Impact of Light Spectra and Substrate Composition on the Bioefficiency, Nutritional Content, and Morphology of Oyster Mushrooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture, Spawn, and Substrate Production
2.2. Mushroom Production
2.3. Nutritional Analyses
2.4. Statistical Analysis
3. Results
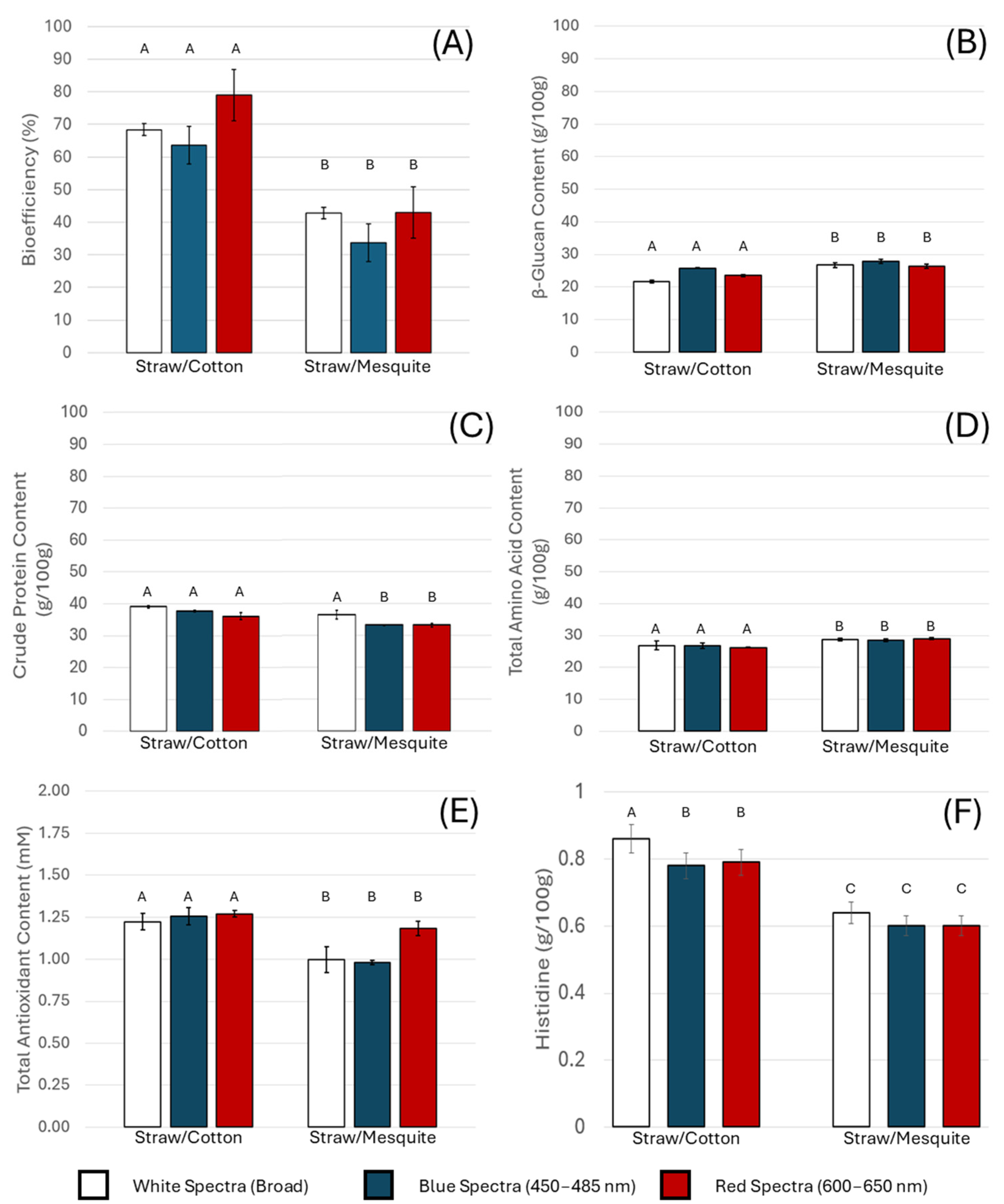
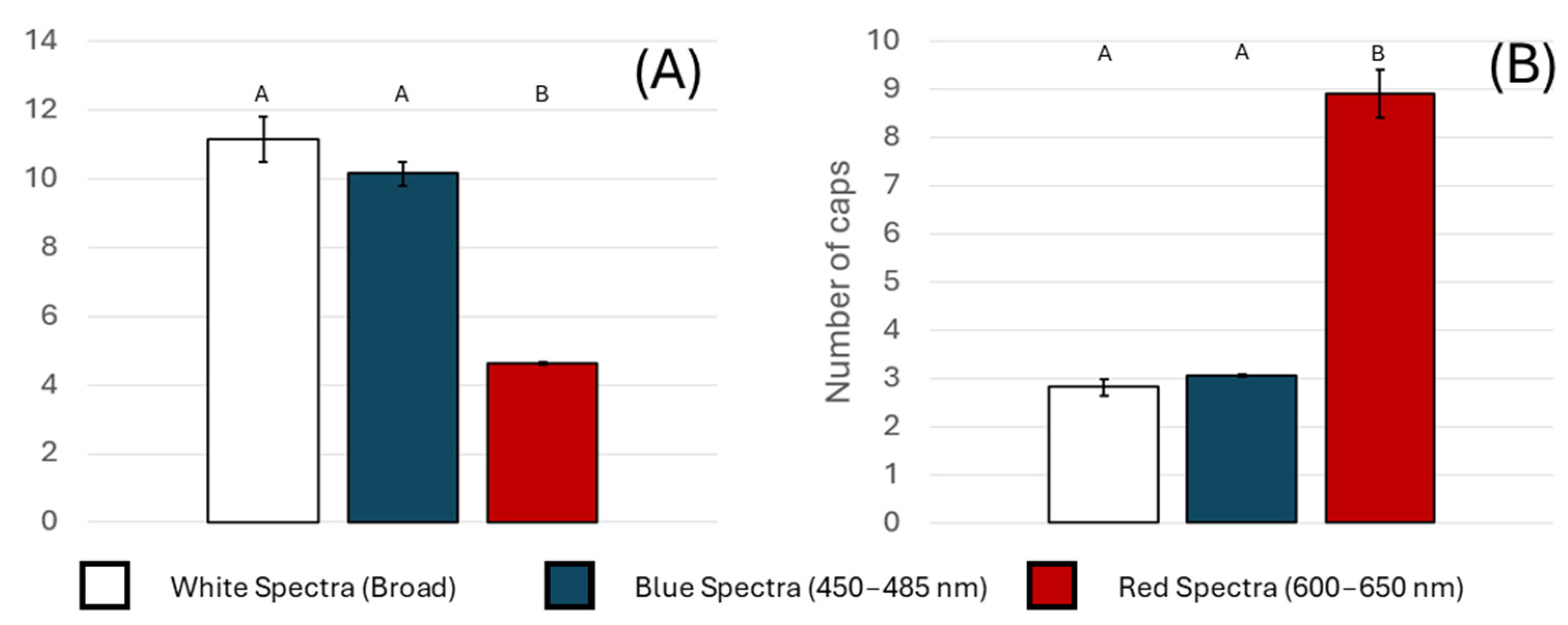
3.1. Impact of Light Spectra
3.2. Effect of Substrate Composition
3.3. Statistical Summary
4. Discussion
4.1. Light Spectra
4.2. Importance of Substrate Selection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Crops and Livestock Products. 2025. Available online: https://www.fao.org/faostat/en/#data/QCL/metadata (accessed on 7 March 2025).
- Prasad, D.; Singh, R.P. Mushroom Production in the World: An Overview. In Proceedings of the Vision 2047: Sustainable Developments Towards Atma Nirbhar Bharat (VSANB-2023), Chandigarh, India, 23–24 December 2023. [Google Scholar]
- Aditya; Neeraj; Jarial, R.S.; Jarial, K.; Bhatia, J.N. Comprehensive Review on Oyster Mushroom Species (Agaricomycetes): Morphology, Nutrition, Cultivation and Future Aspects. Heliyon 2024, 10, e26539. