Exogenous Abscisic Acid Enhances Waterlogging Tolerance in Lindera megaphylla
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experiment Design
2.3. Determination of Morphological Indicators
2.4. Determination of Physiological Indicators
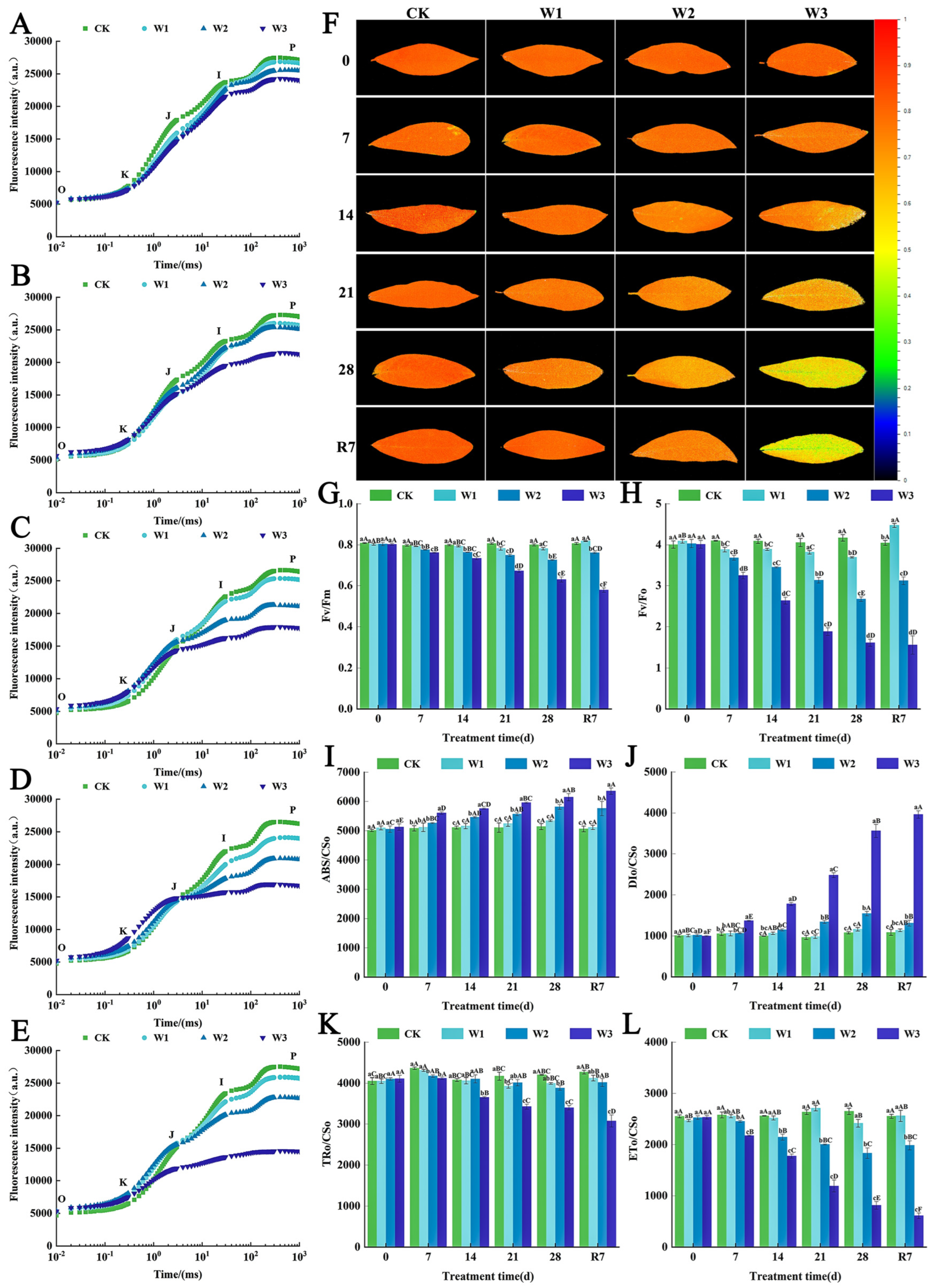
2.5. Determination of Chlorophyll Fluorescence Parameters
2.6. Correlation Analysis of Physiological Parameters
2.7. Data Statistics and Analysis
3. Results
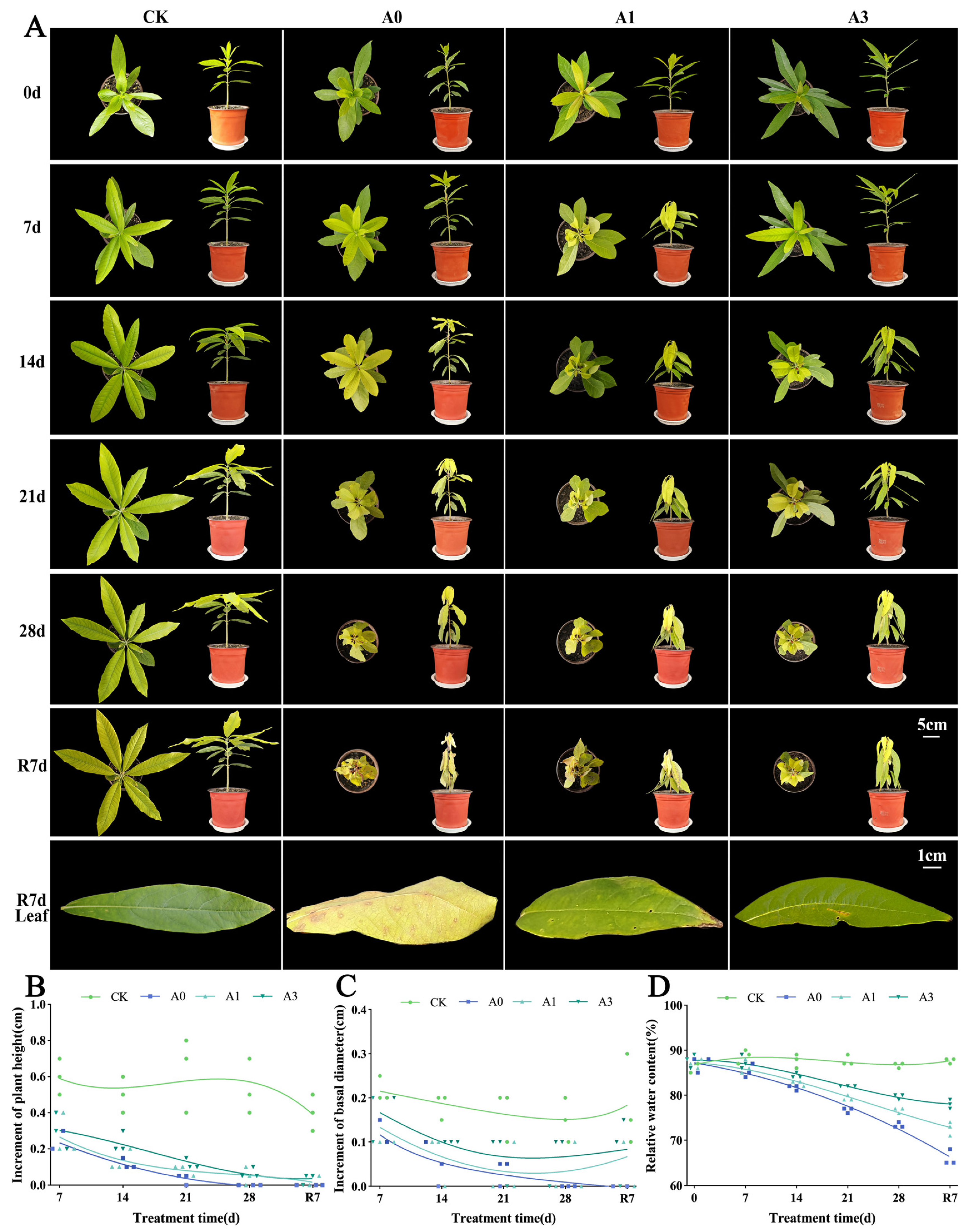
3.1. The Effects of Waterlogging Stress on the Growth, Survival, and Water Homeostasis of L. megaphylla Seedlings
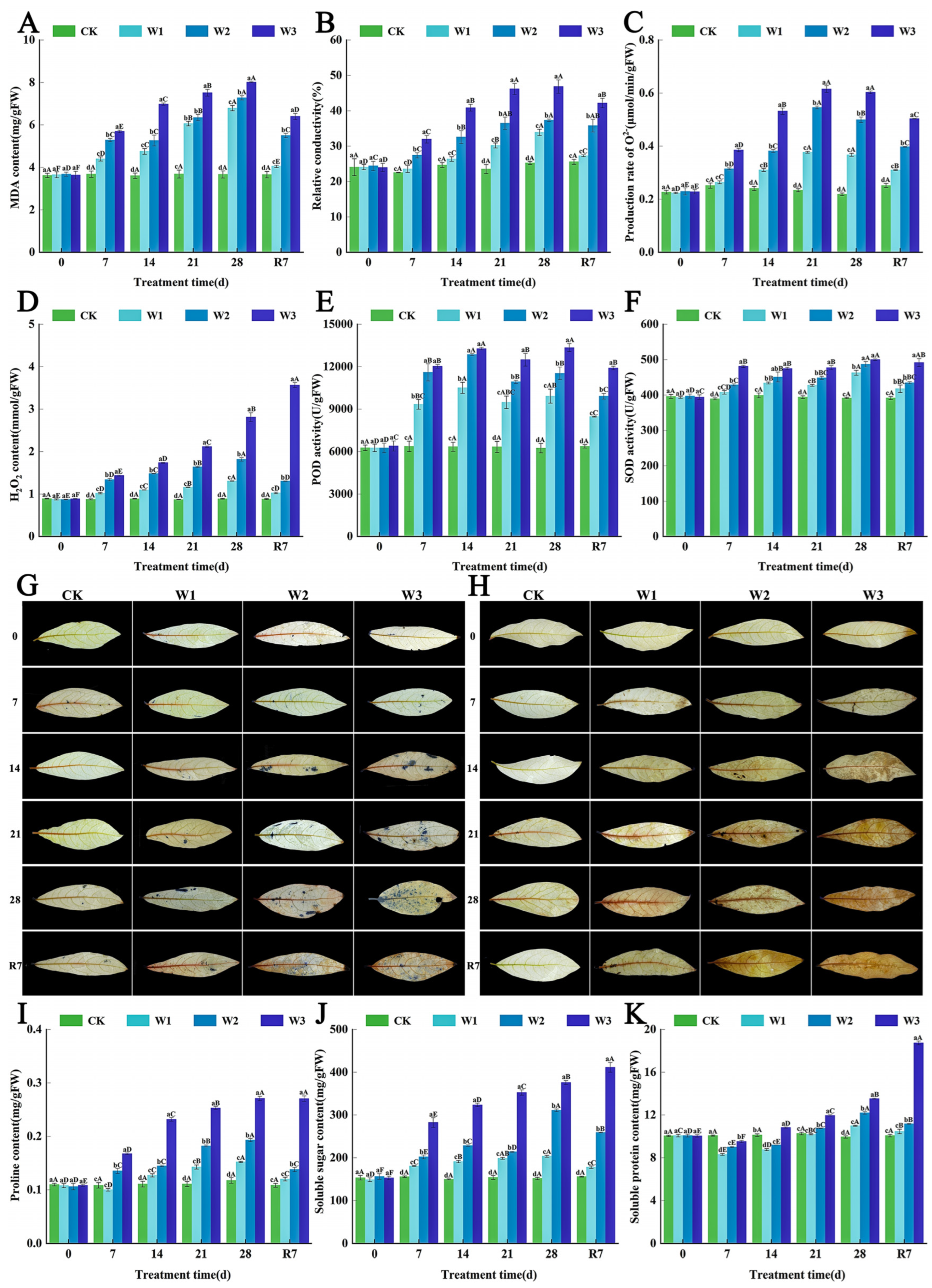
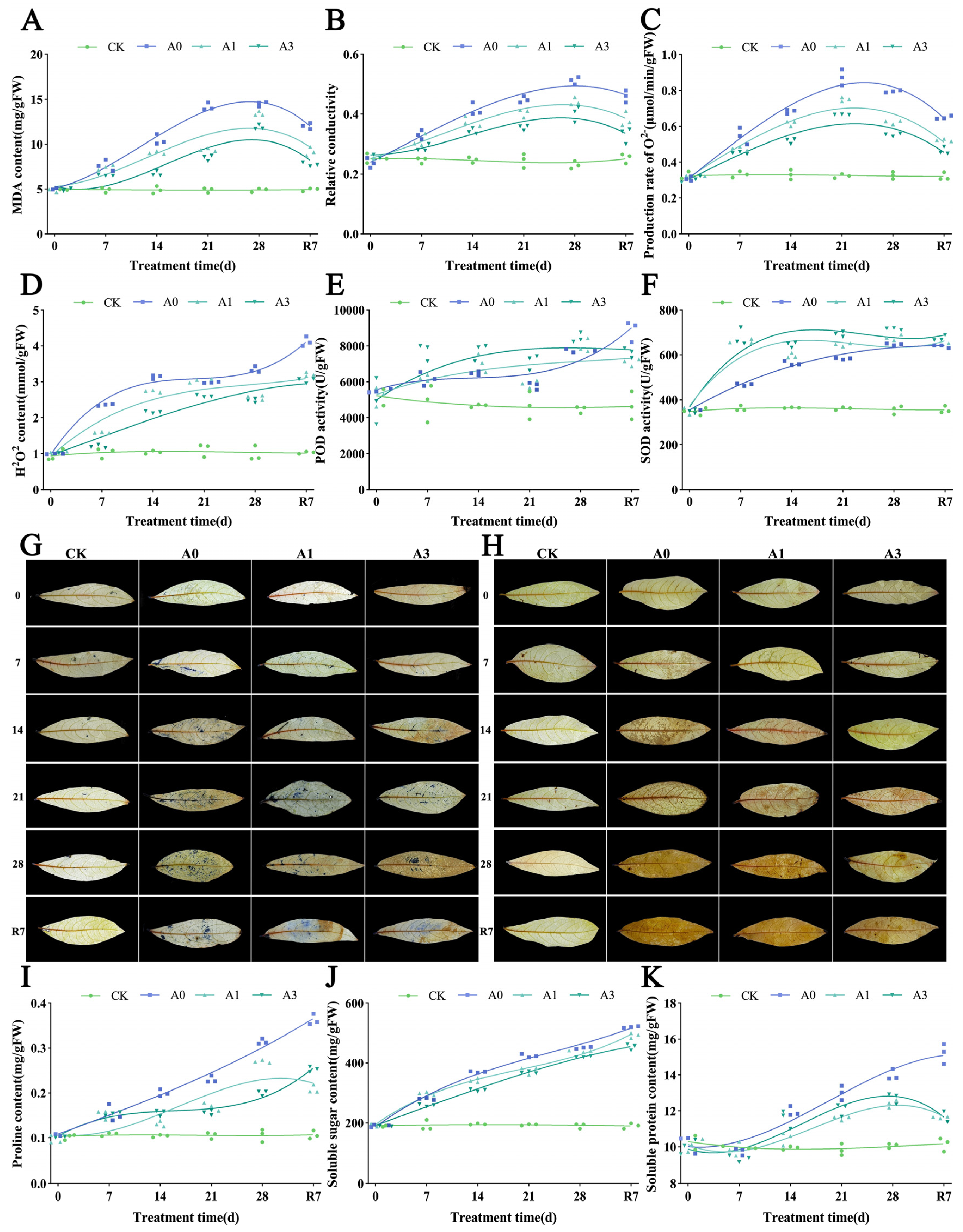
3.2. The Effects of Waterlogging Stress on Membrane Damage, Oxidative Stress, and Osmotic Regulation in the Leaves of L. megaphylla
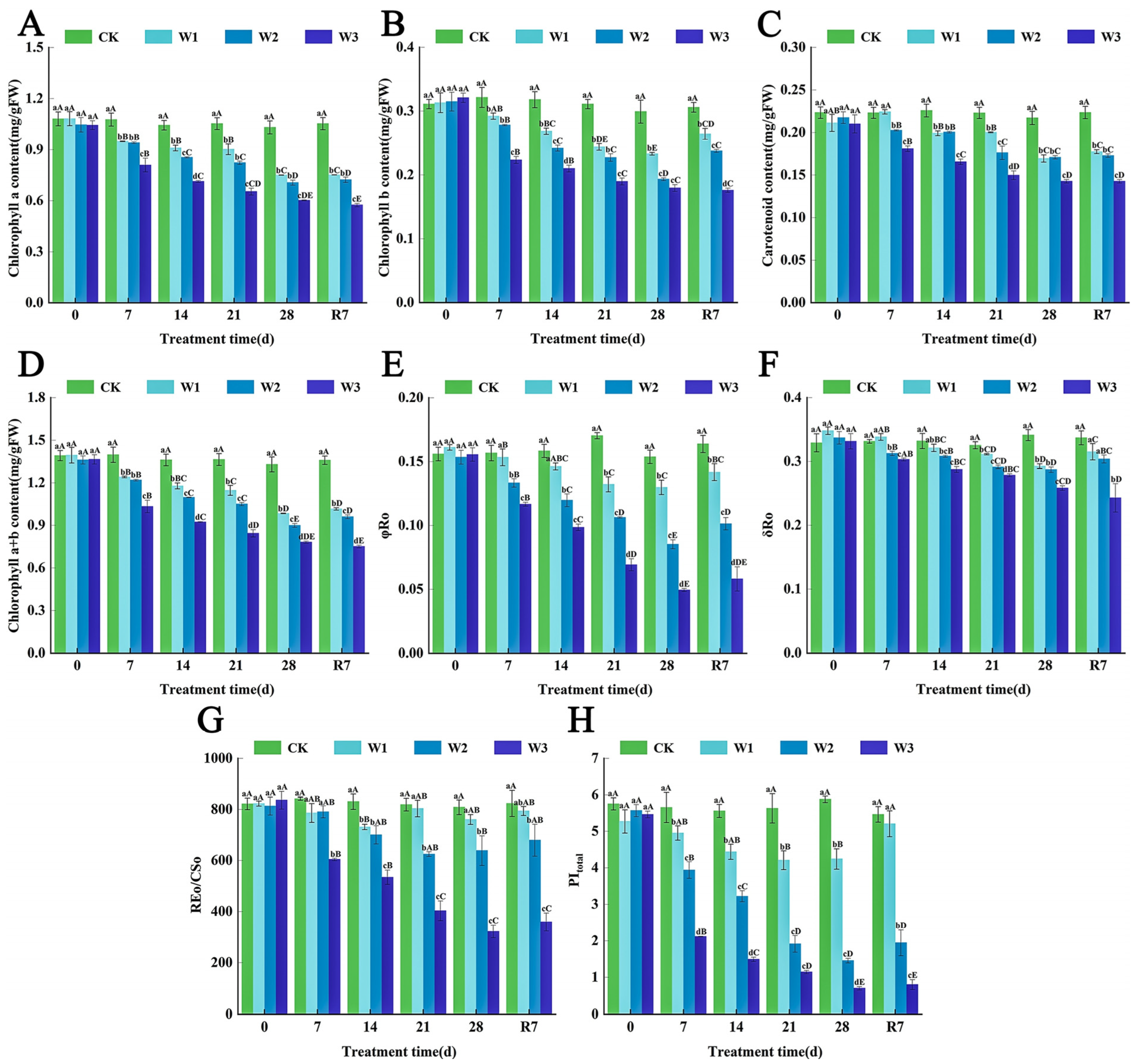
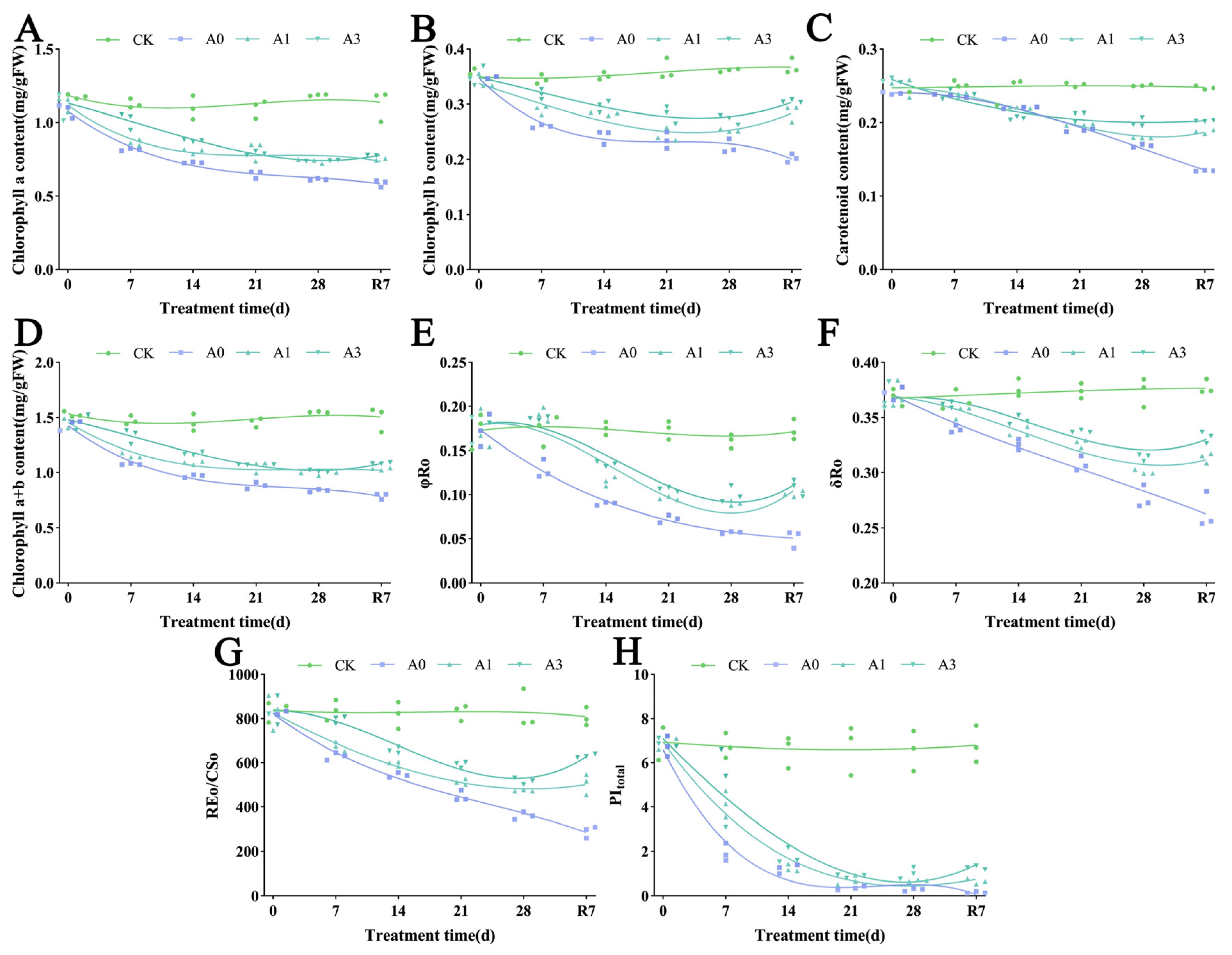
3.3. The Effects of Waterlogging Stress on Photosynthetic Pigment Metabolism and Photosystem Function in the Leaves of L. megaphylla
3.4. Comprehensive Effects of Waterlogging Stress on Physiological and Biochemical Indicators of L. megaphylla Seedlings
3.5. Exogenous ABA Alleviates Growth Inhibition and Water Imbalance
3.6. Regulation of Membrane Damage and Oxidative Stress by Exogenous ABA
3.7. The Synergistic Protective Mechanism of Exogenous ABA on the Photosynthetic System
4. Discussion
4.1. ABA Alleviates Waterlogging Damage by Maintaining Morphology and Water Homeostasis
4.2. ABA Mitigates Oxidative Damage Through an Antioxidant–Membrane Protection Network
4.3. ABA Maintains Cellular Homeostasis Through a Differential Osmotic Regulation Strategy
4.4. ABA Enhances Light Energy Utilization Efficiency by Synergistically Protecting the Photosynthetic Apparatus
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Liu, J.; Chen, P.; Zhang, X.; Wang, K.; Lu, J.; Li, Y. Selection and Validation of Optimal RT-qPCR Reference Genes for the Normalization of Gene Expression under Different Experimental Conditions in Lindera megaphylla. Plants 2023, 12, 2185. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Bai, Y.; Zhang, X.; Jiao, Q.; Chen, P.; Li, R.; Li, Y.; Xu, W.; Fu, Y.; et al. High-Quality Lindera megaphylla Genome Analysis Provides Insights into Genome Evolution and Allows for the Exploration of Genes Involved in Terpenoid Biosynthesis. Hortic. Res. 2025, 12, uhaf116. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-L.; Chen, C.-C.; Huang, Y.-L.; Ou, J.-C.; Hu, C.-P.; Chen, C.-F.; Chang, C. Anti-Tumor Effects of d-Dicentrine from the Root of Lindera megaphylla. Planta Medica 1998, 64, 212–215. [Google Scholar] [CrossRef]
