Blue Light Suppresses Pepper Resistance Against Phytophthora capsici Through CRY2-Mediated ROS and SA Signaling Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Cultivation of P. capsici and Inoculation Assay
2.3. Real-Time Quantitative PCR Assay
2.4. Subcellular Localization
2.5. Sequence Comparison and Phylogenetic Analysis
2.6. Virus-Induced Gene Silencing
2.7. Tobacco Transformation
2.8. Measurement of Reactive Oxygen Content and Enzyme Activity
2.9. SA Measurement
2.10. Trypan Blue Staining
2.11. Statistical Analysis
2.12. Accession Numbers
3. Results
3.1. Light Quality Regulates Pepper Resistance to P. capsici
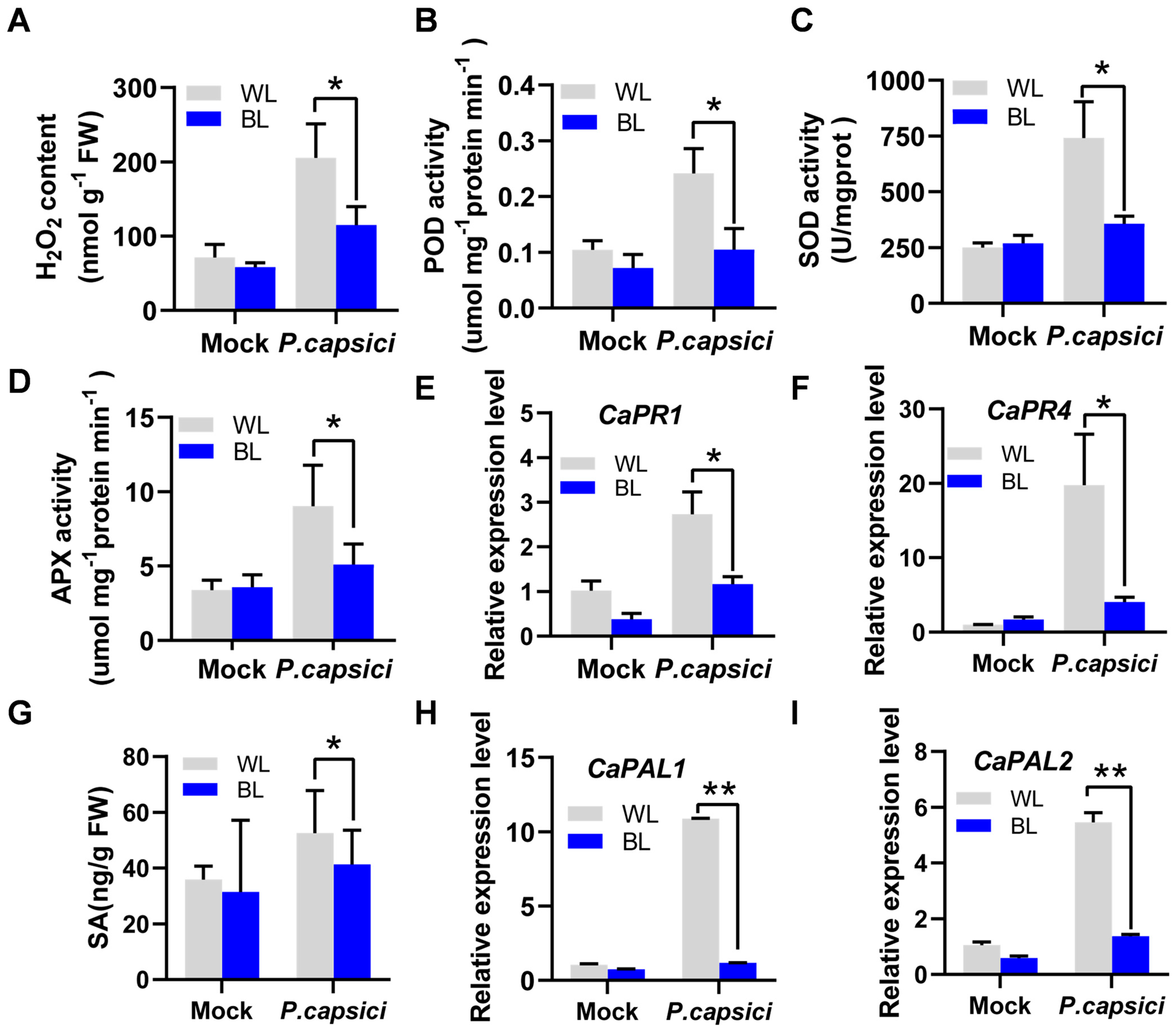
3.2. BL Regulates Pepper Resistance to P. capsici Through ROS and SA Signaling Pathways
3.3. Characterization of CaCRY2
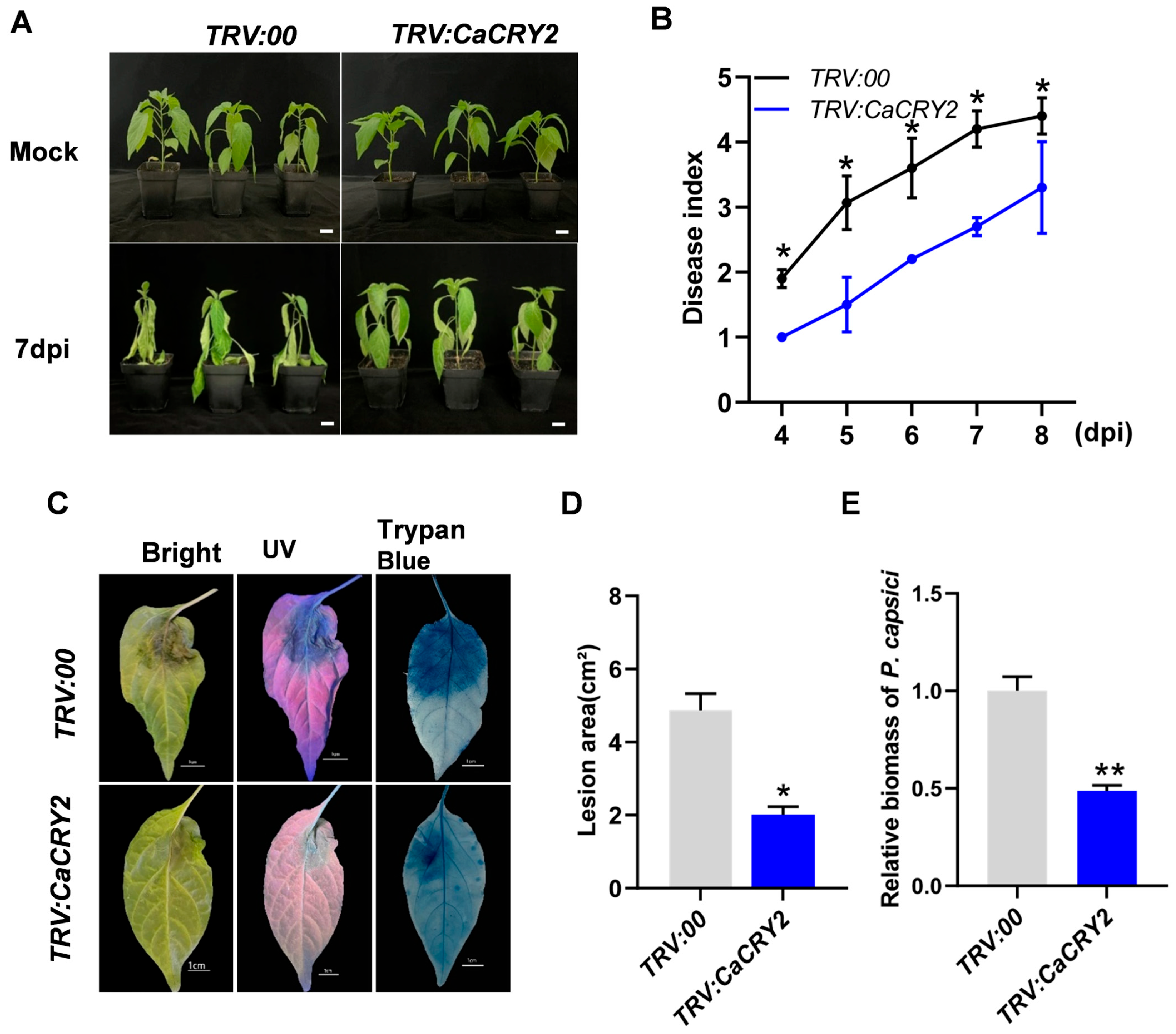
3.4. Silencing of CaCRY2 Enhances Pepper Plant Resistance to P. capsici
3.5. CaCRY2 Participates in ROS and SA Signaling Pathways
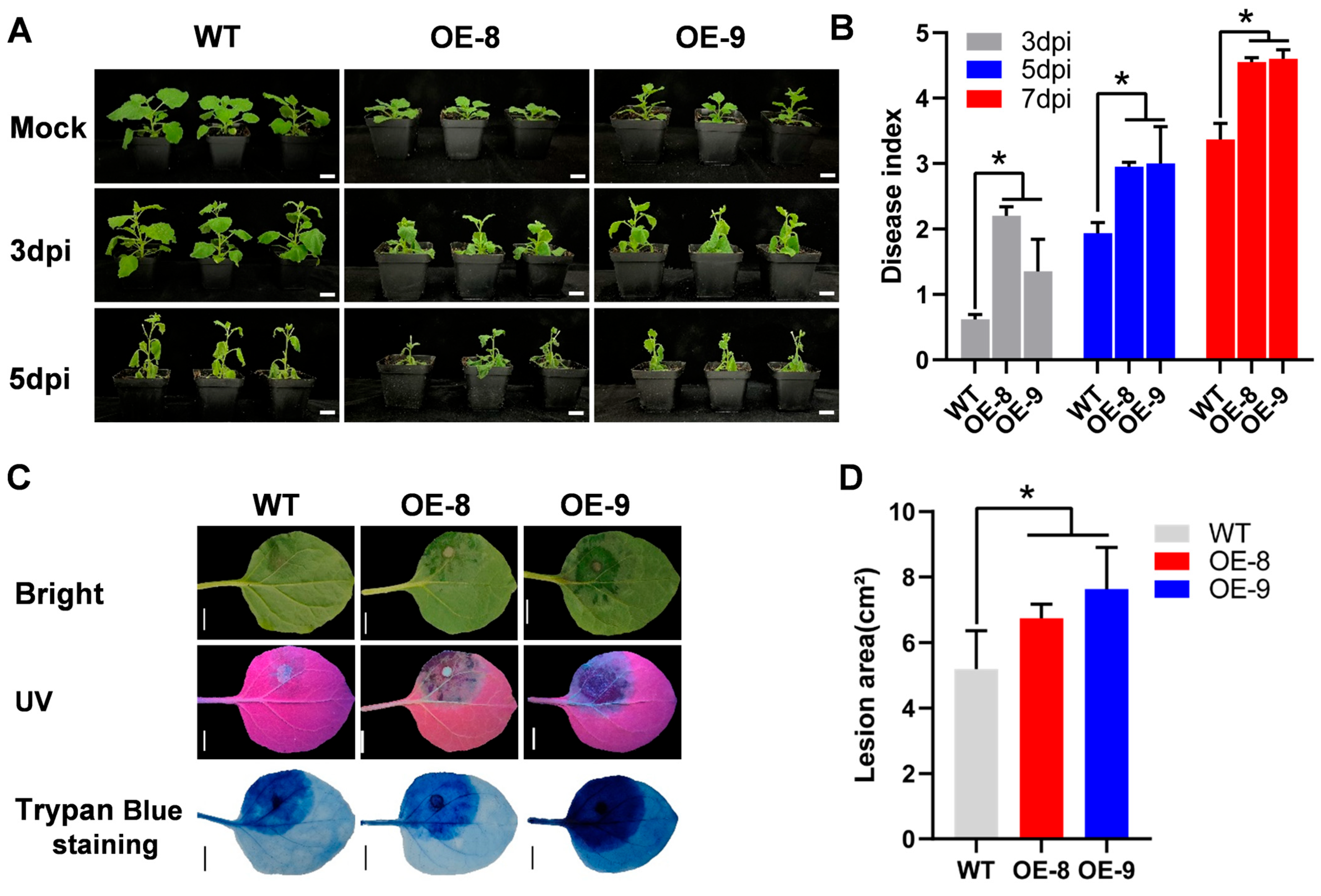
3.6. Heterologous Expression of CaCRY2 Weakens Tobacco Resistance to P. capsici
4. Discussion
4.1. BL Suppresses Pepper Defense Against PCI
4.2. CaCRY2 Regulates Pepper Resistance to P. capsici
4.3. CaCRY2 Enhances Pepper Susceptibility to P. capsici via ROS and SA Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Wang, X.; Sun, J.; Wang, X.; Zhu, Z.; Ayhan, D.; Yi, S.; Yan, M.; Zhang, L.; Meng, T.; et al. Two telomere-to-telomere gapless genomes reveal insights into Capsicum evolution and capsaicinoid biosynthesis. Nat. Commun. 2024, 15, 4295. [Google Scholar] [CrossRef] [PubMed]
- Hausbeck, M.; Lamour, K. Phytophthora capsici on vegetable crops: Research progress and management challenges. Plant Dis. 2004, 88, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.; Giles, G.; Robbins, K.; Gore, M.; Smart, C. Quantitative genetic analysis of interactions in the pepper-Phytophthora capsici pathosystem. Mol. Plant Microbe Interact. 2021, 35, 1018–1033. [Google Scholar] [CrossRef] [PubMed]
- Barchenger, D.W.; Lamour, K.H.; Bosland, P.W. Challenges and strategies for breeding resistance in Capsicum annuum to the multifarious pathogen, Phytophthora capsici. Front. Plant Sci. 2018, 9, 628. [Google Scholar] [CrossRef]