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An Insight into the Nutritional and Medicinal Value of Edible Mushrooms: A Natural Treasury for Human Health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic Composition and Amino Acid Contents of Mushrooms Cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef]
- Stastny, J.; Marsik, P.; Tauchen, J.; Bozik, M.; Mascellani, A.; Havlik, J.; Landa, P.; Jablonsky, I.; Treml, J.; Herczogova, P.; et al. Antioxidant and Anti-Inflammatory Activity of Five Medicinal Mushrooms of the Genus Pleurotus. Antioxidants 2022, 11, 1569. [Google Scholar] [CrossRef]
- Effiong, M.E.; Umeokwochi, C.P.; Afolabi, I.S.; Chinedu, S.N. Comparative Antioxidant Activity and Phytochemical Content of Five Extracts of Pleurotus Ostreatus (Oyster Mushroom). Sci. Rep. 2024, 14, 3794. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production Through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Royse, D. Influence of Spawn Rate and Commercial Delayed Release Nutrient Levels on Pleurotus Cornucopiae (Oyster Mushroom) Yield, Size, and Time to Production. Appl. Microbiol. Biotechnol. 2002, 58, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Rossi, J. Using Cotton Byproducts in Beef Cattle Diets. Available online: https://fieldreport.caes.uga.edu/publications/B1311/using-cotton-byproducts-in-beef-cattle-diets (accessed on 17 May 2023).
