Physiological Response and Transcriptome Analysis of Waxy Near-Isogenic Lines in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Drought Treatment
2.2. Relative Water Content
2.3. Determination of Physiological Index
2.4. RNA Extraction, DNB Sequencing, and Identification of DEGs
2.5. Identification of Key Candidate Genes in Waxy/Non-Waxy Chinese Cabbage in Response to Drought Stress
2.6. qPCR Validation of DEGs
3. Results
3.1. Phenotypic and Physiological Differences of Waxy/Non-Waxy Chinese Cabbage in Response to Drought Stress
3.2. Transcriptome Analysis of Waxy/Non-Waxy Chinese Cabbage in Response to Drought Stress
3.2.1. Statistics of Transcriptome Sequencing Data
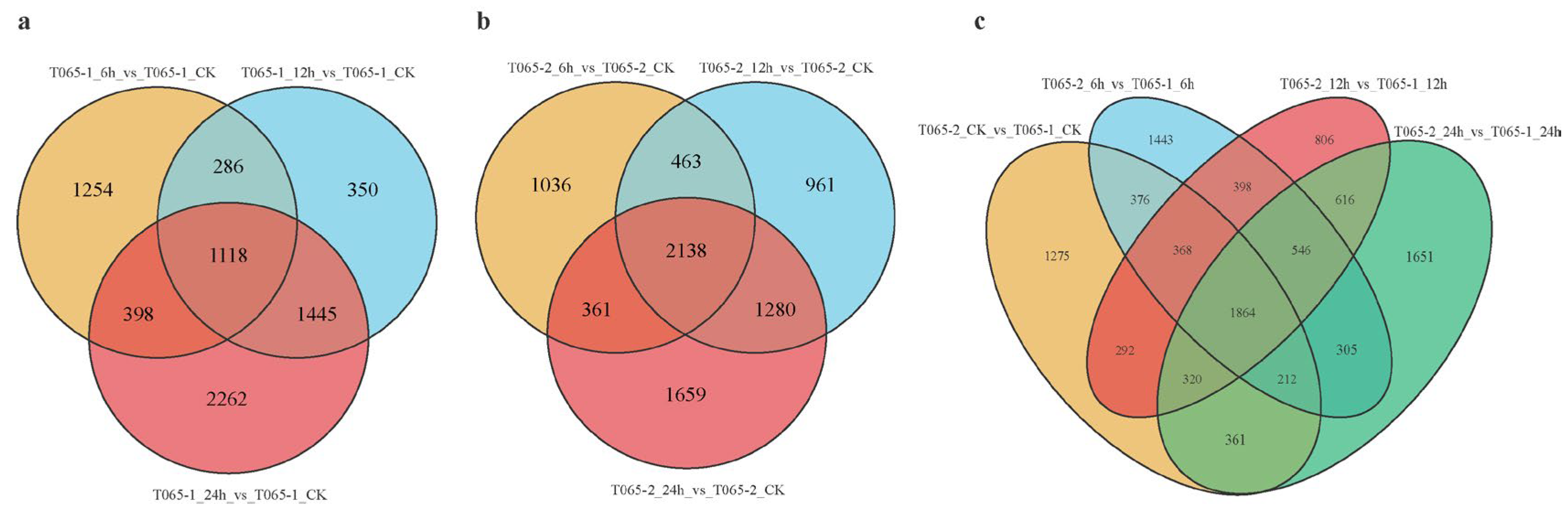
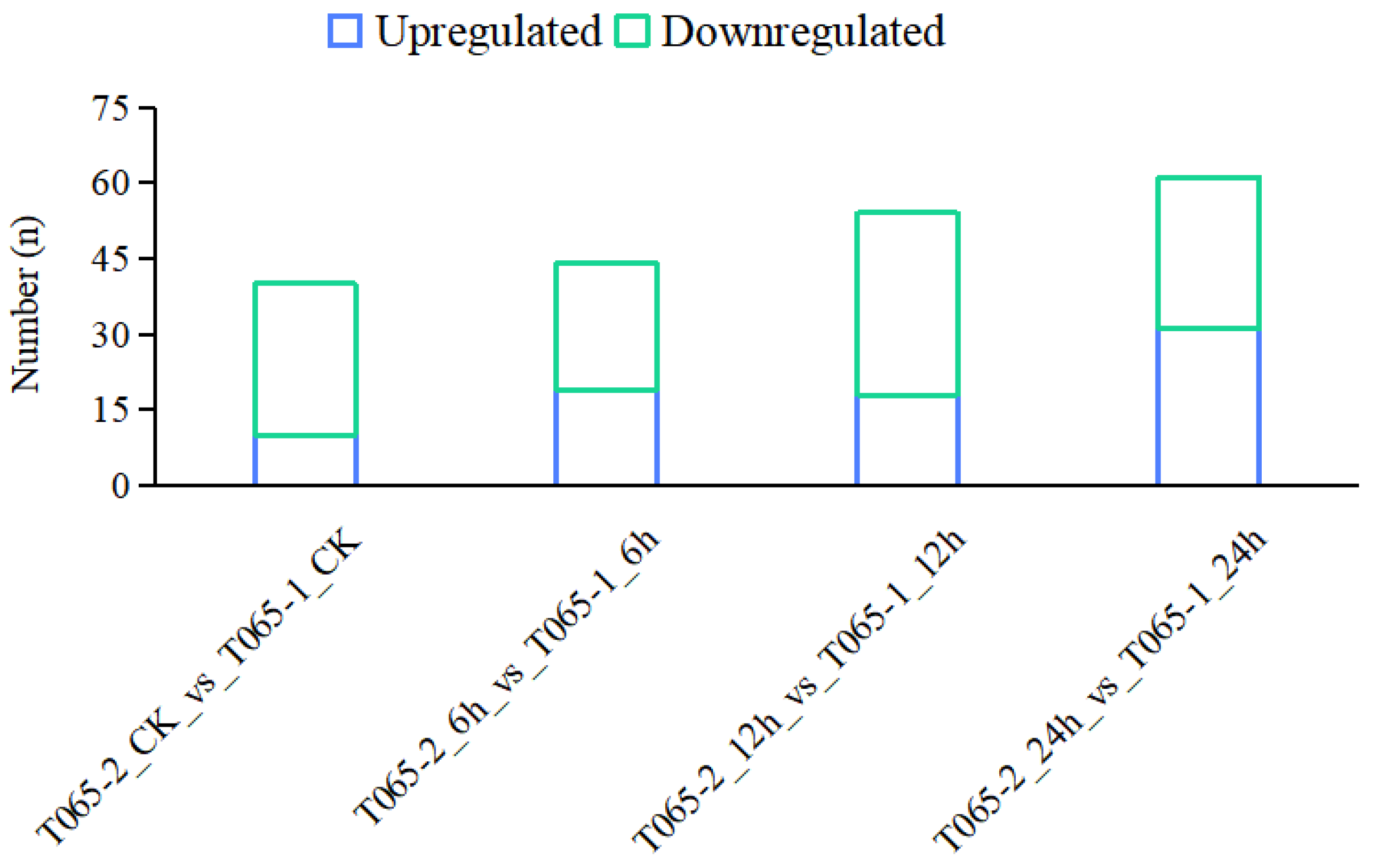
3.2.2. Identification and Analysis of DEGs
