Abstract
The production of carrot (D. carota L.) doubled haploids (DH) for the acceleration of this important vegetable crop breeding requires genome doubling of haploid regenerants. If spontaneous doubling does not occur, artificial chromosome doubling can be complicated by the lack of efficient genome-doubling protocols. We tested an antimitotic agent treatment of carrot at the embryo stage. It allowed us to produce and treat a large number of clonal carrot embryos (at least 30 embryos per treatment condition) in small volumes with minimal reagent amounts. We showed that 0.01–1 g/L colchicine did not perturb carrot development. Trifluralin showed no signs of toxicity at 0.001 and 0.01 g/L concentrations, but 0.1 g/L trifluralin reduced survival by 40% and delayed plantlet regeneration. We showed via DNA content flow cytometry that 0.01–1 g/L colchicine and 0.001–0.1 g/L trifluralin could double the carrot genome. The highest diploid percent was observed at 1 g/L colchicine (34%) and 0.1 g/L trifluralin (28%). The highest percent of diploids together with mixoploids (partial diploids) was at 0.01 and 0.1 g/L trifluralin (over 70%), followed by 1 g/L colchicine (56%). To our knowledge, this is the first report on trifluralin application for genome doubling in Apiaceae. In our study, we determined colchicine and trifluralin toxicity and doubling efficiency at different concentrations that can be used for carrot DH-line production and further improvement of genome doubling methods.
1. Introduction
DH technology can bring exceptional advantages to breeding of carrot (D. carota L.), one of the top ten most consumed vegetables worldwide [1]. Carrot line production is complicated by a two-year life cycle, self-incompatibility, and inbreeding depression. DH technologies can significantly shorten the line production and reduce its cost almost five-fold [2]. Moreover, DH lines are fully homozygous, which is challenging to achieve by conventional inbreeding for parental lines of cross-pollinated species.
Despite the obvious advantages of DH technologies for parental line production, carrot remains a relatively recalcitrant crop for DH induction. Ovule, anther, and microspore isolation is labor-intensive since carrot plants have small flowers. Moreover, flower buds in the umbrella mature at different times, hindering the selection of flowers with microspores at the required developmental stage. The microspores at earlier and later stages die out, which interferes with embryo development from the responsive microspore stage. Moreover, haploid plant growth induction is genotype-dependent [3]. Another serious complication for carrot DH line production is genome doubling, if it does not occur spontaneously.
Various spontaneous genome-doubling rates were observed in carrots in different studies. Only haploid and aneuploid plants regenerated from anther culture in the study by Hu and co-authors [4]. In other studies, the number of haploid regenerants in anther, microspore or ovule cultures ranged from 8.3% to 100% [3,5,6,7,8,9,10,11,12,13]. The incidence of spontaneous diploidization can be genetically determined, which is why this process occurs at different frequencies in different varieties. These studies show that it is not necessary to use antimitotic agents to produce DH-lines in varieties with a high spontaneous doubling rate. However, when the spontaneous genome-doubling rate is low, genome doubling is required to produce doubled haploids. Moreover, when the total number of regenerants is low, even a small number of haploid plants cannot be discarded. Therefore, artificial carrot genome-doubling protocols are required; however, a limited number of studies on genome doubling in carrots exist [14]. Two studies show that colchicine treatment can be successfully used on microspores [15,16]. The authors added 0.5 mg/L colchicine to the culture medium for 24 [15] or 48 h [16], and all regenerating plantlets were diploid. However, no comparison with an untreated control was performed in the first study [15]. In the second study, the regenerants from microspores that were not treated with colchicine were also diploid [16]. Both research groups concluded that the addition of colchicine might be advantageous for microspore development. It can also be a promising genome-doubling approach [15,16].
Ferrie and co-authors treated different Apiaceae haploid plantlet roots with 3.4 g/L colchicine for 1.5 h and then planted them in soil. However, plant survival was significantly reduced for the anise, dill, and caraway. The information about the carrot survival rate after colchicine treatment was not explicitly stated [17]. Our preliminary studies showed that carrot haploid plantlet treatment with colchicine can successfully double genomes. We treated carrot haploid plantlets in vitro with 0.5 g/L colchicine for 48 h. As a result, two plantlets became diploid (25%) and the other six regenerants became tetraploid (75%). However, we did not test the plant adaptation to ex vitro conditions [18].
In this study, we performed antimitotic agent treatment at the embryo stage. This approach proved to be effective in other species, such as onion (Allium cepa L.) [19,20] and rapeseed (Brassica napus L.) [21]. Our experiments showed that this is an advantageous approach for carrot since carrot embryos have a high potential for secondary embryogenesis, i.e., a large number of embryos of the same genotype can be produced for testing various treatment regimens. We tested different concentrations of colchicine and trifluralin with at least 30 embryos per treatment condition to find the most efficient and least toxic concentrations. To our knowledge, trifluralin has not yet been tested in any Apiaceae species. The developed genome-doubling protocol can be applied for haploid carrot doubling if spontaneous doubling fails to occur.
