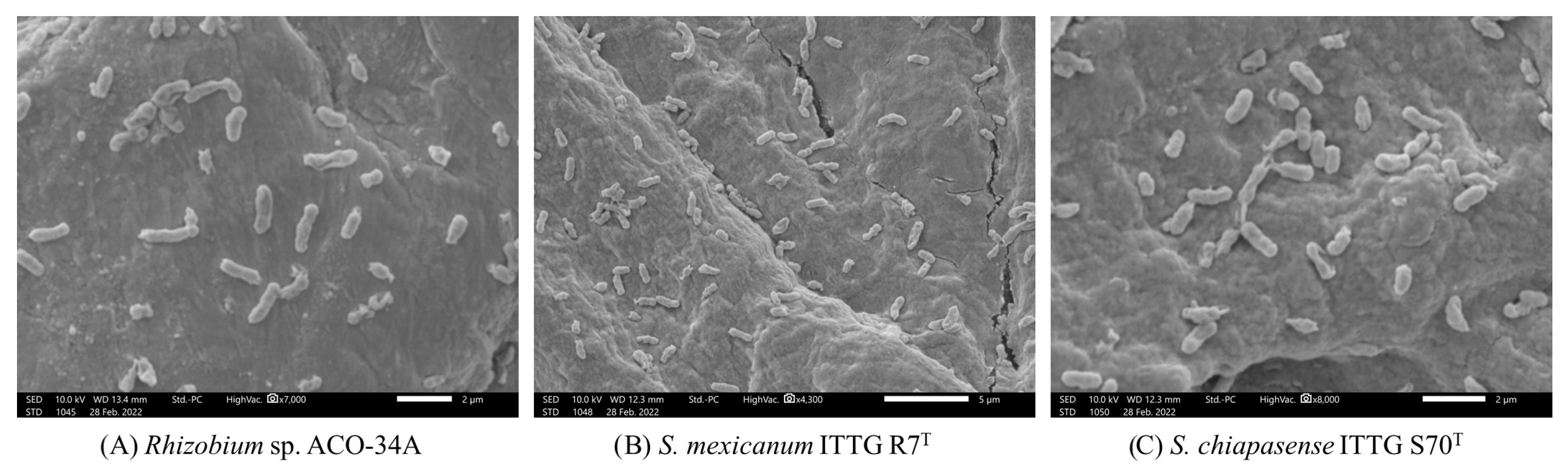
3.5. Colonization Capacity of Native Rhizobial Strains
3.6. Growth Parameters of Agave tequilana Inoculated with Plant Probiotic Bacteria
3.7. Effect of Biofertilization on Sugar Content in Agave tequilana Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tetreault, D.; McCulligh, C.; Lucio, C. Extractive Agave and Tequila Production in Jalisco, Mexico. In Agrarian Extractivism in Latin America, 1st ed.; Routledge: New York, NY, USA, 2021; p. 21. ISBN 9780367822958. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The prebiotic potential of inulin-type fructans: A systematic review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Venema, K.; Tabernero, M.; Sarriá, B.; Bravo, L.; Mateos, R. Bioconversion of polyphenols and organic acids by gut microbiota of predigested Hibiscus sabdariffa L. calyces and Agave (A. tequilana Weber) fructans assessed in a dynamic in vitro model (TIM-2) of the human colon. Food Res. Int. 2021, 143, 110301. [Google Scholar] [CrossRef]
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular Structures of Fructans from Agave tequilana Weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef]
- Tsafrakidou, P.; Michaelidou, A.-M.; Biliaderis, C.G. Fermented Cereal-based Products: Nutritional Aspects, Possible Impact on Gut Microbiota and Health Implications. Foods 2020, 9, 734. [Google Scholar] [CrossRef]
- Ruiz Rivera, J.A.; Ramírez Matheus, A.O. Yogurt Making by Using Probiotics (Bifidobacterium spp. and Lactobacillus acidophilus) and Inulin. Rev. Fac. Agron. 2009, 26, 223–242. [Google Scholar]
- Longoria-García, S.; Cruz-Hernández, M.A.; Flores-Verástegui, M.I.M.; Contreras-Esquivel, J.C.; Montañez-Sáenz, J.C.; Belmares-Cerda, R.E. Potential functional bakery products as delivery systems for prebiotics and probiotics health enhancers. J. Food Sci. Technol. 2018, 55, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Mojica, E.; López, M.G. Fructan metabolism in A. tequilana Weber Blue variety along its developmental cycle in the field. J Agric Food Chem. 2012, 60, 11704–11713. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Mahanty, T.; Bhattacharjee, S.; Goswami, M.; Bhattacharjee, S.; Das, B.; Ghosh, A.; Tribedi, P. Biofertilizers: A Potential Approach for Sustainable Agriculture Development. Environ. Sci. Pollut. Res. 2017, 24, 3315–3335. [Google Scholar] [CrossRef] [PubMed]
- Menendez, E.; Garcia-Fraile, P. Plant Probiotic Bacteria: Solutions to Feed the World. AIMS Microbiol. 2017, 3, 502–524. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, A.; Celador-Lera, L.; Fradejas-Bayón, M.; Rivas, R. Plant probiotic bacteria enhance the quality of fruit and horticultural crops. AIMS Microbiol. 2017, 3, 483. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- Pathania, P.; Rajta, A.; Singh, P.C.; Bhatia, R. Role of Plant Growth-Promoting Bacteria in Sustainable Agriculture. Biocatal. Agric. Biotechnol. 2020, 30, 101842. [Google Scholar] [CrossRef]