- Li, Q.; Ai, G.; Shen, D.; Zou, F.; Wang, J.; Bai, T.; Chen, Y.; Li, S.; Zhang, M.; Jing, M.; et al. A Phytophthora capsici effector targets ACD11 binding partners that regulate ROS-mediated defense response in Arabidopsis. Mol. Plant 2019, 12, 565–581. [Google Scholar] [CrossRef]
- Lei, G.; Zhou, K.; Chen, X.; Huang, Y.; Yuan, X.; Li, G.; Xie, Y.; Fang, R. Transcriptome and metabolome analyses revealed the response mechanism of pepper roots to Phytophthora capsici infection. BMC Genom. 2023, 24, 626. [Google Scholar] [CrossRef]
- Rogers, H.; Munné-Bosch, S. Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: Similar but different. Plant Physiol. 2016, 171, 1560–1568. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, N.; Meng, J.; Yang, G.; Liu, W.; Zhou, X.; Ma, N.; Hou, X.; Luan, Y. LncRNA33732-respiratory burst oxidase module associated with WRKY1 in tomato- Phytophthora infestans interactions. Plant J. 2019, 97, 933–946. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Li, R.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 2017, 170, 114–126. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, M.; Sun, X.; Zhao, M.; Chow, W.; Sun, G.; Zhang, Z.; Hu, Y. Light suppresses bacterial population through the accumulation of hydrogen peroxide in tobacco leaves infected with Pseudomonas syringae pv. tabaci. Front. Plant Sci. 2016, 7, 512. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Yu, B.; Han, Y.; Zhang, Y.; Xu, D.; Qi, H. Red light activates H2O2 signal involved in powdery mildew resistance in oriental melon seedlings. Environ. Exp. Bot. 2023, 215, 105508. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, C.; Ahammed, G.; Wu, C.; Yang, Z.; Wan, C.; Chen, J. Red light-induced systemic resistance against root-knot nematode is mediated by a coordinated regulation of salicylic acid, jasmonic acid and redox signaling in watermelon. Front. Plant Sci. 2018, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luan, Y.; Liu, Z. Overexpression of SpWRKY1 promotes resistance to Phytophthora nicotianae and tolerance to salt and drought stress in transgenic tobacco. Physiol. Plantarum 2015, 155, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.; Xing, Q.; Zhao, Y.; Yu, B.; Ma, Y.; Wang, F.; Qi, H. PIF8-WRKY42-mediated salicylic acid synthesis modulates red light induced powdery mildew resistance in oriental melon. Plant Cell Environ. 2023, 46, 1726–1742. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Cai, J.; Sun, W.; Yang, Q.; Hu, G.; Wang, T. Tomato SlWRKY3 negatively regulates Botrytis cinerea resistance via TPK1b. Plants 2024, 13, 1597. [Google Scholar] [CrossRef]