2. Materials and Methods
2.1. Growing Conditions for Donor Plants
The carrot (D. carota L.) cultivar Lobbericher was used in this study. Donor plants were grown from seeds, and the developed taproots were vernalized for five months. Next, the taproots were planted in plastic pots (22 cm in diameter) filled with a mixture of peat and perlite (7:3, v/v). The plants were cultivated in a growing chamber with an illuminance of 65 μmol m−2 s−1 using Plantastar HPS, 600 W 220 V E40 lamps (Osram, Nove Zamky, Slovakia) with a photoperiod of 16 h/8 h (day/night) at 22–25 °C. Donor plants were watered as needed and fertilized weekly with a liquid commercial fertilizer Akvarin (Buyskiy himicheskiy zavod, Bui, Russia), containing N (13%), P2O5 (5%), K2O (25%), MgO (2%), S (8%), Fe (EDTA) (0.054%), Zn (EDTA) (0.014%), Cu (EDTA) (0.01%), Mn (EDTA) (0.042%), Mo (0.004%), and B (0.02%).
2.2. Microspore and Embryo Culture
The outermost umbellets were collected from flowering carrot plants in the early flowering phase with immature anthers. The flower buds from one or two outermost umbellet rows containing late vacuolated microspores and young bicellular pollen grains were collected with tweezers. The flower buds were surface-sterilized with 96% ethanol solution for 30 s and with 2.5% sodium hypochlorite with the addition of Tween 20 (Panreac, Barcelona, Spain) for 15 min, followed by three 7 min washes in sterile dH2O. Then, the flower buds were macerated on a magnetic stirrer in small glass bottles with MSm medium [22] supplemented with 13% (w/v) sucrose (MSm-13). Next, the microspores and pollen grains were filtered through a 40 µm nylon mesh. They were washed in MSm-13 three times, followed by centrifugation at 120 g for 5 min. Then, the microspores were plated in 60 mm culture dishes with MSm-13 supplemented with 0.1 mg/L 2,4-D, 0.1 mg/L kinetin, and 100 µg/mL ampicillin. The microspores were incubated in the dark at 32 °C for 60 h and then cultured in the incubator at 40 rpm, 25 °C in the dark until embryos were visible. After that, the embryos were transferred to the MSm medium with 0.1 mg/L kinetin and 100 µg/mL ampicillin at 40 rpm, 23 °C under natural illumination. Under these conditions, the embryos actively propagated via secondary embryogenesis.
2.3. Cytometric Genome Ploidy Determination
Embryo fragments or leaves removed from regenerated plantlets were used for cytometric analysis. A diploid carrot donor plant grown from a seed was used as an external reference standard. A leaf or an embryo fragment was chopped with a razor blade in 300 µL of ice-cold Galbraith lysis buffer (45 mM MgCl2, 20 mM MOPS, 30 mM sodium citrate, 0.1% (v/v) Triton X-100, pH 7.0) with the addition of 50 µg/mL RNase A (Syntol, Moscow, Russia). Then, the plant material was filtered through a 30 µm pre-separation filter (Miltenyi Biotec, Bergisch Gladbach, Germany) and stained with 50 μg/mL propidium iodide (PI) (Sigma, Saint Louis, MO, USA) [23]. PI-stained nuclei were analyzed with the CytoFLEX cytometer (Beckman Coulter, Brea, CA, USA). At least 6000 events were recorded for each sample. Data acquisition and processing were performed using the CytExpert 2.4 software (Beckman Coulter, USA).
2.4. Antimitotic Treatment and Plantlet Regeneration
A haploid embryo was selected using DNA content flow cytometry and propagated for antimitotic agent treatments. For trifluralin treatment experiments, haploid embryos were placed in glass jars with culture medium, and trifluralin (Treflan, Dow AgroSciences, Indianapolis, IN, USA) was added at different concentrations (0.001, 0.01, 0.1, and 1 g/L). Glass jars were used because Treflan is dissolved in acetone, which damages plastic culture dishes. For colchicine treatment, haploid embryos were cultured in 6-well plates with medium and colchicine (CDH, India; the stock solution was prepared in dH2O) at concentrations of 0.01, 0.1, and 1 g/L. Embryos cultivated under the same conditions without the addition of antimitotic agents were used as a control. The jars and plates were covered with foil to prevent trifluralin and colchicine light degradation (Figure 1). After 48 h of treatment, the embryos were thoroughly washed with sterile dH2O and transferred to the fresh medium.
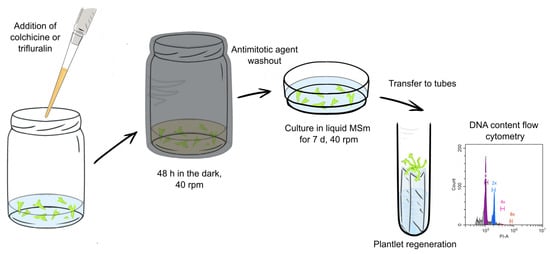
Figure 1.
The antimitotic agent treatment workflow.