- Chaudhary, P.; Singh, S.; Chaudhary, A.; Sharma, A.; Kumar, G. Overview of Biofertilizers in Crop Production and Stress Management for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 930340. [Google Scholar] [CrossRef] [PubMed]
- Flores-Félix, J.D.; Silva, L.R.; Rivera, L.P.; Marcos-García, M.; García-Fraile, P.; Martínez-Molina, E.; Mateos, P.F.; Velázquez, E.; Andrade, P.; Rivas, R. Plants Probiotics as a Tool to Produce Highly Functional Fruits: The Case of Phyllobacterium and Vitamin C in Strawberries. PLoS ONE 2015, 10, e0122281. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Azevedo, J.; Pereira, M.J.; Carro, L.; Velazquez, E.; Peix, A.; Mateos, P.F.; Rivas, R. Inoculation of the Non-Legume Capsicum annuum (L.) with Rhizobium Strains. 1. Effect on Bioactive Compounds, Antioxidant Activity, and Fruit Ripeness. J. Agric. Food Chem. 2014, 62, 557–564. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Velázquez, E.; Martínez-Molina, E.; González-Andrés, F.; Squartini, A.; Rivas, R. Connecting the lab and the field: Genome analysis of Phyllobacterium and Rhizobium strains and field performance on two vegetable crops. Agronomy 2021, 11, 1124. [Google Scholar] [CrossRef]
- Jiménez-Gómez, A.; Flores-Félix, J.D.; García-Fraile, P.; Mateos, P.F.; Menéndez, E.; Velázquez, E.; Rivas, R. Probiotic activities of Rhizobium laguerreae on growth and quality of spinach. Sci. Rep. 2018, 8, 295. [Google Scholar] [CrossRef]
- Naserzadeh, Y.; Nafchi, A.M.; Mahmoudi, N.; Nejad, D.K.; Gadzhikurbanov, A.S. Effect of combined use of fertilizer and plant growth stimulating bacteria Rhizobium, Azospirillum, Azotobacter and Pseudomonas on the quality and components of corn forage in Iran. RUDN J. Agron. Anim. Ind. 2019, 14, 209–224. [Google Scholar] [CrossRef]
- Gen-Jiménez, A.; Flores-Félix, J.D.; Rincón-Molina, C.I.; Manzano-Gomez, L.A.; Rogel, M.A.; Ruíz-Valdiviezo, V.M.; Rincón-Molina, F.A.; Rincón-Rosales, R. Enhance of Tomato Production and Induction of Changes on the Organic Profile Mediated by Rhizobium Biofortification. Front. Microbiol. 2023, 14, 1235930. [Google Scholar] [CrossRef] [PubMed]
- Flores-Núñez, V.M.; Camarena-Pozos, D.A.; Chávez-González, J.D.; Alcalde-Vázquez, R.; Vázquez-Sánchez, M.N.; Hernández-Melgar, A.G.; Xool-Tamayo, J.; Moreno-Ulloa, A.; Martínez, L.P.P. Synthetic Communities Increase Microbial Diversity and Productivity of Agave tequilana Plants in the Field. Phytobiomes J. 2023, 7, 435–448. [Google Scholar] [CrossRef]
- Guardado-Fierros, B.G.; Tuesta-Popolizio, D.A.; Lorenzo-Santiago, M.A.; Rubio-Cortés, R.; Camacho-Ruíz, R.M.; Castañeda-Nava, J.J.; Gutiérrez-Mora, A.; Contreras-Ramos, S.M. PGPB Consortium Formulation to Increase Fermentable Sugar in Agave tequilana Weber var. Blue: A Study in the Field. Plants 2024, 13, 1371. [Google Scholar] [CrossRef]