- Aslam, A.; Mahmood, A.; Ur-Rehman, H.; Li, C.; Liang, X.; Shao, J.; Negm, S.; Moustafa, M.; Aamer, M.; Hassan, M.U. Plant Adaptation to Flooding Stress under Changing Climate Conditions: Ongoing Breakthroughs and Future Challenges. Plants 2023, 12, 3824. [Google Scholar] [CrossRef]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant Morphological, Physiological and Anatomical Adaption to Flooding Stress and the Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Gai, C.; Li, X.; Feng, X.; Lai, R.; Song, Y.; Zeng, R.; Chen, D.; Chen, Y. Waterlogging Tolerance of Actinidia valvata Dunn Is Associated with High Activities of Pyruvate Decarboxylase, Alcohol Dehydrogenase and Antioxidant Enzymes. Plants 2023, 12, 2872. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Alam, I.; Khoso, M.A.; Ali, Q.; Liu, F. Waterlogging Stress in Plants: Unraveling the Mechanisms and Impacts on Growth, Development, and Productivity. Environ. Exp. Bot. 2024, 224, 105824. [Google Scholar] [CrossRef]
- Olorunwa, O.J.; Adhikari, B.; Brazel, S.; Popescu, S.C.; Popescu, G.V.; Barickman, T.C. Short Waterlogging Events Differently Affect Morphology and Photosynthesis of Two Cucumber (Cucumis sativus L.) Cultivars. Front. Plant Sci. 2022, 13, 896244. [Google Scholar] [CrossRef]
- Sharma, S.; Bhatt, U.; Sharma, J.; Kalaji, H.M.; Mojski, J.; Soni, V. Ultrastructure, Adaptability, and Alleviation Mechanisms of Photosynthetic Apparatus in Plants under Waterlogging: A Review. Photosynthetica 2022, 60, 430–444. [Google Scholar] [CrossRef]
- Su, Q.; Sun, Z.; Liu, Y.; Lei, J.; Zhu, W.; Liao, N. Physiological and Comparative Transcriptome Analysis of the Response and Adaptation Mechanism of the Photosynthetic Function of Mulberry (Morus alba L.) Leaves to Flooding Stress. Plant Signal. Behav. 2022, 17, 2094619. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Zhang, L.; Li, J.; Fang, Y.; Zhao, L.; Ren, Y.; Chen, F. Abscisic Acid Enhances Tolerance to Spring Freeze Stress and Regulates the Expression of Ascorbate–Glutathione Biosynthesis-Related Genes and Stress-Responsive Genes in Common Wheat. Mol. Breed. 2020, 40, 108. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Q.; Li, Y.; Wang, Q.; Xu, F.; Dang, X.; Xu, W.; Zhang, J.; Miao, R. Abscisic Acid Is Required for Root Elongation Associated with Ca2+ Influx in Response to Water Stress. Plant Physiol. Biochem. 2021, 169, 127–137. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High Temperature and Drought Stress Cause Abscisic Acid and Reactive Oxygen Species Accumulation and Suppress Seed Germination Growth in Rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Li, M.; Zhao, W.; Du, Q.; Xiao, H.; Li, J.; Wang, J.; Shang, F. Abscisic Acid and Hydrogen Peroxide Regulate Proline Homeostasis in Melon Seedlings under Cold Stress by Forming a Bidirectional Closed Loop. Environ. Exp. Bot. 2023, 205, 105102. [Google Scholar] [CrossRef]
- Didi, D.A.; Su, S.; Sam, F.E.; Tiika, R.J.; Zhang, X. Effect of Plant Growth Regulators on Osmotic Regulatory Substances and Antioxidant Enzyme Activity of Nitraria tangutorum. Plants 2022, 11, 2559. [Google Scholar] [CrossRef]
- Zhang, D.; Du, Q.; Sun, P.; Lou, J.; Li, X.; Li, Q.; Wei, M. Physiological and Transcriptomic Analyses Revealed the Implications of Abscisic Acid in Mediating the Rate-Limiting Step for Photosynthetic Carbon Dioxide Utilisation in Response to Vapour Pressure Deficit in Solanum lycopersicum (Tomato). Front. Plant Sci. 2021, 12, 745110. [Google Scholar] [CrossRef]