- Correa Uriburu, F.M.; Cattaneo, F.; Maldonado, L.M.; Zampini, I.C.; Alberto, M.R.; Isla, M.I. Prosopis Alba Seed as a Functional Food Waste for Food Formulation Enrichment. Foods 2022, 11, 2857. [Google Scholar] [CrossRef]
- Patil, S.S.; Ahmed, S.A.; Telang, S.; Mushtaq Vaseem Baig, M. The Nutritional Value of Pleurotus Ostreatus (Jacq.:Fr.) Kumm Cultivated on Different Lignocellulosic Agrowastes. Innov. Rom. Food Biotechnol. 2010, 7, 66–76. [Google Scholar]
- Badu, M.; Twumasi, S.K.; Boadi, N.O. Effects of Lignocellulosic in Wood Used as Substrate on the Quality and Yield of Mushrooms. FNS 2011, 2, 780–784. [Google Scholar] [CrossRef]
- Chang, S.-T.; Miles, P. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-8493-1043-0. [Google Scholar]
- Hoa, H.T.; Wang, C.-L.; Wang, C.-H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Coon, D.; Lindow, L.; Boz, Z.; Martin-Ryals, A.; Zhang, Y.; Correll, M.J. Reporting and Practices of Sustainability in Controlled Environment Agriculture: A Scoping Review. Environ. Syst. Decis. 2024, 44, 301–326. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Jankauskienė, J.; Viršilė, A.; Sirtautas, R.; Novičkovas, A.; Sakalauskienė, S.; Sakalauskaitė, J.; Duchovskis, P. LED Irradiance Level Affects Growth and Nutritional Quality of Brassica Microgreens. Open Life Sci. 2013, 8, 1241–1249. [Google Scholar] [CrossRef]
- Hytönen, T.; Pinho, P.; Rantanen, M.; Kariluoto, S.; Lampi, A.; Edelmann, M.; Joensuu, K.; Kauste, K.; Mouhu, K.; Piironen, V.; et al. Effects of LED Light Spectra on Lettuce Growth and Nutritional Composition. Light. Res. Technol. 2018, 50, 880–893. [Google Scholar] [CrossRef]
- Huang, M.-Y.; Lin, K.-H.; Lu, C.-C.; Chen, L.-R.; Hsiung, T.-C.; Chang, W.-T. The Intensity of Blue Light-Emitting Diodes Influences the Antioxidant Properties and Sugar Content of Oyster Mushrooms (Lentinus sajor-caju). Sci. Hortic. 2017, 218, 8–13. [Google Scholar] [CrossRef]
- Jang, M.-J.; Lee, Y.-H.; Ju, Y.-C.; Kim, S.-M.; Koo, H.-M. Effect of Color of Light Emitting Diode on Development of Fruit Body in Hypsizygus marmoreus. Mycobiology 2013, 41, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.-J.; Lee, Y.-H.; Kim, J.-H.; Joo, Y. Effect of LED Light on Primordium Formation, Morphological Properties, Ergosterol Content and Antioxidant Activity of Fruit Body in Pleurotus eryngii. Korean J. Mycol. 2011, 39, 175–179. [Google Scholar] [CrossRef]
- Corrochano, L.M. Light in the Fungal World: From Photoreception to Gene Transcription and Beyond. Annu. Rev. Genet. 2019, 53, 149–170. [Google Scholar] [CrossRef]
- Chen, M.; Chory, J. Phytochrome Signaling Mechanisms and the Control of Plant Development. Trends Cell Biol. 2011, 21, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Todo, T. The Cryptochromes. Genome Biol. 2005, 6, 220. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Masuno, K.; Abe, M.; Nishizawa, H.; Matsumoto, T.; Kunitomo, S.; Sakata, H.; Nakamura, K.; Koyama, T.; Ito, M.; et al. Light-Stimulative Effects on the Cultivation of Edible Mushrooms by Using Blue Led. In Proceedings of the 7th International Conference on Mushroom Biology and Mushroom Products, Arcachon, France, 4–7 October 2011. [Google Scholar]