- Jing, Y.; Lin, R. Transcriptional regulatory network of the light signaling pathways. New Phytol. 2020, 227, 683–697. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Xie, J.; Wu, Y.; Tang, Z.; Liu, Z.; Lv, J.; Yu, J. Physiological responses of cucumber seedlings to different supplemental light duration of red and blue LED. Front. Plant Sci. 2021, 12, 709313. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Yu, H.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur. J. Plant Pathol. 2010, 127, 125–135. [Google Scholar] [CrossRef]
- Jung, J.; Li, Z.; Chen, H.; Yang, S.; Li, D.; Priatama, R.; Kumar, V.; Xuan, Y. Mutation of phytochrome B promotes resistance to sheath blight and saline–alkaline stress via increasing ammonium uptake in rice. Plant J. 2022, 113, 277–290. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Guang, Y.; Lin, J.; Zhou, Y.; Yu, T.; Ding, F.; Wang, Y.; Chen, J.; Zhou, Y.; et al. Red light induces salicylic acid accumulation by activating CaHY5 to enhance pepper resistance against Phytophthora capsici. Hortic. Res. 2023, 10, uhad213. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Inoue, S.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H+-ATPase. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef]
- Naqvi, S.; He, Q.; Trusch, F.; Qiu, H.; Pham, J.; Sun, Q.; Christie, J.; Gilroy, E.; Birch, P. Blue-light receptor phototropin 1 suppresses immunity to promote Phytophthora infestans infection. New Phytol. 2022, 233, 2282–2293. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.; van der Horst, G.; Batschauer, A.; Ahmad, M. The cryptochromes: Blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Lin, C.; Shalitin, D. Cryptochrome structure and signal transduction. Annu. Rev. Plant Biol. 2003, 54, 469–496. [Google Scholar] [CrossRef] [PubMed]