- Gu, J.; Liu, P.; Nie, W.; Wang, Z.; Cui, X.; Fu, H.; Wang, F.; Qi, M.; Sun, Z.; Li, T.; et al. Abscisic Acid Alleviates Photosynthetic Damage in the Tomato Abscisic Acid-Deficient Mutant Sitiens and Protects Photosystem II from Damage via the WRKY22–PsbA under Low-Temperature Stress. J. Integr. Agric. 2024, 24, 546–563. [Google Scholar] [CrossRef]
- Chen, H.; Shi, Y.; An, L.; Yang, X.; Liu, J.; Dai, Z.; Zhang, Y.; Li, T.; Ahammed, G.J. Overexpression of SlWRKY6 enhances drought tolerance by strengthening antioxidant defense and stomatal closure via ABA signaling in Solanum lycopersicum L. Plant Physiol. Biochem. 2024, 213, 108855. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.; Ferrer-Gallego, R.; Pou, A.; Rivera-Moreno, M.; Benavente, J.L.; Mayordomo, C.; Deis, L.; Carbonell-Bejerano, P.; Pizzio, G.A.; Navarro-Payá, D.; et al. Chemical Activation of ABA Signaling in Grapevine through the iSB09 and AMF4 ABA Receptor Agonists Enhances Water Use Efficiency. Physiol. Plant. 2024, 176, e14635. [Google Scholar] [CrossRef]
- Wei, Y.-S.; Javed, T.; Liu, T.-T.; Ali, A.; Gao, S.-J. Mechanisms of Abscisic Acid (ABA)-Mediated Plant Defense Responses: An Updated Review. Plant Stress 2025, 15, 100724. [Google Scholar] [CrossRef]
- Fagerstedt, K.V.; Pucciariello, C.; Pedersen, O.; Perata, P. Recent Progress in Understanding the Cellular and Genetic Basis of Plant Responses to Low Oxygen Holds Promise for Developing Flood-Resilient Crops. J. Exp. Bot. 2024, 75, 1217–1233. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, G.; Cui, Z.; Kong, X.; Yu, X.; Gui, R.; Han, Y.; Li, Z.; Lang, H.; Hua, Y.; et al. Regain Flood Adaptation in Rice through a 14-3-3 Protein OsGF14h. Nat. Commun. 2022, 13, 5664. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, X.; Liao, J.; Ji, P.; Yang, J.; Cao, Z.; Duan, X.; Xiong, J.; Wang, Y.; Xu, C.; et al. Exogenous Abscisic Acid Improves Grain Filling Capacity under Heat Stress by Enhancing Antioxidative Defense Capability in Rice. BMC Plant Biol. 2023, 23, 619. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Zhang, Q.; Cui, M.; Zhao, M.; Li, N.; Wang, S.; Wu, R.; Zhang, L.; Cao, Y.; et al. The Interaction of ABA and ROS in Plant Growth and Stress Resistances. Front. Plant Sci. 2022, 13, 1050132. [Google Scholar] [CrossRef] [PubMed]
- Verslues, P.E.; Sharma, S. Proline Metabolism and Its Implications for Plant-Environment Interaction. Arab. Book/Am. Soc. Plant Biol. 2010, 8, e0140. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Teng, K.; Li, J.; Liu, L.; Han, Y.; Du, Y.; Zhang, J.; Sun, H.; Zhao, Q. Exogenous ABA Induces Drought Tolerance in Upland Rice: The Role of Chloroplast and ABA Biosynthesis-Related Gene Expression on Photosystem II during PEG Stress. Acta Physiol. Plant 2014, 36, 2219–2227. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Xu, Z.; Liu, G.; Fei, Y. Effects of water stress on the physiological characteristics of Lindera megaphylla and Cinnamomum camphora seedlings. J. South. Agriculture. 2015, 46, 1449–1454. (In Chinese) [Google Scholar]