- Ruíz-Valdiviezo, V.M.; Rogel-Hernández, M.A.; Guerrero, G.; Rincón-Molina, C.I.; García-Pérez, L.G.; Gutiérrez-Miceli, F.A.; Rincón-Rosales, R. Complete Genome Sequence of a Novel Nonnodulating Rhizobium Species Isolated from Agave americana L. Rhizosphere. Genome Announc. 2017, 5, e01280-17. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Lloret, L.; Ponce, E.; Martínez-Romero, E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov.; which shares common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol. Ecol. 2009, 67, 103–117. [Google Scholar] [CrossRef]
- Gomila, M.; Peña, A.; Mulet, M.; Lalucat, J.; García-Valdés, E. Phylogenomics and systematics in Pseudomonas. Front. Microbiol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Arkin, A.P.; Cottingham, R.W.; Henry, C.S.; Harris, N.L.; Stevens, R.L.; Maslov, S.; Dehal, P.; Ware, D.; Perez, F.; Canon, S.; et al. KBase: The United States Department of Energy Systems Biology Knowledgebase. Nat. Biotechnol. 2018, 36, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela Ruiz, V.; Santoyo, G.; Gómez Godínez, L.J.; Cira Chávez, L.A.; Parra Cota, F.I.; de los Santos Villalobos, S. Complete Genome Sequencing of Bacillus cabrialesii TE3T: A Plant Growth-Promoting and Biological Control Agent Isolated from Wheat (Triticum turgidum subsp. durum) in the Yaqui Valley. Curr. Res. Microb. Sci. 2023, 4, 100193. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Rosales, R.; Rogel, M.A.; Guerrero, G.; Rincón-Molina, C.I.; López-López, A.; Manzano-Gómez, L.A.; Ruíz-Valdiviezo, V.M.; Martínez-Romero, E. Genomic Data of Acaciella Nodule Ensifer mexicanus ITTG R7T. Microbiol. Resour. Announc. 2021, 10, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, O.O.; Adekanmbi, A.; Dianda, M.; Fagade, O.E. Metal-Tolerating ability of some selected Rhizobia strains. J. Eng. Res. 2020, 25, 1–5. [Google Scholar]
- Liu, Z.; Li, Y.C.; Zhang, S.; Fu, Y.; Fan, X.; Patel, J.S.; Zhang, M. Characterization of Phosphate-Solubilizing Bacteria Isolated from Calcareous Soils. Appl. Soil Ecol. 2015, 96, 217–224. [Google Scholar] [CrossRef]
- O’Halloran, I.P.; Cade-Menun, B.J. Total and organic phosphorus. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 265–291. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, T.P.; Kotasthane, A.S.; Kosharia, A.; Kushwah, R.; Zaidi, N.W.; Singh, U.S. Crop-specific plant growth-promoting effects of ACC deaminase enzyme, siderophore production, and cyanogenic fluorescent Pseudomonas. 3 Biotech 2017, 7, 27. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstonet, S.E. Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Robledo, M.; Rivera, L.; Jiménez-Zurdo, J.I.; Rivas, R.; Dazzo, F.; Velázquez, E.; Mateos, P.F.; Martínez-Molina, E.; Hirsch, A.M. Role of Rhizobium Endoglucanase CelC2 in Cellulose Biosynthesis and Biofilm Formation on Plant Roots and Abiotic Surfaces. Microb. Cell Fact. 2012, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Hankin, L.; Anagnostakis, S.L. Solid Media Containing Carboxymethylcellulose to Detect CX Cellulase Activity of Microorganisms. J. Gen. Microbiol. 1977, 98, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Flores-Félix, J.D. Caracterización Molecular y Funcional de Biofertilizantes Bacterianos, y Análisis de su Potencial para Mejorar la Producción de Cultivos de Maíz, Guisante, Lechuga, Fresa y Zanahoria. Universidad de Salamanca. 2018. Available online: https://gredos.usal.es/handle/10366/139502 (accessed on 27 January 2025).
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Chin-A-Woeng, T.F.; de Priester, W.; van der Bij, A.J.; Lugtenberg, B.J. Description of the colonization of a gnotobiotic tomato rhizosphere by Pseudomonas fluorescens biocontrol strain WCS365, using scanning electron microscopy. Mol. Plant Microbe Interact. 1997, 10, 79–86. [Google Scholar] [CrossRef]
- Knights, H.E.; Jorrin, B.; Haskett, T.L.; Poole, P.S. Deciphering Bacterial Mechanisms of Root Colonization. Environ. Microbiol. Rep. 2021, 13, 428–444. [Google Scholar] [CrossRef]
- De La Torre-Ruiz, N.; Ruiz-Valdiviezo, V.M.; Rincón-Molina, C.I.; Rodríguez-Mendiola, M.; Arias-Castro, C.; Gutiérrez-Miceli, F.A.; Palomeque-Dominguez, H.; Rincón-Rosales, R. Effect of plant growth-promoting bacteria on the growth and fructan production of Agave americana L. Braz. J. Microbiol. 2016, 47, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- Maurya, R.; Verma, S.; Bahadur, I. Advances in the Application of Plant Growth-Promoting Rhizobacteria in Horticulture. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability; Kumar, A., Meena, V., Eds.; Springer: Singapore, 2019; pp. 67–76. [Google Scholar] [CrossRef]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and Phosphate Solubilizing Bacteria: Keys for Sustainable Agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Datta, B.; Chakrabartty, P.K. Siderophore Biosynthesis Genes of Rhizobium sp. Isolated from Cicer arietinum L. 3 Biotech 2014, 4, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Erlacher, A.; Ramadan, E.M.; El-Arabi, T.F.; Müller, H.; Bragina, A.; Berg, G. Comparisons of Diazotrophic Communities in Native and Agricultural Desert Ecosystems Reveal Plants as Important Drivers in Diversity. FEMS Microbiol. Ecol. 2016, 92, fiv166. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, E.M.; AbdelHafez, A.A.; Hassan, E.A.; Saber, F.M. Plant Growth Promoting Rhizobacteria and Their Potential for Biocontrol of Phytopathogens. Afr. J. Microbiol. Res. 2016, 10, 486–504. [Google Scholar] [CrossRef]