- Montoya, S.; López, D.M.; Segura, B. Influencia de la luz azul sobre la productividad del cultivo sólido de Ganoderma lucidum. Rev. Colomb. Biotecnol. 2018, 20, 51–58. [Google Scholar] [CrossRef]
- ZubaïR, M.F.; IbrahïM, O.S.; Atolanï, O.; Hamid, A.A. Chemical Composition and Nutritional Characterization of Cotton Seed as Potential Feed Supplement. J. Turk. Chem. Soc. Sect. A Chem. 2021, 8, 977–982. [Google Scholar] [CrossRef]
- Sciammaro, L.P.; Qunitero Ruiz, N.A.; Ferrero, C.; Puppo, M.C. Chapter 21—Chemical and Nutritional Composition of Prosopis Spp. Seeds and Pods. In Prosopis as a Heat Tolerant Nitrogen Fixing Desert Food Legume; Academic Press: Cambridge, MA, USA, 2022; pp. 297–304. [Google Scholar]
- Latimer, G. AOAC Official Method 984.13A Protein (Crude) in Animal Feed: Copper Catalyst Kjeldahl Method. In Official Methods of Analysis of AOAC International; AOAC Publications: New York, NY, USA, 2023. [Google Scholar]
- Latimer, G. AOAC Official Method 982.30 Protein Efficiency Ratio: Calculation Method. In Official Methods of Analysis of AOAC International; AOAC Publications: New York, NY, USA, 2023. [Google Scholar]
- Latimer, G. AOAC Official Method 995.16 β-D-Glucan in Oats: Streamlined Enzymatic Method. In Official Methods of Analysis of AOAC International; AOAC Publications: New York, NY, USA, 2023. [Google Scholar]
- Yu, Z.; Streng, C.; Seibeld, R.F.; Igbalajobi, O.A.; Leister, K.; Ingelfinger, J.; Fischer, R. Genome-Wide Analyses of Light-Regulated Genes in Aspergillus Nidulans Reveal a Complex Interplay between Different Photoreceptors and Novel Photoreceptor Functions. PLoS Genet. 2021, 17, e1009845. [Google Scholar] [CrossRef]
- De Bonis, M.; Locatelli, S.; Sambo, P.; Zanin, G.; Pecchia, J.A.; Nicoletto, C. Effect of Different LED Light Wavelengths on Production and Quality of Pleurotus Ostreatus Grown on Different Commercial Substrates. Horticulturae 2024, 10, 349. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Tian, F.; Jia, C.; Li, C.; Li, Y. Transcriptomic Profiling Sheds Light on the Blue-Light and Red-Light Response of Oyster Mushroom (Pleurotus ostreatus). AMB Expr. 2020, 10, 10. [Google Scholar] [CrossRef]
- Yue, Z.; Zhang, W.; Liu, W.; Xu, J.; Liu, W.; Zhang, X. Effect of Different Light Qualities and Intensities on the Yield and Quality of Facility-Grown Pleurotus Eryngii. J. Fungi 2022, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Telang, S.M.; Patil, S.S.; Baig, M.M.V. Biological Efficiency and Nutritional Value of Pleurotus Sapidus Cultivated on Different Substrates. Food Sci. Res. J. 2010, 1, 127–129. [Google Scholar]
- Gupta, A.; Sharma, S.; Saha, S.; Walia, S. Yield and Nutritional Content of Pleurotus Sajor Caju on Wheat Straw Supplemented with Raw and Detoxified Mahua Cake. Food Chem. 2013, 141, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yadav, R.K.P.; Pokhrel, C.P. Growth and Yield of Oyster Mushroom (Pleurotus ostreatus) on Different Substrates. AMB Express 2016, 6, 87. [Google Scholar]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive Mill Waste Enhances α-Glucan Content in the Edible Mushroom Pleurotus Eryngii. Int. J. Mol. Sci. 2017, 18, 1564. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The Effect of Oat β -Glucan on LDL-Cholesterol, Non-HDL-Cholesterol and apoB for CVD Risk Reduction: A Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Gürgen, A.; SeviïNdiïK, M.; Yildiz, S.; Akgül, H. Determination of Antioxidant and Oxidant Potentials of Pleurotus Citrinopileatus Mushroom Cultivated on Various Substrates. Kahraman. Sütçü İmam Üniv. Tarım Doğa Derg. 2020, 23, 586–591. [Google Scholar] [CrossRef]