- Chen, Y.; Zhang, X.; Liu, J.; Chen, P.; Li, Y.; Liu, H. Effects of low-temperature stress on growth and physiological characteristics of Lindera megaphylla seedlings. Plant Physiol. J. 2024, 60, 1759–1768. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.; Yin, F.; Ge, Z.; Xiang, J.; Fei, Y. Study on the resistance physiology of Lindera megaphylla under high-temperature stress. J. Yangtze Univ. (Nat. Sci. Ed.). 2012, 9, 8–10, 4. (In Chinese) [Google Scholar]
- Ding, Q.; Zhang, Y.; Liu, G.; Xu, Z.; Fei, Y. Effects of Water Stress on Photosynthesis Characteristics of Cinnamomum camphora and Lindera megaphylla Seedlings. J. Southwest For. Univ. 2015, 35, 14–20. (In Chinese) [Google Scholar]
- Salvatierra, A.; Pimentel, P.; Almada, R.; Hinrichsen, P. Exogenous GABA Application Transiently Improves the Tolerance to Root Hypoxia on a Sensitive Genotype of Prunus Rootstock. Environ. Exp. Bot. 2016, 125, 52–66. [Google Scholar] [CrossRef]
- Sha, S.; Wang, G.; Liu, J.; Wang, M.; Wang, L.; Liu, Y.; Geng, G.; Liu, J.; Wang, Y. Regulation of Photosynthetic Function and Reactive Oxygen Species Metabolism in Sugar Beet (Beta vulgaris L.) Cultivars under Waterlogging Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2024, 210, 108651. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Ma, T.; Zhou, C.; Sang, S.; Li, J.; Ji, S. Integrative Physiology and Transcriptome Sequencing Reveal Differences between G. Hirsutum and G. Barbadense in Response to Salt Stress and the Identification of Key Salt Tolerance Genes. BMC Plant Biol. 2024, 24, 787. [Google Scholar] [CrossRef]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderón-Urrea, A.; Wu, Y.; Tang, Z. Foliar Application of Abscisic Acid Mitigates Cadmium Stress and Increases Food Safety of Cadmium-Sensitive Lettuce (Lactuca sativa L.) Genotype. PeerJ 2020, 8, e9270. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhou, Q.; Yan, C.; Song, S.; Wang, X.; Wu, Z.; Wang, X.; Ma, C. Comparative Protein Profiling of Two Soybean Genotypes with Different Stress Tolerance Reveals Major Components in Drought Tolerance. Front. Sustain. Food Syst. 2023, 7, 1200608. [Google Scholar] [CrossRef]
- Bello, A.S.; Ben-Hamadou, R.; Hamdi, H.; Saadaoui, I.; Ahmed, T. Application of Cyanobacteria (Roholtiella sp.) Liquid Extract for the Alleviation of Salt Stress in Bell Pepper (Capsicum annuum L.) Plants Grown in a Soilless System. Plants 2021, 11, 104. [Google Scholar] [CrossRef]
- Grellet Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative Determination of Superoxide in Plant Leaves Using a Modified NBT Staining Method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The Apoplastic Oxidative Burst Peroxidase in Arabidopsis Is a Major Component of Pattern-Triggered Immunity. Plant Cell 2012, 24, 275–287. [Google Scholar] [CrossRef]