In 7–10 days after treatment, the embryos were transferred onto the filter paper bridges soaked with the medium in glass tubes (20 mm × 200 mm) for plant regeneration. The plantlets were cultured under fluorescent lamps OSRAM Fluora L36W/77 (predominantly blue and red spectrum) and Philips 36W/54-765 (predominantly white spectrum) with total illumination of 24 μmol m−2 s−1, photoperiod 16 h/8 h (day/night) at 23 °C for 8 weeks until plantlets were developed.
2.5. Plantlet Adaptation to Ex Vitro Conditions and Growing in a Growth Chamber
The plantlets with developed roots and leaves were planted into pots with a mixture of peat and perlite (7:3, v/v) and covered with perforated plastic cups for acclimatization to ex vitro conditions. The cups were gradually removed from the pots after two to three weeks of cultivation. The plants were grown under the same conditions as the donor plants.
2.6. Statistical Analysis and Graph Preparation
Bar graphs and donut plots were plotted using Microsoft Excel 2016 for Windows 10. Statistical analysis was done using Statistica 7.0 software (StatSoft, Tulsa, OK, USA). One-way ANOVA, followed by the Tukey multiple comparison test and the Chi-square test, was used in this study. Statistical analysis was considered significant at p < 0.05 for the ANOVA test. Bonferroni correction was used for pairwise comparisons after the Chi-square test. A 0.05 p-value was divided by the maximum number of pairwise comparisons for 7 groups (21). Therefore, the differences were considered significant at p < 0.0024.
3. Results
Isolated microspores from the Lobbericher carrot (D. carota L.) cultivar were cultured until embryos developed. Individual embryos were transferred for further cultivation in Petri dishes with the MSm medium. Subsequently, the obtained embryos were analyzed on the flow cytometer to determine ploidy. For genome-doubling experiments, we selected a haploid embryo genotype that successfully propagated via secondary embryogenesis and could regenerate into plantlets.
The propagated embryos were treated with different concentrations of trifluralin and colchicine for 48 h. Then, plantlets were washed from the antimitotic compound with sterile dH2O and cultured in the MSm medium for survival and genome-doubling rate assessment (Figure 1).
3.1. Colchicine and Trifluralin Toxicity for Carrot Embryoids at Different Concentrations
The embryo survival was assessed 7 days after the antimitotic agent removal. Then, the embryos were transferred from Petri dishes with MSm to the paper bridges in the tubes with the same medium. Carrot embryos regenerated into plantlets on the paper bridges and were assessed again after 45 days of culture. Thus, we assessed acute and long-term toxic effects of the antimitotic agents in carrot embryos.
One week after treatment, no signs of toxicity were visible in samples treated with 0.01, 0.1, and 1 g/L colchicine (Figure 2A). No embryo damage or slowing of development was observed at later stages either (Figure 2B). Quantifications showed no significant difference in plantlet survival rate 45 days after treatment (Figure 2C).
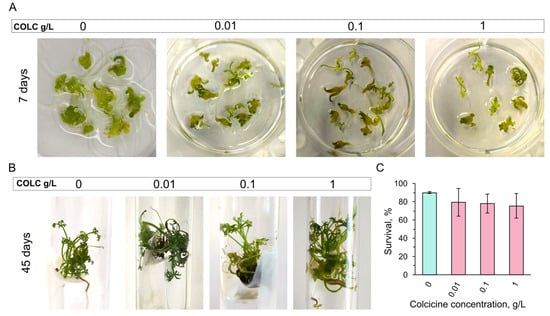
Figure 2.
Colchicine toxicity at different concentrations in carrot embryos. (A) Embryos at day 7 after colchicine treatment. Untreated control (0 g/L) and embryos after 0.01, 0.1, and 1 g/L colchicine exposure are shown. (B) Regenerated plantlets in control (0 g/L) and after 0.01, 0.1, and 1 g/L colchicine treatment at day 45 after colchicine removal. (C) Plantlet survival quantifications after colchicine exposure at day 45 of culture show no significant difference between control and treated samples. P-values were calculated by one-way ANOVA, followed by Tukey’s multiple comparison test, p > 0.05. COLC stands for colchicine.
Similar to colchicine, no major toxic effects were observed for trifluralin at concentrations of 0.001, 0.01, and 0.1 g/L (Figure 3A). However, no embryos survived at 1 g/L concentration. Embryo browning was observed right after the 1 g/L trifluralin treatment, and no embryo recovery occurred after 7 days in culture (Figure 3A). Embryos developed into plantlets after 0.001 and 0.01 g/L trifluralin treatment in 45 days, similar to an untreated control (Figure 3B). However, 0.1 g/L trifluralin delayed plantlet development (Figure 3B), but surviving embryos regenerated into plantlets in about 3 months in culture (Figure 3C). Plantlet survival was significantly decreased after 0.1 g/L trifluralin treatment, but not after 0.001 and 0.01 g/L trifluralin exposure (Figure 3D).