- Lugtenberg, B. Principles of Plant-Microbe Interactions. In Microbes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Kang, J.P.; Huo, Y.; Kim, Y.; Ahn, J.C.; Hurh, J.; Yang, D.; Kim, J.J. Rhizobium panacihumi sp. nov.; an Isolate from Ginseng-Cultivated Soil, as a Potential Plant Growth-Promoting Bacterium. Arch. Microbiol. 2019, 201, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The Biofilm Matrix: Multitasking in a Shared Space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Uc-Chuc, M.A.; Loyola-Vargas, V.M.; Santiago-García, P.A.; López, M.G. Fructosyltransferases in Plants: Structure, Function, and Application: A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100343. [Google Scholar] [CrossRef]

| Characteristic | Rhizobium sp. ACO-34A | S. mexicanum ITTG R7T | S. chiapasense ITTG S70T |
|---|---|---|---|
| Origin | Agave americana (Mexico) | Acaciella angustissima (Mexico) | Acaciella angustissima (Mexico) |
| Cell morphology | Rods (0.4 × 1.1 μm) | Rods (0.7 × 1.2 μm) | Rods (0.6 × 1.4 μm) |
| Flagella | Peritrichous | peritrichous | peritrichous |
| DNA G+C content (mol%) | 61.1 | 62.0 | 61.8 |
| pH range for growth | 5.0–8.0 | 5.0–8.0 | 5.0–8.0 |
| Growth at/in: | |||
| 37 °C | (−) | (−) | (−) |
| 1% NaCl | (+) | (+) | (+) |
| 2% NaCl | (+) | (+) | (+) |
| 5% NaCl | (−) | (−) | (−) |
| Antibiotic resistance | |||
| (mg mL−1): | |||
| Netilmicin (10) | (−) | (+) | (+) |
| Penicillin (10) | (+) | (+) | (+) |
| Chloramphenicol (30) | (+) | (+) | (+) |
| Gentamicin (10) | (+) | (−) | (−) |
| Ciprofloxacin (5) | (+) | (+) | (+) |
| Cefalexin (30) | (−) | (−) | (−) |
| Amikacin (30) | (−) | (+) | (−) |
| Ampicillin (10) | (+) | (+) | (+) |
| Tolerance to heavy metals (μM): | |||
| Al3+ (500 μM) | (+) | (+) | (+) |
| Cu2+ (100 μM) | (+) | (+) | (+) |
| Zn2+ (100 μM) | (−) | (+) | (+) |
| Pb2+ (100 μM) | (−) | (−) | (−) |
| Strain | P-Solubilization | Siderophore Production | IAA Production (mg L−1) | |||
|---|---|---|---|---|---|---|
| PSI ¥ | Ca3(PO4)2 (mg L−1) | CaHPO4 (mg L−1) | SID ≠ | % Siderophore | ||
| Rhizobium sp. ACO 34A | 1.17 ± (0.11) * | 34.8 ± (0.65) | 28.4 ± (0.76) | 1.22 ± (0.06) | 32.4 ± (0.84) | 22.5 ± (0.65) |
| Sinorhizobium mexicanum ITTG R7 | 2.12 ± (0.18) | 51.4 ± (1.39) | 32.5 ± (0.93) | 1.51 ± (0.08) | 50.4 ± (0.83) | 20.7 ± (0.57) |
| Sinorhizobium chiapasense ITTG S70 | 2.24 ± (0.21) | 58.2 ± (1.27) | 28.2 ± (0.87) | 1.61 ± (0.07) | 58.5 ± (0.92) | 20.8 ± (0.66) |
| Treatments | Total Height (cm) | Total Fresh Weight (g) | Piña Weight (g) | Number of Leaves | Chlorophyll (mg/g FW) |
|---|---|---|---|---|---|
| ACO-34A | 77.20 (±8.79) *A | 1416.80 (±181.33) A | 852.45 (±154.67) A | 14.25 (±0.96) A | 861.12 (±46.27) A |
| ITTG R7T | 50.95 (±6.52) C | 835.70 (±152.47) B | 637.87 (±90.13) B | 13.75 (±1.03) B | 758.82 (±17.40) B |