- Liu, Y. Physiological Response of Lindera megaphylla to High Temperature Stress and Alleviating Effect of Exogenous Salicylic Acid; Henan Agricultural University: Zhengzhou, China, 2024; (In Chinese). [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in Vivo Recording of Prompt and Delayed Fluorescence and 820-Nm Reflection Changes during Drying and after Rehydration of the Resurrection Plant Haberlea rhodopensis. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-Tocopherol Methyl Transferase Gene in Transgenic Brassica Juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Tyagi, A.; Ali, S.; Park, S.; Bae, H. Exploring the Potential of Multiomics and Other Integrative Approaches for Improving Waterlogging Tolerance in Plants. Plants 2023, 12, 1544. [Google Scholar] [CrossRef]
- Langan, P.; Bernád, V.; Walsh, J.; Henchy, J.; Khodaeiaminjan, M.; Mangina, E.; Negrão, S. Phenotyping for Waterlogging Tolerance in Crops: Current Trends and Future Prospects. J. Exp. Bot. 2022, 73, 5149–5169. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, S.; Wang, J.; Chen, Z.; Zhang, Y.; Duan, J.; Liu, P.; Wang, X.; Guo, J. Responses of Physiological, Morphological and Anatomical Traits to Abiotic Stress in Woody Plants. Forests 2023, 14, 1784. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, X. Reprogramming of Plant Central Metabolism in Response to Abiotic Stresses: A Metabolomics View. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.; Abou-Elwafa, S.F.; Shabala, S.; Xu, L. Understanding a Mechanistic Basis of ABA Involvement in Plant Adaptation to Soil Flooding: The Current Standing. Plants 2021, 10, 1982. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xi, B.; Wu, X.; Choat, B.; Feng, J.; Jiang, M.; Tissue, D. Unlocking Drought-Induced Tree Mortality: Physiological Mechanisms to Modeling. Front. Plant Sci. 2022, 13, 835921. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, N.; Yan, J. PYR/PYL/RCAR Abscisic Acid Receptors Regulate Root Cell Hydraulic Conductivity through Activating Aquaporin Expression. Int. J. Biol. 2018, 10, 7. [Google Scholar] [CrossRef]
- Lian, H.-L.; Yu, X.; Lane, D.; Sun, W.-N.; Tang, Z.-C.; Su, W.-A. Upland Rice and Lowland Rice Exhibited Different PIP Expression under Water Deficit and ABA Treatment. Cell Res. 2006, 16, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Q.; Jiao, F.; Yan, Z.; Zhang, H.; Zhang, Y.; Ding, Z.; Mu, C.; Liu, X.; Li, Y.; et al. Research Progress on the Mechanism of Salt Tolerance in Maize: A Classic Field That Needs New Efforts. Plants 2023, 12, 2356. [Google Scholar] [CrossRef]
- Pei, D.; Hua, D.; Deng, J.; Wang, Z.; Song, C.; Wang, Y.; Wang, Y.; Qi, J.; Kollist, H.; Yang, S.; et al. Phosphorylation of the Plasma Membrane H+-ATPase AHA2 by BAK1 Is Required for ABA-Induced Stomatal Closure in Arabidopsis. Plant Cell 2022, 34, 2708–2729. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, F. Exogenous Abscisic Acid Priming Modulates Water Relation Responses of Two Tomato Genotypes with Contrasting Endogenous Abscisic Acid Levels to Progressive Soil Drying under Elevated CO2. Front. Plant Sci. 2021, 12, 733658. [Google Scholar] [CrossRef] [PubMed]
