Interaction of BPC1 and ALDH2 Affects Natural De-Astringency in Chinese PCNA Persimmon (Diospyros kaki)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Analysis of Tannin Content
2.3. Phylogenetic Analysis
2.4. Extraction of RNA and Synthesis of cDNA
2.5. qRT-PCR Expression Analysis
2.6. Isolation of Genes and Promoters
2.7. Dual Luciferase Activity Assay
2.8. Subcellular Localization
2.9. Vector Construction and Plant Transformation
3. Results
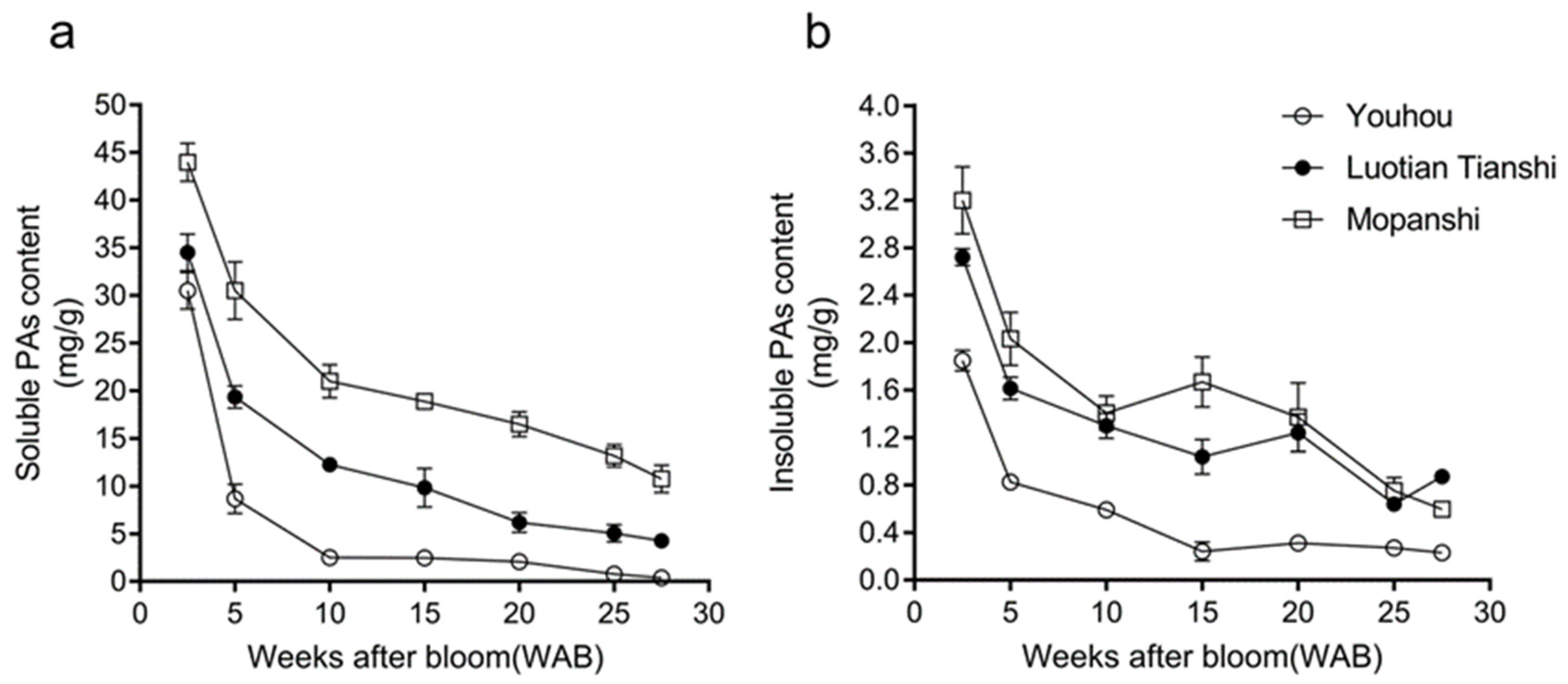
3.1. Changes in Tannin Content During Fruit Development
3.2. Sequence Analysis of the DkBPC1 Gene
3.3. Expression Patterns of DkBPC1 During Fruit Development
3.4. DkBPC1 Is Located in the Nucleus
3.5. DkBPC1 Enhances the Activity of the DkALDH2b Promoter
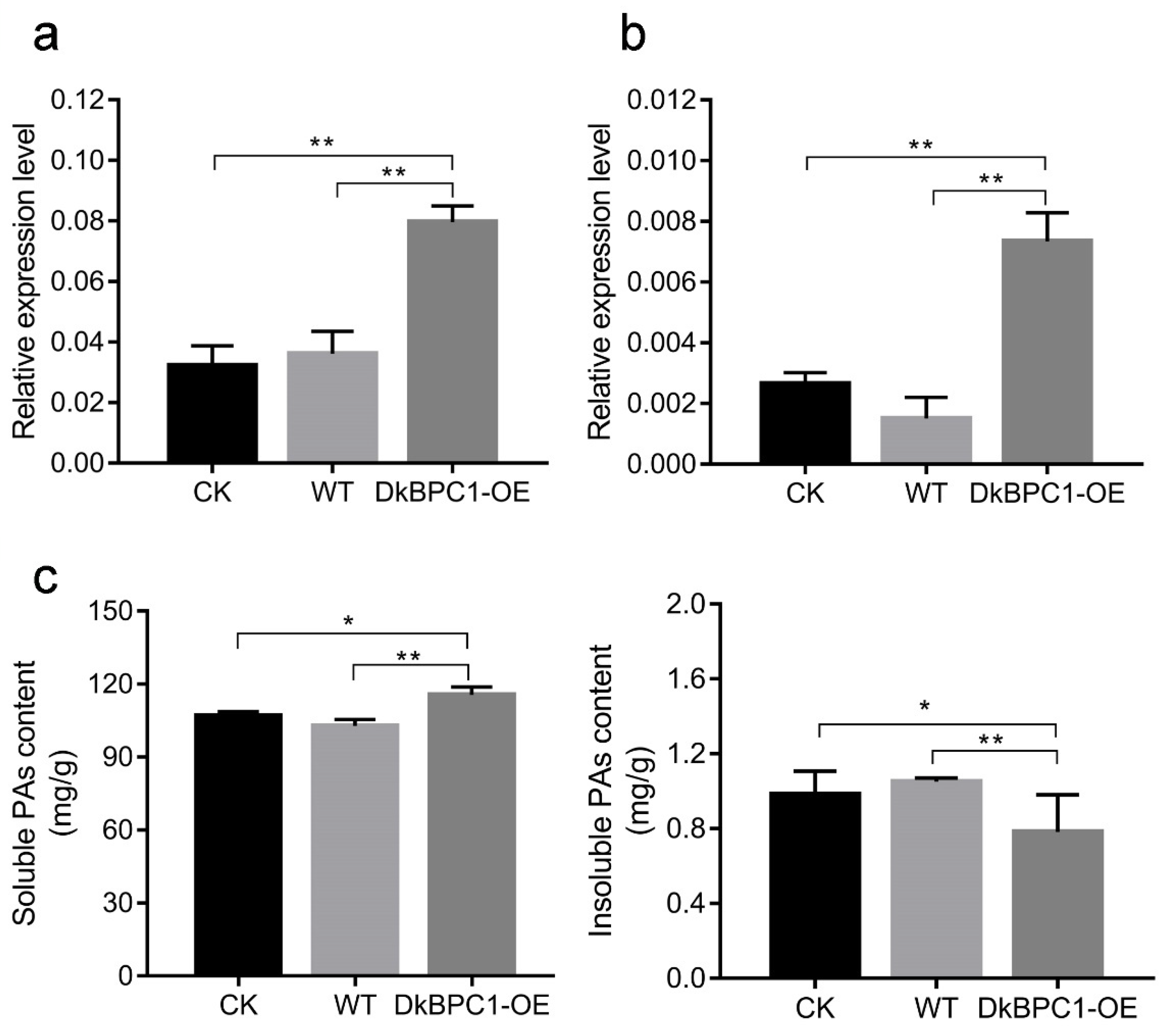
3.6. Transient Expression of DkBPC1 in Persimmon Leaves In Vivo
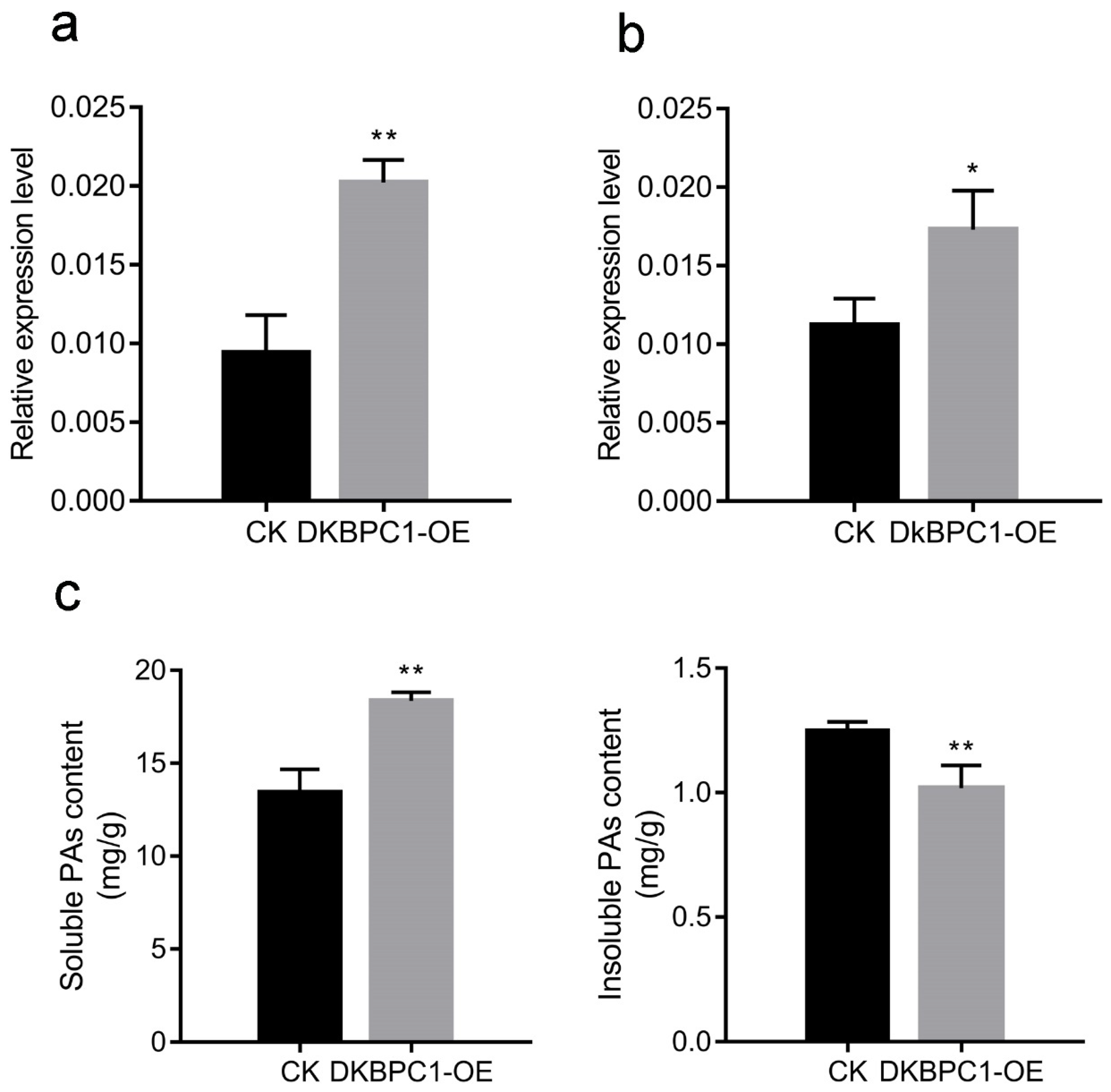
3.7. Transient Overexpression of DkBPC1 in Persimmon Fruit Discs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Z.; Wang, R. Persimmon in China: Domestication and traditional utilizations of genetic resources. Adv. Hortic. Sci. 2008, 22, 239–243. [Google Scholar]
- Tang, D.; Zhang, Q.; Xu, L.; Guo, D.; Luo, Z. Number of species and geographical distribution of Diospyros L. (Ebenaceae) in China. Hortic. Plant J. 2019, 2, 59–69. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, D.; Sheng, Y.; Tao, J.; Yang, Y. Carotenoids in fruits of different persimmon cultivars. Molecules 2011, 16, 624–636. [Google Scholar] [CrossRef]
- Akagi, T.; Katayama-Ikegami, A.; Yonemori, K. Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci. Hortic. 2011, 130, 373–380. [Google Scholar] [CrossRef]