| ITTG S70T | 52.97 (±5.60) C | 801.97 (±176.71) B | 370.70 (±119.27) C | 11.00 (±0.5) C | 629.97 (±57.80) C |
| Chemical fertilizer (Triple 17) | 57.21 (±4.12) B | 1431.71 (±191.53) A | 604.50 (±62.35) B | 10.52 (±0.5) C | 649.9 (±35.67) C |
| Negative control | 40.30 (±10.00) D | 586.12 (±190.21) C | 280.27 (±159.85) D | 8.00 (±1.34) D | 664.15 (±43.09) C |
| p-value | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| HSD Tukey ¥ (p < 0.05) | 3.9804 | 90.2026 | 47.062 | 2.2334 | 50.2347 |
| Treatments | Inulin (mg g−1) | Sucrose (mg g−1) | Glucose (mg g−1) | Fructose (mg g−1) |
|---|---|---|---|---|
| ACO-34A | 4.03 (±0.15) A * | 2.77 (±0.08) A | 3.07 (±0.18) A | 2.49 (0.09) A |
| ITTG R7T | 2.29 (±0.13) B | 1.70 (±0.06) B | 2.22 (±0.06) B | 2.16 (0.09) A |
| ITTG S70T | 2.15 (±0.13) B | 1.66 (±0.07) B | 2.19 (±0.11) B | 2.14 (0.14) A |
| Chemical fertilizer (Triple 17) | 2.24 (±0.11) B | 1.68 (±0.10) B | 2.32 (±0.06) B | 2.29 (0.13) A |
| Negative control | 1.13 (±0.02) C | 1.17 (±0.04) C | 1.6 (±0.03) C | 1.25 (0.06) B |
| p-value | 0.0000 | 0.0003 | 0.0005 | 0.0065 |
| HSD ¥ (p < 0.05) | 0.3485 | 0.6425 | 0.6204 | 0.7988 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranto-Gómez, V.M.; Rincón-Molina, C.I.; Manzano-Gómez, L.A.; Gen-Jiménez, A.; Maldonado-Gómez, J.C.; Villalobos-Maldonado, J.J.; Ruiz-Valdiviezo, V.M.; Rincón-Rosales, R.; Rincón-Molina, F.A. Plant Probiotic Potential of Native Rhizobia to Enhance Growth and Sugar Content in Agave tequilana Weber var. Blue. Horticulturae 2025, 11, 137. https://doi.org/10.3390/horticulturae11020137
Maranto-Gómez VM, Rincón-Molina CI, Manzano-Gómez LA, Gen-Jiménez A, Maldonado-Gómez JC, Villalobos-Maldonado JJ, Ruiz-Valdiviezo VM, Rincón-Rosales R, Rincón-Molina FA. Plant Probiotic Potential of Native Rhizobia to Enhance Growth and Sugar Content in Agave tequilana Weber var. Blue. Horticulturae. 2025; 11(2):137. https://doi.org/10.3390/horticulturae11020137
Chicago/Turabian StyleMaranto-Gómez, Víctor Manuel, Clara Ivette Rincón-Molina, Luis Alberto Manzano-Gómez, Adriana Gen-Jiménez, Julio César Maldonado-Gómez, Juan José Villalobos-Maldonado, Víctor Manuel Ruiz-Valdiviezo, Reiner Rincón-Rosales, and Francisco Alexander Rincón-Molina. 2025. "Plant Probiotic Potential of Native Rhizobia to Enhance Growth and Sugar Content in Agave tequilana Weber var. Blue" Horticulturae 11, no. 2: 137. https://doi.org/10.3390/horticulturae11020137
APA StyleMaranto-Gómez, V. M., Rincón-Molina, C. I., Manzano-Gómez, L. A., Gen-Jiménez, A., Maldonado-Gómez, J. C., Villalobos-Maldonado, J. J., Ruiz-Valdiviezo, V. M., Rincón-Rosales, R., & Rincón-Molina, F. A. (2025). Plant Probiotic Potential of Native Rhizobia to Enhance Growth and Sugar Content in Agave tequilana Weber var. Blue. Horticulturae, 11(2), 137. https://doi.org/10.3390/horticulturae11020137