- Brudler, R.; Hitomi, K.; Daiyasu, H.; Toh, H.; Kucho, K.; Ishiura, M.; Kanehisa, M.; Roberts, V.; Todo, T.; Tainer, J.; et al. Identification of a new cryptochrome class: Structure, function, and evolution. Mol. Cell 2003, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, B.; Zhao, C.; Pepper, M.; Lin, C. The action mechanisms of plant cryptochromes. Trends Plant Sci. 2011, 16, 684–691. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. A structural view of plant CRY2 photoactivation and inactivation. Nat. Struct. Mol. Biol. 2020, 27, 401–403. [Google Scholar] [CrossRef]
- Ye, Y.; Li, R.; Pu, W.; Zhang, Y.; Jiang, L.; Li, H.; Liu, Y.; Ye, Y.; Yue, M.; Lin, Y.; et al. Expression analysis and interaction protein screening of CRY1 in strawberry. Horticulturae 2022, 8, 460. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.; Li, T.; Xu, F.; Mao, Z.; Cao, X.; Miao, L.; Du, S.; Hua, J.; Zhao, J.; et al. Blue light-dependent interactions of CRY1 with GID1 and DELLA proteins regulate gibberellin signaling and photomorphogenesis in Arabidopsis. Plant Cell 2021, 33, 2375–2394. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, H.; Liu, B.; Liu, X.; Lin, C. Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr. Biol. 2011, 21, 841–847. [Google Scholar] [CrossRef]
- Somers, D.; Devlin, P.; Kay, S. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Y.; Sang, Y.; Li, Q.; Yang, H. A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 2005, 102, 12270–12275. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Lian, H.; Wang, F.; Huang, J.; Yang, H. Cryptochromes, phytochromes, and COP1 regulate light-controlled stomatal development in Arabidopsis. Plant Cell 2009, 21, 2624–2641. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Li, X.; Guo, Y.; Chu, J.; Fang, S.; Yan, C.; Noel, J.; Liu, H. Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light. Proc. Natl. Acad. Sci USA 2016, 113, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Li, M.; Fu, D.; Wu, S.; Li, J.; Gong, Z.; Liu, H.; Yang, S. The CRY2-COP1-HY5-BBX7/8 module regulates blue light-dependent cold acclimation in Arabidopsis. Plant Cell 2021, 33, 3555–3573. [Google Scholar] [CrossRef]
- Wu, L.; Yang, H. Cryptochrome 1 is implicated in promoting R protein-mediated plant pesistance to Pseudomonas syringae in Arabidopsis. Mol. Plant 2010, 3, 539–548. [Google Scholar] [CrossRef]