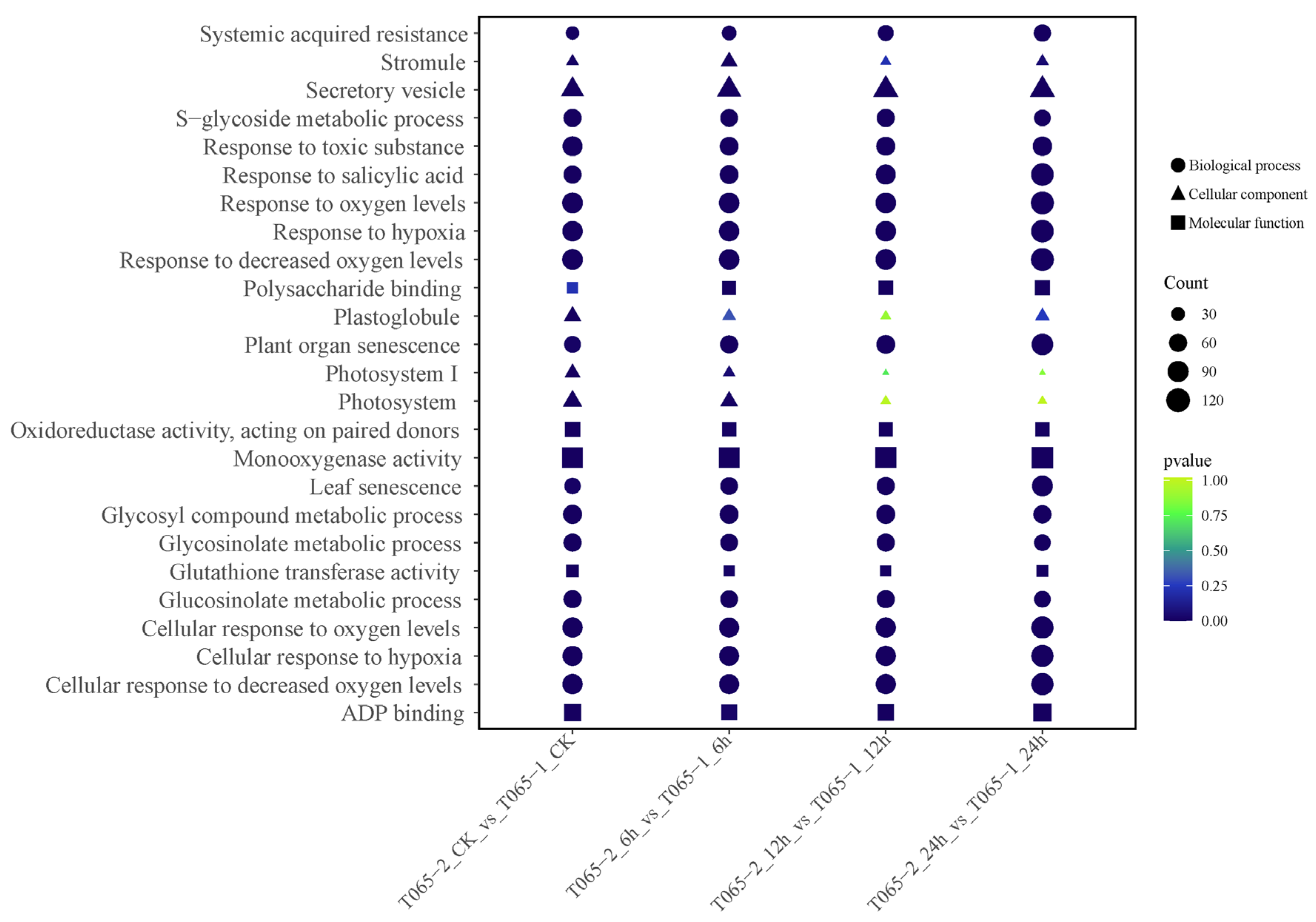
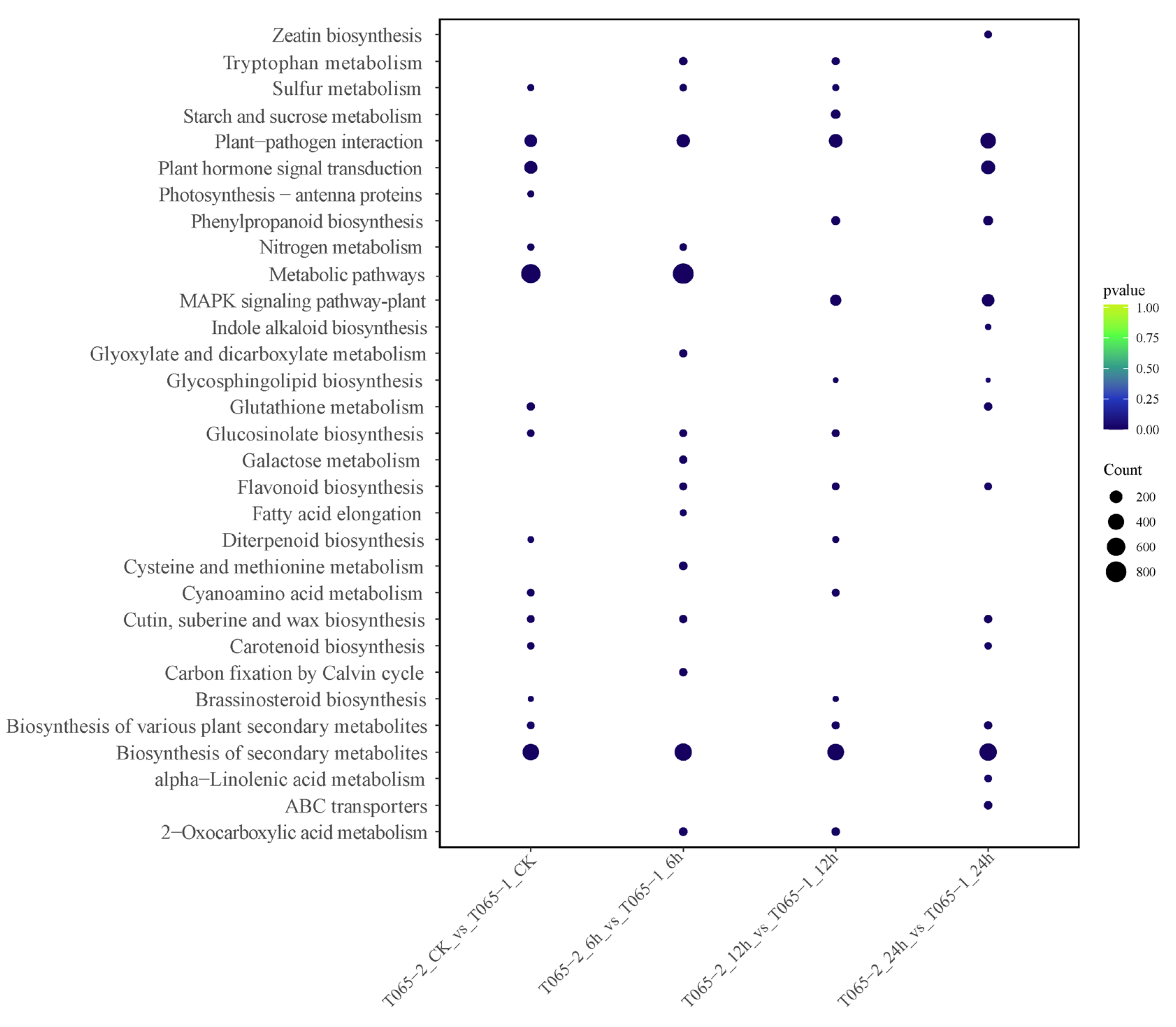
3.2.3. GO Enrichment and KEGG Pathway Analysis of DEGs
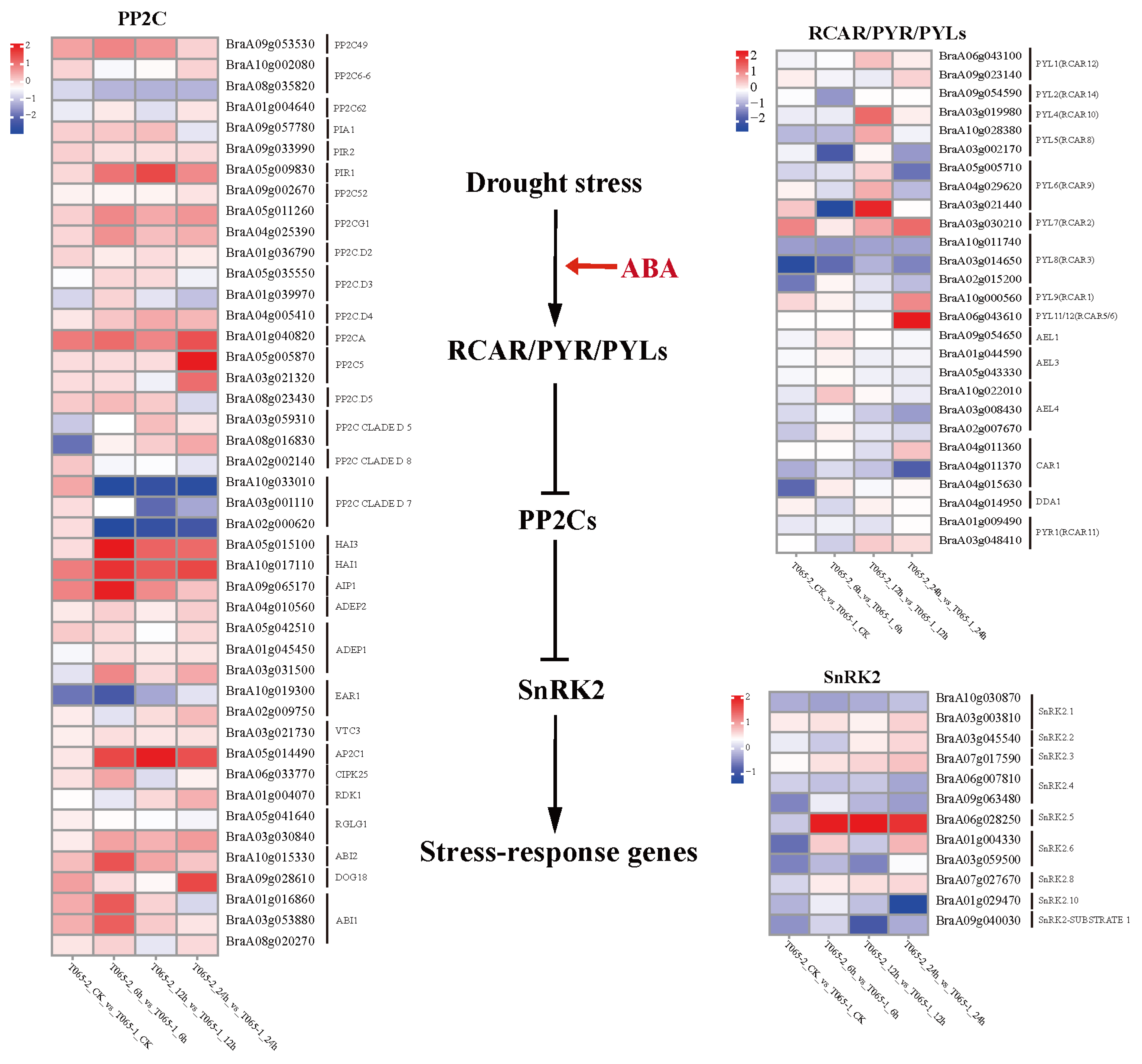
3.3. Expression Analysis of Genes Related to