- Kanzaki, S. The origin and cultivar development of Japanese persimmon (Diospyros kaki Thunb.). J. Soc. Food Sci. Technol. Jpn. 2016, 63, 328–330. [Google Scholar] [CrossRef]
- Sato, A.; Yamada, M. Persimmon breeding in Japan for pollination-constant non-astringent (PCNA) type with marker-assisted selection. Breed. Sci. 2016, 66, 60–68. [Google Scholar] [CrossRef]
- You, W.; Ma, Y.; Chen, W.; Zhang, Q.; Luo, Z. Quantitative genotyping of CPCNA locus advances genetic strategies in PCNA persimmon breeding. Sci. Hortic. 2024, 330, 113105. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, Q.; Guo, D.; Luo, Z. Effectiveness of the RO2 marker for the identification of non-astringency trait in Chinese PCNA persimmon and its possible segregation ratio in hybrid F1 population. Sci. Hortic. 2013, 150, 227–231. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, M.; Xu, L.; Zhang, Q.; Zhou, C.; Hu, X.; Luo, Z. Recent advances in natural deastringency and genetic improvement of Chinese PCNA persimmon (Diospyros kaki). Horticulturae 2023, 9, 1273. [Google Scholar] [CrossRef]
- Xu, J. Functional Characterization of ALDH2 Genes Involved in Natural Deastringency in Chinese PCNA Persimmon. Ph.D. Thesis, Huazhong Agricuture University, Wuhan, China, 2018. [Google Scholar]
- Sangwan, I.; O’brian, M.R. Identification of a soybean protein that interacts with GAGA element dinucleotide repeat DNA. Plant Physiol. 2002, 129, 1788–1794. [Google Scholar] [CrossRef]
- Santi, L.; Wang, Y.; Stile, M.R.; Berendzen, K.; Wanke, D.; Roig, C.; Pozzi, C.; Müller, K.; Müller, J.; Rohde, W.; et al. The GA octodinucleotide repeat binding factor BBR participates in the transcriptional regulation of the homeobox gene Bkn3. Plant J. 2003, 34, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Kooiker, M.; Airoldi, C.A.; Losa, A.; Manzotti, P.S.; Finzi, L.; Kater, M.M.; Colombo, L. BASIC