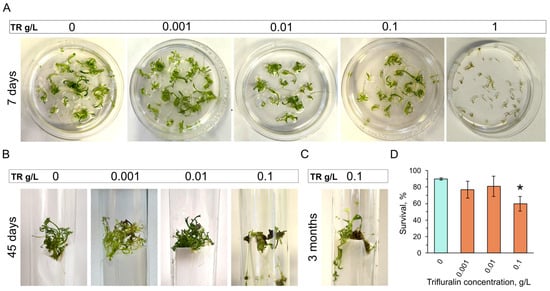
Figure 3.
Trifluralin toxicity at different concentrations in carrot embryos. (A) Untreated control (0 g/L) and embryos after 0.001, 0.01, and 0.1, 1 g/L trifluralin treatment at day 7 after trifluralin exposure. (B) Regeneration in control (0 g/L) and after 0.001, 0.01, and 0.1 g/L trifluralin treatment at day 45. (C) Plantlet regeneration from embryos treated with 0.1 g/L trifluralin 3 months after treatment. (D) Plantlet survival quantifications after trifluralin treatment at day 45 of culture. p-values were calculated by one-way ANOVA, followed by Tukey’s multiple comparison test. p < 0.05 was considered significant (shown by an asterisk). TR stands for trifluralin.
3.2. The Genome Doubling Efficiency After Colchicine and Trifluralin Treatment at Different Concentrations
Regenerant leaves were taken for cytometric analysis two months after antimitotic treatment. The nuclei were isolated, stained with PI, and analyzed with the flow cytometer. A leaf from a diploid carrot plant grown from a seed was used as an external standard (Figure 4A). Untreated regenerant plants were haploid (Figure 4B). Different ploidy was observed in treated samples. Haploid plants with no doubling (Figure 4C), as well as plants with a fold increase in DNA content level, including diploid (Figure 4D), tetraploid, (Figure 4D’), and octoploid (Figure 4D’’) plants, were detected. Various mixoploid plants (Figure 4E–F’’) were observed as well.
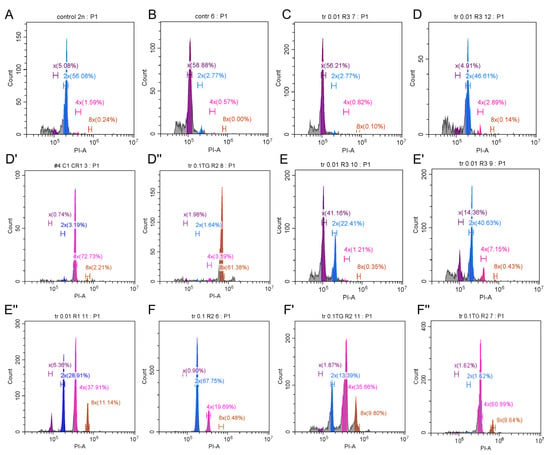
Figure 4.
Ploidy variants detected using flow cytometry after colchicine and trifluralin treatment. (A) A diploid control grown from a seed (2n = 2x = 18). (B) An untreated haploid plantlet (n = x = 9). (C) A plantlet after antimitotic treatment, showing no doubling (haploid). (D–D’’). Plantlets that underwent one (D), two (D’), or three (D’’) rounds of genome doubling: 2x, 4x, and 8x, respectively. (E–E’’) Mixoploids with genome doubling in a fraction of cells: x + 2x (E), x + 2x + 4x (E’), x + 2x + 4x + 8x (E’’). (F–F’’) Mixoploids with no haploid cells left, but some cells underwent more than one round of doubling: 2x + 4x (F), 2x + 4x + 8x (F’), 4x + 8x (F’’).
We performed cytometric analysis of more than 30 plantlets for each experimental condition (Figure 5A). We analyzed an untreated plantlet ploidy level to test if spontaneous doubling occurred during plantlet regeneration. We observed no spontaneous genome doubling in the control (Figure 5A,B). Therefore, the DNA doubling observed after different treatments should be attributed to antimitotic agent activity.
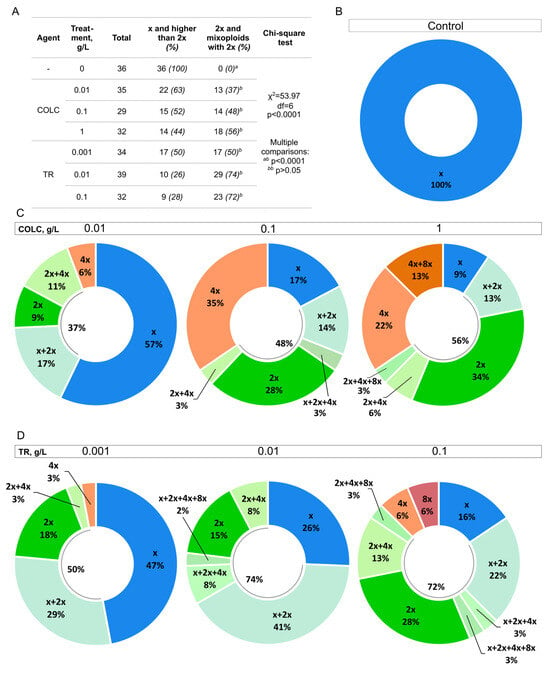
Figure 5.