Signal Transduction Pathways in Waxy/Non-Waxy Chinese Cabbage in Response to Drought Stress
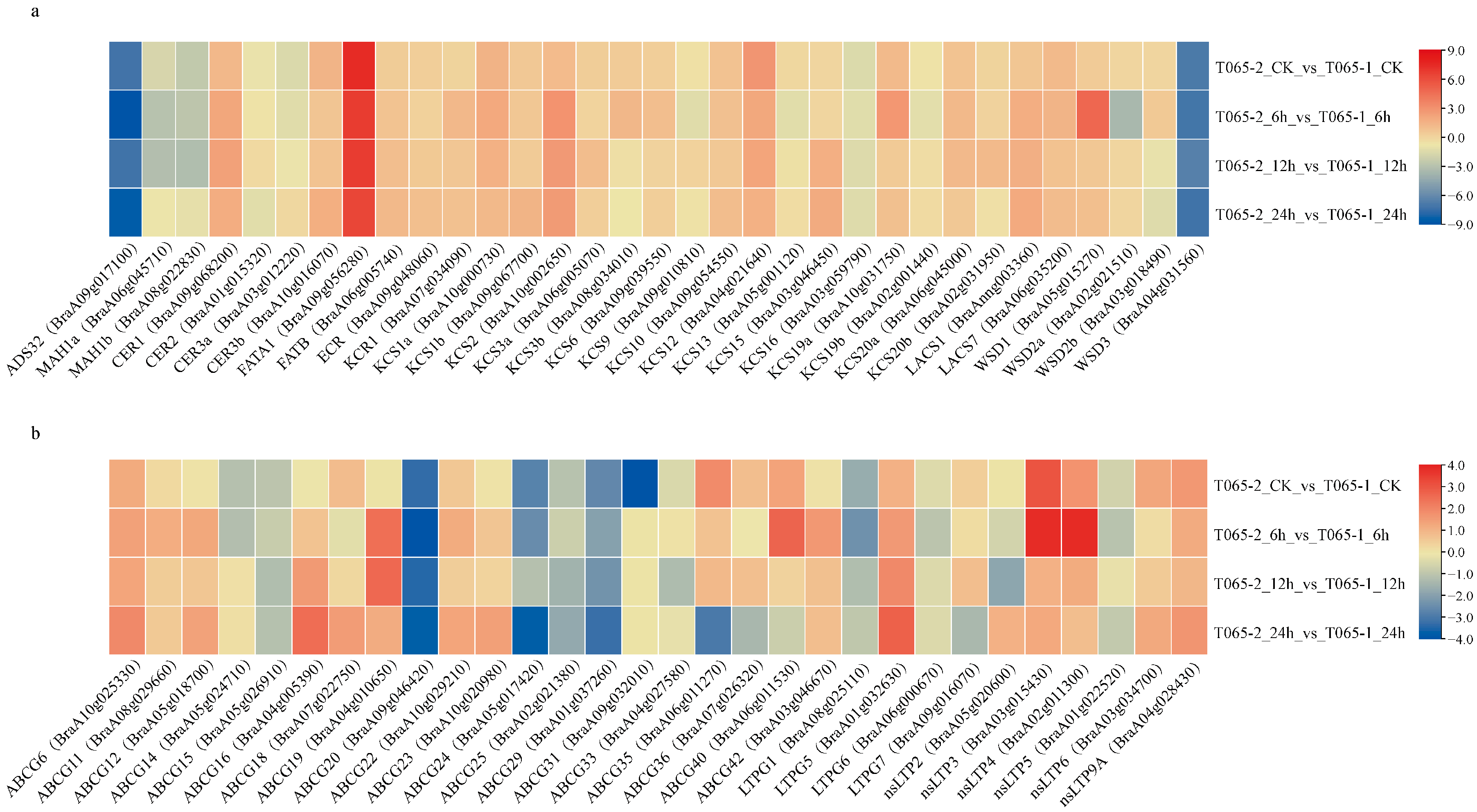
3.4. Expression Analysis of Wax Biosynthesis and Transport-Related Genes in