PENTACYSTEINE1, a GA binding protein that induces conformational changes in the regulatory region of the homeotic Arabidopsis gene SEEDSTICK. Plant Cell 2005, 17, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, S.; Wang, S.; Ji, X.; Liu, Z. Genome-wide identification and characterization of BPC transcription factors in tobacco (Nicotiana tabacum). Agronomy 2025, 15, 2084. [Google Scholar] [CrossRef]
- Wai, A.; Divya, D.; Park, J.; Lae-Hyeon, C.; Dohyeon, K.; Md, M.; Chang-Kil, K.; Chung, M. Genome wide identification of BBP/BPC transcription factor in tomato and its expression profiling in response to abiotic stress. Plant Biotechnol. Rep. 2024, 18, 759–776. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Yan, J. BPC1 and BPC2 positively regulates the waterlogging stress tolerance in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2025, 747, 151296. [Google Scholar] [CrossRef]
- Simonini, S.; Kater, M.M. Class I BASIC PENTACYSTEINE factors regulate HOMEOBOX genes involved in meristem size maintenance. J. Exp. Bot. 2014, 65, 1455–1465. [Google Scholar] [CrossRef]
- Sahu, A.; Singh, R.; Verma, P.K. Plant BBR/BPC transcription factors: Unlocking multilayered regulation in development, stress and immunity. Planta 2023, 258, 31. [Google Scholar] [CrossRef]
- Pang, Y.; Peel, G.J.; Sharma, S.B.; Tang, Y.; Dixon, R.A. A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc. Natl. Acad. Sci. USA 2008, 105, 14210–14215. [Google Scholar] [CrossRef]