- Giday, H.; Fanourakis, D.; Kjaer, K.H.; Fomsgaard, I.S.; Ottosen, C.-O. Threshold Response of Stomatal Closing Ability to Leaf Abscisic Acid Concentration during Growth. J. Exp. Bot. 2014, 65, 4361–4370. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Palta, J.A.; Liu, H.-Y.; Chen, Z.; Li, F.-M. Exogenous ABA Induces Osmotic Adjustment, Improves Leaf Water Relations and Water Use Efficiency, But Not Yield in Soybean under Water Stress. Agronomy 2019, 9, 395. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; She, Y.; Hu, C.; Wang, Q.; Wu, L.; You, C.; Ke, J.; He, H. Physiological Adaptation Mechanisms to Drought and Rewatering in Water-Saving and Drought-Resistant Rice. Int. J. Mol. Sci. 2022, 23, 14043. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, S.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Tavu, L.E.J.; Redillas, M.C.F.R. Oxidative Stress in Rice (Oryza sativa): Mechanisms, Impact, and Adaptive Strategies. Plants 2025, 14, 1463. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Nong, C.; Lin, T.; Fang, L.; Feng, X.; Chen, Y.; Lin, Y.; Lai, Z.; Miao, L. Piriformospora Indica Enhances Resistance to Fusarium Wilt in Strawberry by Increasing the Activity of Superoxide Dismutase, Peroxidase, and Catalase, While Reducing the Content of Malondialdehyde in the Roots. Horticulturae 2024, 10, 240. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Wang, C.; Wang, Q.; Wei, Q.; Liu, X.; Liu, Y.; Chen, A.; Jiang, J.; Zhao, X.; et al. Exogenous Melatonin Improves Drought Tolerance by Regulating the Antioxidant Defense System and Photosynthetic Efficiency in Fodder Soybean Seedings. Plants 2025, 14, 460. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, X.; Chen, Z.; Wang, H.; Shah, S.H.A.; Bai, A.; Liu, T.; Xiao, D.; Hou, X.; Li, Y. BcSRC2 Interacts with BcAPX4 to Increase Ascorbic Acid Content for Responding ABA Signaling and Drought Stress in Pak Choi. Hortic. Res. 2024, 11, uhae165. [Google Scholar] [CrossRef]
- Xiang, H.; Li, W.; Wang, X.; He, N.; Cao, D.; Wang, M.; Liu, J. Exogenous ABA Foliar Spray Treatment Alleviates Flooding Stress in Adzuki Bean (Vigna angularis) during the Seedling Stage. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12210. [Google Scholar] [CrossRef]
- Xu, H.; Dong, C.; Wu, Y.; Fu, S.; Tauqeer, A.; Gu, X.; Li, Q.; Niu, X.; Liu, P.; Zhang, X.; et al. The JA-to-ABA Signaling Relay Promotes Lignin Deposition for Wound Healing in Arabidopsis. Mol. Plant 2024, 17, 1594–1605. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, Z.; Kim, S.; Jeong, E.; Shim, J.S. Modulation of Lignin Biosynthesis for Drought Tolerance in Plants. Front. Plant Sci. 2023, 14, 1116426. [Google Scholar] [CrossRef]
- Shi, F.; Pan, Z.; Dai, P.; Shen, Y.; Lu, Y.; Han, B. Effect of Waterlogging Stress on Leaf Anatomical Structure and Ultrastructure of Phoebe sheareri Seedlings. Forests 2023, 14, 1294. [Google Scholar] [CrossRef]