- Jeong, R.; Chandra-Shekara, A.; Barman, S.; Navarre, D.; Klessig, D.; Kachroo, A.; Kachroo, P.; Jones, J. Cryptochrome 2 and phototropin 2 regulate resistance protein-mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2010, 107, 13538–13543. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, T.; Zhu, L.; Luo, S.; Deng, X.; Lin, H.; Xi, D. The role of photoreceptors in response to cucumber mosaic virus in Arabidopsis thaliana. J. Plant Growth Regul. 2016, 36, 257–270. [Google Scholar] [CrossRef]
- Nie, W.; Li, Y.; Chen, Y.; Zhou, Y.; Yu, T.; Zhou, Y.; Yang, Y. Spectral light quality regulates the morphogenesis, architecture, and flowering in pepper (Capsicum annuum L.). J. Photochem. Photobiol. B Biol. 2023, 241, 112673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Yu, T.; Li, J.; Qiu, X.; Zhu, C.; Liu, J.; Dang, F.; Yang, Y. Characterization of the B-BOX gene family in pepper and the role of CaBBX14 in defense response against Phytophthora capsici infection. Int. J. Biol. Macromol 2023, 237, 124071. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Xing, Y.; Weng, X.; Zhao, Y.; Tang, W.; Wang, L.; Zhou, H.; Yu, S.; Xu, C.; Li, X.; et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat. Genet. 2008, 40, 761–767. [Google Scholar] [CrossRef]
- Thompson, J.; Gibson, T.; Higgins, D. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Dong, S.; Jiang, X.; Wang, L.; Yu, J.; Zhou, Y. Crosstalk of PIF4 and DELLA modulates CBF transcript and hormone homeostasis in cold response in tomato. Plant Biotechnol. J. 2020, 18, 879–1105. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Fernández-Milmanda, G.; Crocco, C.; Reichelt, M.; Mazza, C.; Köllner, T.; Zhang, T.; Cargnel, M.; Lichy, M.; Koo, A.; Austin, A.; et al. A light-dependent molecular link between competition cues and defence responses in plants. Nat. Plants 2019, 6, 223–230. [Google Scholar] [CrossRef]
- Roeber, V.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2020, 44, 645–664. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. Phytochrome A and B function antagonistically to regulate cold tolerance via abscisic acid-aependent jasmonate signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef]