- Hellens, R.P.; Edwards, E.A.; Leyland, N.R.; Bean, S.; Mullineaux, P.M. pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 2000, 42, 819–832. [Google Scholar] [CrossRef]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient plant expression vectors for functional genomics, quantification of promoter activity and RNA silencing. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Mo, R.; Huang, Y.; Yang, S.; Zhang, Q.; Luo, Z. Development of Agrobacterium-mediated transient transformation in persimmon (Diospyros kaki Thunb.). Sci. Hortic. 2015, 192, 29–37. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, C.; Wu, X.; Chen, W.; Luo, Z.; Xu, L.; Zhang, Q. DkDTX1/MATE1 mediates the accumulation of proanthocyanidin and affects astringency in persimmon. Plant Cell Environ. 2024, 47, 5205–5219. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, M.; Li, X.; Song, K.; Shi, D. Fruit Astringency: Mechanisms, Technologies, and Future Directions. Horticulturae 2025, 11, 699. [Google Scholar] [CrossRef]
- Guan, C.; Wang, M.; Zhang, Y.; Ruan, X.; Zhang, Q.; Luo, Z.; Yang, Y. DkWRKY interacts with pyruvate kinase gene DkPK1 and promotes natural deastringency in C-PCNA persimmon. Plant Sci. 2020, 290, 110285. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, Q.; Li, J.; Liu, Y.; Xu, L.; Zhang, Q.; Luo, Z. DkMYB14 is a bifunctional transcription factor that regulates the accumulation of proanthocyanidin in persimmon fruit. Plant J. 2021, 106, 1708–1727. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.J.; Williams, L.A.; Monfared, M.M.; Gallagher, T.L.; Kraft, E.A.; Nelson, C.G.; Gasser, C.S. Definition and interactions of a positive regulatory element of the Arabidopsis INNER NO OUTER promoter. Plant J. 2004, 37, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.; Dubreucq, B.; Roudier, F.; Dubos, C.; Lepiniec, L. Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell 2011, 23, 4065–4078. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mohamed, D.; Dowhanik, S.; Petrella, R.; Gregis, V.; Li, J.; Wu, L.; Gazzarrini, S. Spatiotemporal restriction of FUSCA3 expression by class I BPCs promotes ovule development and coordinates embryo and endosperm growth. Plant Cell 2020, 32, 1886–1904. [Google Scholar] [CrossRef]