- Mihai, R.A.; Melo Heras, E.J.; Florescu, L.I.; Catana, R.D. The Edible Gray Oyster Fungi Pleurotus Ostreatus (Jacq. Ex Fr.) P. Kumm a Potent Waste Consumer, a Biofriendly Species with Antioxidant Activity Depending on the Growth Substrate. J. Fungi 2022, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Osunde, M.O.; Olayinka, A.; Fashina, C.D.; Torimiro, N. Effect of Carbon-Nitrogen Ratios of Lignocellulosic Substrates on the Yield of Mushroom (Pleurotus pulmonarius). OALib 2019, 6, e5777. [Google Scholar] [CrossRef]
- Mdachi, S.J.M.; Nkunya, M.H.H.; Nyigo, V.A.; Urasa, I.T. Amino Acid Composition of Some Tanzanian Wild Mushrooms. Food Chem. 2004, 86, 179–182. [Google Scholar] [CrossRef]
| Bio- Efficiency | Crude Protein | Total Amino Acids | Total Antioxidant Content | β-Glucan | Histidine | Glutamic Acid | Arginine | |
|---|---|---|---|---|---|---|---|---|
| (%) | (g/100 g) | (g/100 g) | (mM) | (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | |
| Cottonseed | 70.4 ± 2.6 | 37.6 ± 0.9 | 26.7 ± 0.6 | 1.2 ± 0.0 | 23.0 ± 1.0 | 0.8 ± 0.0 | 4.2 ± 0.1 | 2.1 ± 0.1 |
| Mesquite | 39.8 ± 2.3 | 34.3 ± 0.6 | 28.8 ± 0.4 | 1.1 ± 0.1 | 27.0 ± 1.0 | 0.6 ± 0.0 | 3.8 ± 0.1 | 1.7 ± 0.0 |
| p | <0.001 | 0.003 | 0.018 | 0.018 | 0.003 | <0.001 | <0.001 | <0.001 |
| Variable | Block | Spectra | Substrate | Substrate × Spectra |
|---|---|---|---|---|
| Bioefficiency | 0.699 | 0.732 | <0.001 | 0.833 |
| Crude Protein | 0.848 | 0.054 | 0.003 | 0.768 |
| Total Amino Acids | 0.744 | 0.326 | 0.018 | 0.715 |
| Glutamic Acid | 0.613 | 0.039 | <0.001 | 0.264 |
| Arginine | 0.933 | 0.016 | <0.001 | 0.632 |
| Histidine | 0.364 | 0.001 | <0.001 | 0.404 |
| Total Antioxidants | 0.868 | 0.482 | 0.018 | 0.600 |
| Beta-Glucan | 0.982 | 0.128 | 0.003 | 0.510 |
| Cap Diameter | 0.542 | <0.001 | ||
| Number of Caps perCluster | 0.859 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitmore, C.; Coon, D.; Rodriguez, B.; Fisher, K.; Pryor, B. Impact of Light Spectra and Substrate Composition on the Bioefficiency, Nutritional Content, and Morphology of Oyster Mushrooms. Horticulturae 2025, 11, 1430. https://doi.org/10.3390/horticulturae11121430
Whitmore C, Coon D, Rodriguez B, Fisher K, Pryor B. Impact of Light Spectra and Substrate Composition on the Bioefficiency, Nutritional Content, and Morphology of Oyster Mushrooms. Horticulturae. 2025; 11(12):1430. https://doi.org/10.3390/horticulturae11121430
Chicago/Turabian StyleWhitmore, Chrisa, Donald Coon, Bree Rodriguez, Karen Fisher, and Barry Pryor. 2025. "Impact of Light Spectra and Substrate Composition on the Bioefficiency, Nutritional Content, and Morphology of Oyster Mushrooms" Horticulturae 11, no. 12: 1430. https://doi.org/10.3390/horticulturae11121430
APA StyleWhitmore, C., Coon, D., Rodriguez, B., Fisher, K., & Pryor, B. (2025). Impact of Light Spectra and Substrate Composition on the Bioefficiency, Nutritional Content, and Morphology of Oyster Mushrooms. Horticulturae, 11(12), 1430. https://doi.org/10.3390/horticulturae11121430