- Yang, F.; Lv, G. Responses of Calligonum leucocladum to Prolonged Drought Stress through Antioxidant System Activation, Soluble Sugar Accumulation, and Maintaining Photosynthetic Homeostasis. Int. J. Mol. Sci. 2025, 26, 4403. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Han, Q.; Ma, D.; Hou, J.; Huang, X.; Wang, C.; Xie, Y.; Kang, G.; Guo, T. Characterizing Physiological and Proteomic Analysis of the Action of H2S to Mitigate Drought Stress in Young Seedling of Wheat. Plant Mol. Biol. Rep. 2018, 36, 45–57. [Google Scholar] [CrossRef]
- Estrada-Melo, A.C.; Ma, C.; Reid, M.S.; Jiang, C.-Z. Overexpression of an ABA Biosynthesis Gene Using a Stress-Inducible Promoter Enhances Drought Resistance in Petunia. Hortic. Res. 2015, 2, 15013. [Google Scholar] [CrossRef]
- Lee, B.-R.; La, V.H.; Park, S.-H.; Mamun, M.A.; Bae, D.-W.; Kim, T.-H. H2O2-Responsive Hormonal Status Involves Oxidative Burst Signaling and Proline Metabolism in Rapeseed Leaves. Antioxidants 2022, 11, 566. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2025, 14, 2. [Google Scholar] [CrossRef]
- Renzetti, M.; Bertolini, E.; Trovato, M. Proline Metabolism Genes in Transgenic Plants: Meta-Analysis under Drought and Salt Stress. Plants 2024, 13, 1913. [Google Scholar] [CrossRef]
- Baghery, M.A.; Kazemitabar, S.K.; Dehestani, A.; Mehrabanjoubani, P. Sesame (Sesamum indicum L.) response to drought stress: Susceptible and tolerant genotypes exhibit different physiological, biochemical, and molecular response patterns. Physiol. Mol. Biol. Plants 2023, 29, 1353–1369. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Rennenberg, H. Molecular and Physiological Responses of Trees to Waterlogging Stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, L.; Yang, X.; Wei, J.; Li, L.; Guo, E.; Kong, Y. Using Spectral Reflectance to Estimate the Leaf Chlorophyll Content of Maize Inoculated with Arbuscular Mycorrhizal Fungi under Water Stress. Front. Plant Sci. 2021, 12, 646173. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, S.; Liu, W.; Zhai, Z.; Pan, Y.; Liu, C.; Chern, M.; Wang, H.; Huang, M.; Zhang, Z.; et al. A Chlorophyll a Oxygenase 1 Gene ZmCAO1 Contributes to Grain Yield and Waterlogging Tolerance in Maize. J. Exp. Bot. 2021, 72, 3155–3167. [Google Scholar] [CrossRef]
- He, L.; Yu, L.; Li, B.; Du, N.; Guo, S. The Effect of Exogenous Calcium on Cucumber Fruit Quality, Photosynthesis, Chlorophyll Fluorescence, and Fast Chlorophyll Fluorescence during the Fruiting Period under Hypoxic Stress. BMC Plant Biol. 2018, 18, 180. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Chen, M.; Zhou, L.; Li, Z.; Zhao, Q.; Pan, G.; Zaidi, S.-H.-R.; Cheng, F. Involvement of Abscisic Acid in PSII Photodamage and D1 Protein Turnover for Light-Induced Premature Senescence of Rice Flag Leaves. PLoS ONE 2016, 11, e0161203. [Google Scholar] [CrossRef] [PubMed]
- Lima-Melo, Y.; Kılıç, M.; Aro, E.-M.; Gollan, P.J. Photosystem I Inhibition, Protection and Signalling: Knowns and Unknowns. Front. Plant Sci. 2021, 12, 791124. [Google Scholar] [CrossRef]