The distribution of different ploidies in the untreated control and after colchicine and trifluralin treatment at different concentrations. (A) The total number of plantlets, the number of target events (plantlets with at least some 2x cells), and unwanted ploidies (haploids and polyploids) are shown for control, colchicine, and trifluralin treatments at different concentrations. The percentage of the total number is shown in brackets. A Chi-square test was performed to compare the groups. The difference between the groups is significant (p < 0.0001). In pairwise comparisons, the groups with the same letter (bb) show no significant difference, while the comparison of treated samples with the control (ab) shows a significant difference (p < 0.0001). The Bonferroni correction was used; therefore, the differences were considered significant at p < 0.0024. (B–D) Donut plots show the ploidy level distribution after different treatments. Blue—haploid plantlets (x), different shades of green—plantlets that have at least some diploid cells (2x), bright green—diploid plantlets, orange and red—plantlets that have ploidy higher than 2x (4x, 4x + 8x, 8x). Data from at least 30 plants were used for each donut plot. COLC and TR stand for colchicine and trifluralin, respectively. (B) The regenerant ploidy distribution in the untreated control. (C) The regenerant ploidy distribution after 0.01, 0.1, and 1 g/L colchicine treatment. (D) The regenerant ploidy distribution after 0.001, 0.01, and 0.1 g/L trifluralin treatment.
Mixoploid plants that have at least some diploid (2x) cells can be included in the breeding process because they are partially fertile, and 2x seeds can be collected from them [24,25]. Therefore, both diploids and mixoploids with some 2x cells can be considered as target events (Figure 5A). All treated samples were significantly different from the untreated control, but the difference between the treated samples was not significant (Figure 5A). Both colchicine and trifluralin treatments can be successfully used for genome doubling at the tested concentrations.
The number of target events did not grow significantly at higher colchicine or trifluralin concentrations (Figure 5C,D). We observed that the percentage of haploid (x) plantlets decreased with the increase of antimitotic agent concentration, but the incidence of higher than 2x ploidy grew (Figure 5C,D). The number of 2x plantlets showed an increasing trend with the increase of antimitotic agent concentration. The highest percentage of diploid plantlets was observed at 1 g/L colchicine (34%) and 0.1 g/L trifluralin (28%). The highest percentage of plantlets with at least some 2x cells was at 0.01 and 0.1 g/L trifluralin (74 and 72%, respectively). The best result for colchicine was at 1 g/L concentration (56%). Although 0.1 g/L trifluralin treatment showed successful chromosome set doubling, it also decreased plantlet survival and delayed development (Figure 3B–D). Therefore, lower trifluralin concentrations are preferred for genome doubling in carrot embryos.
3.3. The Treated Plant Adaptation to Ex Vitro Conditions
The plantlets were transferred to the fresh medium one or two times during the 3–4-month culture period after antimitotic treatment until the plantlets grew in size and developed roots. Then, the plantlets were transferred to a mixture of peat and perlite for adaptation. The plantlets successfully adapted to ex vitro conditions (Figure 6) regardless of the concentration of the antimitotic compound used on them.
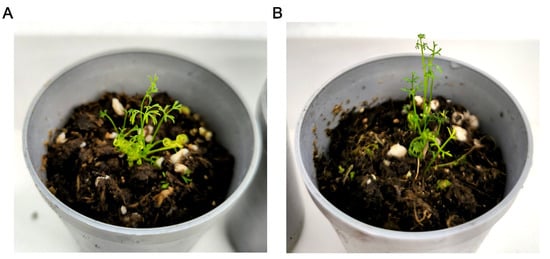
Figure 6.
Plantlets after antimitotic agent treatment adapt to ex vitro conditions. (A,B) Plantlets after two weeks of adaptation. (A) Mixoploid x + 2x plantlet. (B) Diploid plantlet.
4. Discussion
Genome doubling with chemical agents is an important tool for producing breeding material. It is utilized for DH technologies, polyploid production, and overcoming sterility in interspecific hybrids [18]. Genome doubling methods were tested in a variety of cultivars, including vegetable [18], medicinal [26,27], industrial [28], and ornamental [26,29,30] species. It was shown that the protocols must be adjusted for each species individually because species differ in their tolerance to the antimitotic agent’s toxic effects, the tissue accessibility for the agent diffusion, and the rate of cell division [18].
As mentioned above, genome doubling is critical for DH production. DH technologies redirect male or female gametophytes to the sporophyte developmental route, resulting in haploid plant development. The haploid regenerant genome has to be doubled to produce fully homozygous diploid plants that can be included in breeding programs. A fraction of haploids can spontaneously double their genome in many species, including the members of Apiaceae, Brassicaceae, Cucurbitaceae, and Solanaceae families [18]. For haploid plants that did not spontaneously double their genome, artificial genome-doubling methods with antimitotic agents, such as colchicine or antimitotic herbicides (trifluralin, amiprophos-methyl (APM), pronamid, or oryzalin) [31] need to be developed and applied to increase the plantlet ploidy level.