- Kudo, M.; Kidokoro, S.; Yoshida, T.; Mizoi, J.; Todaka, D.; Fernie, A.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Double overexpression of DREB and PIF transcription factors improves drought stress tolerance and cell elongation in transgenic plants. Plant Biotechnol. J. 2016, 15, 458–471. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M.; Yin, Y.; Onac, E.; Zhou, G.; Peng, S.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. RNA-seq analysis reveals the role of red light in resistance against Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Genom. 2015, 16, 120. [Google Scholar] [CrossRef]
- Meng, L.; Mestdagh, H.; Ameye, M.; Audenaert, K.; Höfte, M.; Labeke, M. Phenotypic variation of Botrytis cinerea isolates is influenced by spectral light quality. Front. Plant Sci. 2020, 11, 1233. [Google Scholar] [CrossRef]
- Ahmad, M.; Cashmore, A. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162. [Google Scholar] [CrossRef]
- Yu, X.; Klejnot, J.; Zhao, X.; Shalitin, D.; Maymon, M.; Yang, H.; Lee, J.; Liu, X.; Lopez, J.; Lin, C. Arabidopsis cryptochrome 2 completes its posttranslational life cycle in the nucleus. Plant Cell 2007, 19, 3146–3156. [Google Scholar] [CrossRef]
- Fantini, E.; Sulli, M.; Zhang, L.; Aprea, G.; Jiménez-Gómez, J.; Bendahmane, A.; Perrotta, G.; Giuliano, G.; Facella, P. Pivotal roles of cryptochromes 1a and 2 in tomato development and physiology. Plant Physiol. 2019, 179, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, X.; Li, L.; Lian, H.; Mao, Z.; Xu, P.; Guo, T.; Xu, F.; Du, S.; Cao, X.; et al. Photoexcited Cryptochrome 1 interacts with dephosphorylated BES1 to regulate brassinosteroid signaling and photomorphogenesis in Arabidopsis. Plant Cell 2018, 30, 1989–2005. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Chen, Y.; Luo, Y.; Yang, F.; Yu, T.; Han, H.; Yang, Y.; Zhou, Y. Cryptochromes (CRYs) in pepper: Genome-wide identification, evolution and functional analysis of the negative role of CaCRY1 under Phytophthora capsici infection. Plant Sci. 2025, 355, 112460. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ying, J.; Ye, Y.; Wen, S.; Qian, R. Blue light regulates jasmonic acid synthesis via CRY1a and boosts antioxidant enzymes activity in Solanum lycopersicum to resist Botrytis cinerea. Plant Cell Rep. 2025, 44, 160. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2013, 65, 1229–1240. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J.; Yang, F.; Zhang, G.; Wang, D.; Zhang, L.; Ou, Y.; Yao, Y. The WRKY transcription factor WRKY8 promotes resistance to pathogen infection and mediates drought and salt stress tolerance in Solanum lycopersicum. Physiol. Plant 2019, 168, 98–117. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Ali, M.; Jin, J.; Wei, A.; Khattak, A.; Gong, Z. Identification of pepper CaSBP08 gene in defense response against Phytophthora capsici infection. Front. Plant Sci. 2020, 11, 183. [Google Scholar] [CrossRef]
- Wang, H.; Ma, L.; Li, J.; Zhao, H.; Deng, X. Direct interaction of Arabidopsis Cryptochromes with COP1 in mediation of photomorphogenic development. Science 2001, 294, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pedmale, U.; Huang, S.; Zander, M.; Cole, B.; Hetzel, J.; Ljung, K.; Reis, P.; Sridevi, P.; Nito, K.; Nery, J.; et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Zhong, M.; Lin, G.; Ye, M.; Wang, Y.; Zhang, J.; Wang, Q. The CRY1-COP1-HY5 axis mediates blue-light regulation of Arabidopsis thermotolerance. Plant Commun. 2025, 6, 101264. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, T.; Chen, Y.; Luo, Y.; Liu, H.; Zhou, Y.; Wang, X.; Lin, Y.; Liu, S.; Chen, J.; Yang, Y. Blue Light Suppresses Pepper Resistance Against Phytophthora capsici Through CRY2-Mediated ROS and SA Signaling Pathways. Horticulturae 2025, 11, 1434. https://doi.org/10.3390/horticulturae11121434
Yu T, Chen Y, Luo Y, Liu H, Zhou Y, Wang X, Lin Y, Liu S, Chen J, Yang Y. Blue Light Suppresses Pepper Resistance Against Phytophthora capsici Through CRY2-Mediated ROS and SA Signaling Pathways. Horticulturae. 2025; 11(12):1434. https://doi.org/10.3390/horticulturae11121434
Chicago/Turabian StyleYu, Ting, Yue Chen, Ying Luo, Hongyan Liu, Yong Zhou, Xiaobin Wang, Yachun Lin, Shanjun Liu, Jinyin Chen, and Youxin Yang. 2025. "Blue Light Suppresses Pepper Resistance Against Phytophthora capsici Through CRY2-Mediated ROS and SA Signaling Pathways" Horticulturae 11, no. 12: 1434. https://doi.org/10.3390/horticulturae11121434
APA StyleYu, T., Chen, Y., Luo, Y., Liu, H., Zhou, Y., Wang, X., Lin, Y., Liu, S., Chen, J., & Yang, Y. (2025). Blue Light Suppresses Pepper Resistance Against Phytophthora capsici Through CRY2-Mediated ROS and SA Signaling Pathways. Horticulturae, 11(12), 1434. https://doi.org/10.3390/horticulturae11121434