- Mo, R.; Yang, S.; Huang, Y.; Chen, W.; Zhang, Q.; Luo, Z. ADH and PDC genes involved in tannins coagulation leading to natural de-astringency in Chinese pollination constant and non-astringency persimmon (Diospyros kaki Thunb.). Tree Genet. Genomes 2016, 12, 17. [Google Scholar] [CrossRef]
- Xu, J.; Ding, J.; Gan, J.; Mo, R.; Xu, L.; Zhang, Q.; Luo, Z. ALDH2 genes are negatively correlated with natural deastringency in Chinese PCNA persimmon (Diospyros kaki Thunb.). Tree Geneti. Genomes 2017, 13, 122. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, X.; Zhang, L.; Zhang, F.; Wang, S. Interaction of BPC1 and ALDH2 Affects Natural De-Astringency in Chinese PCNA Persimmon (Diospyros kaki). Horticulturae 2025, 11, 1435. https://doi.org/10.3390/horticulturae11121435
Xu J, Li X, Zhang L, Zhang F, Wang S. Interaction of BPC1 and ALDH2 Affects Natural De-Astringency in Chinese PCNA Persimmon (Diospyros kaki). Horticulturae. 2025; 11(12):1435. https://doi.org/10.3390/horticulturae11121435
Chicago/Turabian StyleXu, Junchi, Xia Li, Li Zhang, Fei Zhang, and Shiping Wang. 2025. "Interaction of BPC1 and ALDH2 Affects Natural De-Astringency in Chinese PCNA Persimmon (Diospyros kaki)" Horticulturae 11, no. 12: 1435. https://doi.org/10.3390/horticulturae11121435
APA StyleXu, J., Li, X., Zhang, L., Zhang, F., & Wang, S. (2025). Interaction of BPC1 and ALDH2 Affects Natural De-Astringency in Chinese PCNA Persimmon (Diospyros kaki). Horticulturae, 11(12), 1435. https://doi.org/10.3390/horticulturae11121435