Carrot (D. carota L.) is an important source of carotenoids, dietary fibers, and other bioactive compounds, which makes it a highly valued vegetable worldwide [1]. Carrot breeding programs require homozygous parental lines for hybrid production, which can be satisfied by traditional inbreeding or DH-line production. The latter method allows for the shortening of the parental line production by several years, but it is complicated by certain technological gaps in DH-technologies. One of these gaps includes haploid regenerant genome-doubling methods. Genome doubling with antimitotic agents in carrot has not been sufficiently explored previously because haploids spontaneously double their genotype at high rates in many cases [7,8,9,10,11]. However, in other studies, spontaneous doubling occurred at 0% [4], 8.3% [5], 16.8% [6], and 18.7% [3] rates. When spontaneous doubling is insufficient, artificial genome-doubling protocols need to be applied [14]. Colchicine treatment was previously tested on microspores [15,16] and plantlets [17]. In the current study, we treated haploid carrot at the embryo stage with colchicine and trifluralin at different concentrations in order to find the least toxic and most efficient approaches.
Colchicine is the most commonly used agent for genome doubling, with a lot of data accumulated for different plant species. A wide range of concentrations from 5 × 10−4 to 20 g/L was tested, showing different toxicity and effectiveness in different species [18]. Therefore, a range of antimitotic agent concentrations should be tested to determine the most effective and cost-efficient treatment protocol for a particular species and plant developmental stage. For the current study, 0.01, 0.1, and 1 g/L colchicine concentrations were tested. All tested concentrations did not cause toxic effects and were able to double the chromosome set in carrot embryos at different rates. The highest percentage of diploid and partial diploid (mixoploid) plantlets was observed at 1 g/L colchicine (34% and 56%, respectively).
Trifluralin was generally used at 3.35 × 10−4 to 0.1 g/L concentrations in other species [18]. Concentrations of about 0.1 g/L or higher caused plant death in sugar beet ovules (B. vulgaris subsp. vulgaris (var. saccharifera)) [32]. Trifluralin was successfully applied in a number of species, such as sugar beet (B. vulgaris subsp. vulgaris (var. saccharifera)) [32,33], onion (A. cepa L.) [19], rape (B. napus L.) [34,35], and others. However, it was not tested in carrot or other Apiaceae species. Based on the previous results for other species, we tested 0.001, 0.01, 0.1, and 1 g/L concentrations. We tested higher concentrations (0.1 and 1 g/L) to find what is the lethal concentration for carrot embryos, since trifluralin has not been tested in carrot before. We found that 1 g/L trifluralin causes 100% embryo death. After 0.1 g/L trifluralin treatment, no immediate embryo death or damage was observed; however, the reduction of growth and increased embryo death rate compared to the control was observed after a few weeks of culturing. Embryo development was not affected by 0.001 and 0.01 g/L trifluralin treatment. Although the highest number of diploids was observed at 0.1 g/L trifluralin (28%), the number of partial diploids was comparable for 0.01 and 0.1 g/L trifluralin (74 and 72%, respectively). Since 0.01 g/L trifluralin did not slow down carrot regeneration, this concentration is preferable for carrot embryo genome doubling.
For both antimitotic agents, we observed that the increase in concentration led to a decrease in haploid plantlet number and an increase in the polyploid incidence. A trend towards an increase in the number of diploids and mixoploids (partial diploids) at higher antimitotic agent concentrations was also observed. Probably, the further increase in antimitotic agent concentration will lead to a further decrease in haploid plantlet number and an increase in the fraction of polyploids. We suppose that the percentage of target plantlets will not increase significantly at higher concentrations.
We analyzed the untreated control plantlet ploidy similar to treated plantlets because the spontaneous genome doubling can occur during plantlet regeneration, which would affect the study conclusions regarding antimitotic treatment efficiency. Spontaneous genome doubling and ploidy anomalies were previously shown for androgenic carrot embryos and plantlets during their culturing in vitro [8]. In our study, we observed that an untreated haploid embryo or plantlet subculturing for 3–4 months did not lead to spontaneous genome doubling.
We showed that embryo antimitotic agent treatment has several advantages. First, carrot embryos quickly multiply via secondary embryogenesis, which allows the use of a large number of genetically identical embryos for testing different treatment conditions. Second, the small embryo size allows for the performance of treatments in small volumes, which saves both space and reagents. Plantlet treatment lacks the mentioned advantages. Plantlet colchicine treatment with subsequent transfer to soil was not successful in different Apiaceae species, as tested previously [17]. We also observed an increased plantlet death when we tried to adapt treated plantlets of different genotypes, even a few weeks after plantlet colchicine or trifluralin treatment (unpublished data). However, in this study, we showed that plantlets developed from treated embryos successfully adapted to ex vitro conditions. Possibly, it can be attributed to the long recovery and regeneration time (3–4 months) after treatment.
Different carrot genotypes may vary in their response to antimitotic agent exposure in terms of treatment toxicity and effectiveness. Also, the cell division rate can vary between genotypes, which would require different treatment times. Therefore, testing a number of concentrations and different exposure times might be required for individual genotypes, which can be successfully performed on embryos. Besides, it can be a useful approach for improving the genome-doubling protocol by testing the addition of DMSO, Tween 20, or applying other antimitotic agents, such as APM, pronamid, or oryzalin [18]. In a broader sense, carrot embryos can be used for testing different compound toxicity and other specific effects.