- Gu, L.; Grodzinski, B.; Han, J.; Marie, T.; Zhang, Y.-J.; Song, Y.C.; Sun, Y. Granal Thylakoid Structure and Function: Explaining an Enduring Mystery of Higher Plants. New Phytol. 2022, 236, 319–329. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research Progress in the Interconversion, Turnover and Degradation of Chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef]
- Wang, P.; Richter, A.S.; Kleeberg, J.R.W.; Geimer, S.; Grimm, B. Post-Translational Coordination of Chlorophyll Biosynthesis and Breakdown by BCMs Maintains Chlorophyll Homeostasis during Leaf Development. Nat. Commun. 2020, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Pospíšilová, J.; Synková, H.; Haisel, D.; Baťková, P. Effect of Abscisic Acid on Photosynthetic Parameters during Ex Vitro Transfer of Micropropagated Tobacco Plantlets. Biol. Plant 2009, 53, 11–20. [Google Scholar] [CrossRef]
- Wang, K.; Shen, Y.; Wang, H.; He, S.; Kim, W.S.; Shang, W.; Wang, Z.; Shi, L. Effects of Exogenous Salicylic Acid (SA), 6-Benzylaminopurine (6-BA), or Abscisic Acid (ABA) on the Physiology of Rosa Hybrida ‘Carolla’ under High-Temperature Stress. Horticulturae 2022, 8, 851. [Google Scholar] [CrossRef]




| Groups | ABA Concentration (μmol/L) | Waterlogging Stress |
|---|---|---|
| CK | 0 | − |
| A0 | 0 | + |
| A1 | 1 | + |
| A3 | 3 | + |
| Time | 0 d | 7 d | 14 d | 21 d | 28 d | R7d | Survival Rate | |
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| CK | - | - | - | - | - | - | 100% | |
| W1 | - | - | - | - | c | c+ | 100% | |
| W2 | - | a | a, b | a, b+, c | a+, b++, c+ | a++, b++, c+, d | 91.7% | |
| W3 | - | a, b | a, b+ | a+, b++, c, d | a++, b++, c+, d | a++, b++, c++, d+, e | 30% | |
| Time | 0 d | 7 d | 14 d | 21 d | 28 d | R7d | Survival Rate | |
|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||
| CK | - | - | - | - | - | - | 100% | |
| A0 | - | a | a+, b, c | a++, b+, c++ | a++, b++, c++, d, e | a++, b++, c++, d+, e+ | 33% | |
| A1 | - | a, b | a+, b+ | a+, b++, c+ | a++, b++, c+ | a++, b++, c++, d | 43% | |
| A3 | - | - | a, b+ | a+, b++, c | a+, b++, c+ | a++, b++, c+ | 53% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yu, Y.; Niu, X.; Zhao, Y.; Jiang, J.; Lu, J.; Li, Y.; Chen, P.; Liu, H. Exogenous Abscisic Acid Enhances Waterlogging Tolerance in Lindera megaphylla. Horticulturae 2025, 11, 1433. https://doi.org/10.3390/horticulturae11121433
Xu Y, Yu Y, Niu X, Zhao Y, Jiang J, Lu J, Li Y, Chen P, Liu H. Exogenous Abscisic Acid Enhances Waterlogging Tolerance in Lindera megaphylla. Horticulturae. 2025; 11(12):1433. https://doi.org/10.3390/horticulturae11121433
Chicago/Turabian StyleXu, Yijie, Yuhan Yu, Xinya Niu, Yahui Zhao, Jutang Jiang, Jiuxing Lu, Yonghua Li, Peng Chen, and Hongli Liu. 2025. "Exogenous Abscisic Acid Enhances Waterlogging Tolerance in Lindera megaphylla" Horticulturae 11, no. 12: 1433. https://doi.org/10.3390/horticulturae11121433
APA StyleXu, Y., Yu, Y., Niu, X., Zhao, Y., Jiang, J., Lu, J., Li, Y., Chen, P., & Liu, H. (2025). Exogenous Abscisic Acid Enhances Waterlogging Tolerance in Lindera megaphylla. Horticulturae, 11(12), 1433. https://doi.org/10.3390/horticulturae11121433