5. Conclusions
Carrot embryo treatment with different trifluralin and colchicine concentrations showed that both compounds can be successfully used for genome doubling in carrot embryos in the tested range (0.01–1 g/L and 0.001–0.1 g/L, respectively). The highest doubling rate with the highest plantlet survival was obtained after 1 g/L colchicine and 0.01 g/L trifluralin treatment for 48 h. The highest percentage of diploid plantlets was observed at 1 g/L colchicine (34%) and 0.1 g/L trifluralin (28%). The highest percentage of plantlets with at least some diploid cells was after 0.01 and 0.1 g/L trifluralin treatment (over 70%). For the first time, we demonstrated that trifluralin can be successfully used for carrot genome doubling. We also showed that carrot embryos can serve as an efficient platform for testing different treatment regimens.
Author Contributions
Conceptualization, M.F., E.K. and E.D.; methodology, investigation, formal analysis, data curation, M.F.; resources, M.F. and E.D.; writing—original draft preparation, M.F.; writing—review and editing, M.F., E.K. and E.D.; supervision, E.D.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by a grant from the Russian Science Foundation, grant number 23-76-01034 (https://rscf.ru/en/project/23-76-01034/ (accessed on 5 May 2025)).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DH | Doubled haploid |
| PI | Propidium Iodide |
| APM | Amiprophos-methyl |
References
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Vurtz, T.S.; Domblides, E.A.; Soldatenko, A.V. Economic efficiency of obtaining carrot lines using classical and biotechnological methods. IOP Conf. Ser. Earth Environ. Sci. 2019, 395, 012–084. [Google Scholar] [CrossRef]
- Shmykova, N.; Domblides, E.; Vjurtts, T.; Domblides, A. Haploid Embryogenesis in Isolated Microspore Culture of Carrots (Daucus carota L.). Life 2020, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Matsubara, S.; Murakami, K. Haploid Plant Production by Anther Culture in Carrot (Daucus carota L.). Engei Gakkai Zasshi 1993, 62, 561–565. [Google Scholar] [CrossRef]
- Matsubara, S.; Dohya, N.; Murakami, K. Callus formation and regeneration of adventitious embryos from carrot, fennel and mitsuba microspores by anther and isolated microspore cultures. Acta Hortic. 1994, 392, 129–138. [Google Scholar] [CrossRef]
- Andersen, S.B.; Christiansen, I.; Farestveit, B. Carrot (Daucus carota L.): In Vitro Productionof Haploids and Field Trials. In Haploids in Crop Improvement I; Bajaj, Y.P.S., Ed.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 1990; Volume 12, pp. 393–402. [Google Scholar] [CrossRef]
- Li, J.-R.; Zhuang, F.-Y.; Ou, C.-G.; Hu, H.; Zhao, Z.-W.; Mao, J.-H. Microspore embryogenesis and production of haploid and doubled haploid plants in carrot (Daucus carota L.). Plant Cell Tissue Organ Cult. 2013, 112, 275–287. [Google Scholar] [CrossRef]
- Tyukavin, G.B.; Shmykova, N.A.; Monakhova, M.A. Cytological study of embryogenesis in cultured carrot anthers. Russ. J. Plant Physiol. 1999, 46, 767–773. [Google Scholar]
- Kiszczak, W.; Krzyżanowska, D.; Strycharczuk, K.; Kowalska, U.; Wolko, B.; Górecka, K. Determination of ploidy and homozygosity of carrot plants obtained from anther cultures. Acta Physiol. Plant. 2011, 33, 401–407. [Google Scholar] [CrossRef]
- Kiszczak, W.; Kowalska, U.; Kapuścińska, A.; Burian, M.; Górecka, K. Effect of low temperature on in vitro androgenesis of carrot (Daucus carota L.). In Vitro Cell. Dev. Biol.-Plant 2015, 51, 135–142. [Google Scholar] [CrossRef]
- Adamus, A.; Michalik, B. Anther cultures of carrot (Daucus carota L.). Folia Hortic. Pol. 2003, 15, 49–58. [Google Scholar]
- Kiełkowska, A.; Adamus, A. In vitro culture of unfertilized ovules in carrot (Daucus carota L.). Plant Cell Tissue Organ Cult. 2010, 102, 309–319. [Google Scholar] [CrossRef]
- Kiszczak, W.; Kowalska, U.; Burian, M.; Górecka, K. Induced androgenesis as a biotechnology method for obtaining DH plants in Daucus carota L. J. Hortic. Sci. Biotechnol. 2018, 93, 625–633. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Kiszczak, W. History and Current Status of Haploidization in Carrot (Daucus carota L.). Agronomy 2023, 13, 676. [Google Scholar] [CrossRef]
- Górecka, K.; Kowalska, U.; Krzyżanowska, D.; Kiszczak, W. Obtaining carrot (Daucus carota L.) plants in isolated microspore cultures. J. Appl. Genet. 2010, 51, 141–147. [Google Scholar] [CrossRef]
- Voronina, A.V.; Vishnyakova, A.V.; Monakhos, S.G.; Monakhos, G.F.; Ushanov, A.A.; Mironov, A.A. Effect of cultivation factors on embryogenesis in isolated microspore culture of carrot (Daucus carota L.). J. Water Land Dev. 2022, 55, 125–128. [Google Scholar] [CrossRef]
- Ferrie, A.M.R.; Bethune, T.D.; Mykytyshyn, M. Microspore embryogenesis in Apiaceae. Plant Cell Tissue Organ Cult. 2011, 104, 399–406. [Google Scholar] [CrossRef]
- Fomicheva, M.; Kulakov, Y.; Alyokhina, K.; Domblides, E. Spontaneous and Chemically Induced Genome Doubling and Polyploidization in Vegetable Crops. Horticulturae 2024, 10, 551. [Google Scholar] [CrossRef]
- Grzebelus, E.; Adamus, A. Effect of anti-mitotic agents on development and genome doubling of gynogenic onion (Allium cepa L.) embryos. Plant Sci. 2004, 167, 569–574. [Google Scholar] [CrossRef]
- Fayos, O.; Valles, M.P.; Garces-Claver, A.; Mallor, C.; Castillo, A.M. Doubled haploid production from Spanish onion (Allium cepa L.) germplasm: Embryogenesis induction, plant regeneration and chromosome doubling. Front. Plant Sci. 2015, 6, 384. [Google Scholar] [CrossRef]
- Mohammadi, P.P.; Moieni, A.; Ebrahimi, A.; Javidfar, F. Doubled haploid plants following colchicine treatment of microspore-derived embryos of oilseed rape (Brassica napus L.). Plant Cell Tissue Organ Cult. 2012, 108, 251–256. [Google Scholar] [CrossRef]
- Masuda, K.; Kikuta, Y.; Okazawa, Y. A revision of the medium for somatic embryogenesis in carrot suspension culture. J. Fac. Agric. Hokkaido Univ. 1981, 60, 183–193. [Google Scholar]
- Fomicheva, M.; Domblides, E. Mastering DNA Content Estimation by Flow Cytometry as an Efficient Tool for Plant Breeding and Biodiversity Research. Methods Protoc. 2023, 6, 18. [Google Scholar] [CrossRef]
- Garcia-Arias, F.; Sánchez-Betancourt, E.; Núñez, V. Fertility recovery of anther-derived haploid plants in Cape gooseberry (Physalis peruviana L.). Agron. Colomb. 2018, 36, 201–209. [Google Scholar] [CrossRef]
- Smýkalová, I.; Větrovcová, M.; Klíma, M.; Macháčková, I.; Griga, M. Efficiency of Microspore Culture for Doubled Haploid Production in the Breeding Project “Czech Winter Rape”. Czech J. Genet. Plant Breed. 2006, 42, 58–71. [Google Scholar] [CrossRef]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Madani, H.; Escrich, A.; Hosseini, B.; Sanchez-Muñoz, R.; Khojasteh, A.; Palazon, J. Effect of Polyploidy Induction on Natural Metabolite Production in Medicinal Plants. Biomolecules 2021, 11, 899. [Google Scholar] [CrossRef]
- Trojak-Goluch, A.; Kawka-Lipińska, M.; Wielgusz, K.; Praczyk, M. Polyploidy in Industrial Crops: Applications and Perspectives in Plant Breeding. Agronomy 2021, 11, 2574. [Google Scholar] [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, M.; Hafiz, I.; Silvestri, C. Studies on Colchicine Induced Chromosome Doubling for Enhancement of Quality Traits in Ornamental Plants. Plants 2019, 8, 194. [Google Scholar] [CrossRef]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In vitro Ploidy Manipulation for Crop Improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef]
- Ahmadi, B.; Ebrahimzadeh, H. In vitro androgenesis: Spontaneous vs. artificial genome doubling and characterization of regenerants. Plant Cell Rep. 2020, 39, 299–316. [Google Scholar] [CrossRef]
- Hansen, A.L.; Gertz, A.; Joersbo, M.; Andersen, S.B. Antimicrotubule herbicides for in vitro chromosome doubling in Beta vulgaris L. ovule culture. Euphytica 1998, 101, 231–237. [Google Scholar] [CrossRef]
- Gürel, S.; Gürel, E.; Kaya, Z. Doubled haploid plant production from unpollinated ovules of sugar beet (Beta vulgaris L.). Plant Cell Rep. 2000, 19, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Simmonds, D.H. Application of trifluralin to embryogenic microspore cultures to generate doubled haploid plants in Brassica napus. Physiol. Plant. 1995, 95, 304–309. [Google Scholar] [CrossRef]
- Hansen, N.J.P.; Andersen, S.B. In vitro chromosome doubling potential of colchicine, oryzalin, trifluralin, and APM in Brassica napus microspore culture. Euphytica 1996, 88, 159–164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).