Chinese Cabbage in Response to Drought Stress
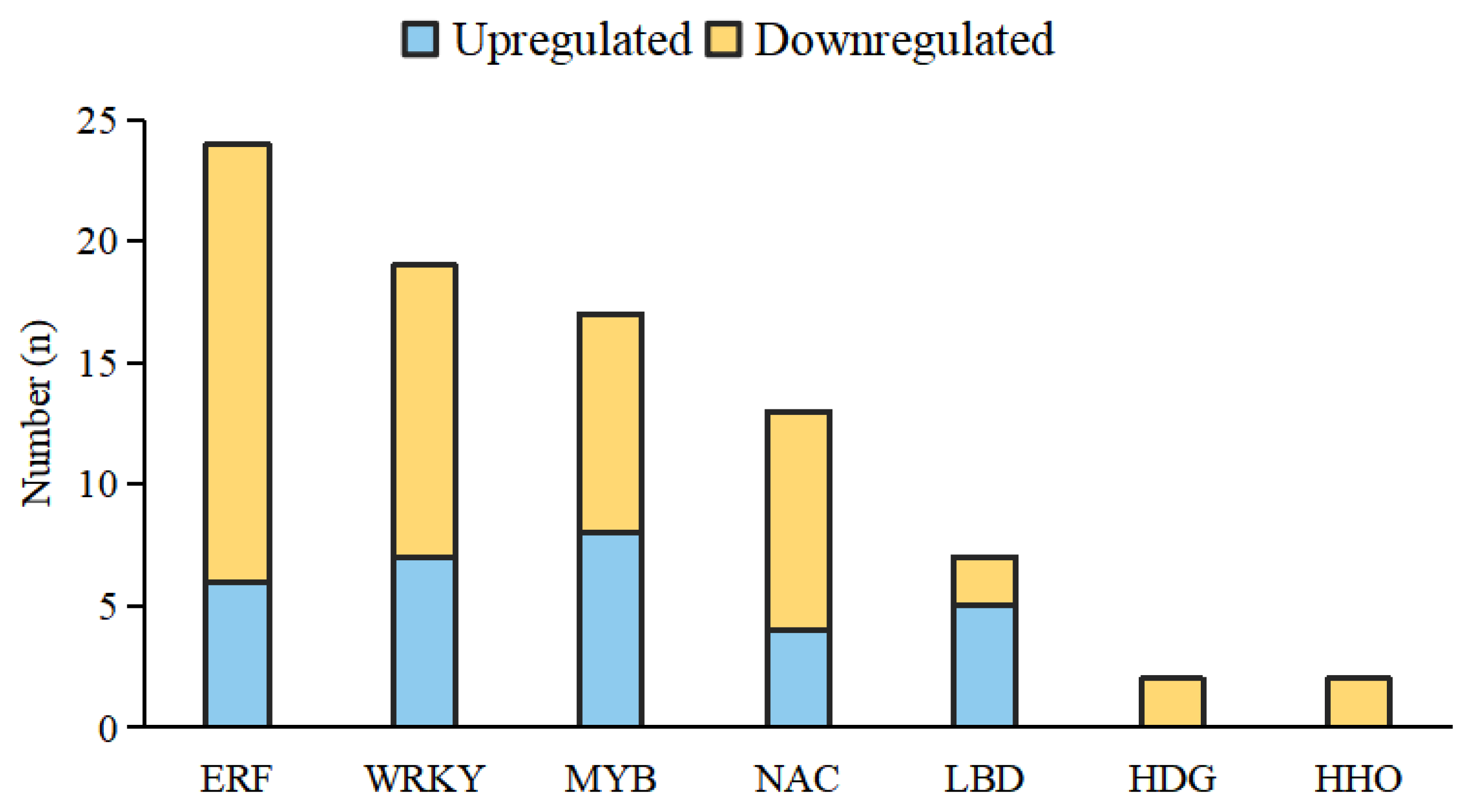
3.5. Expression Analysis of Transcription Factors (TFs) in Waxy/Non-Waxy Chinese Cabbage in Response to Drought Stress
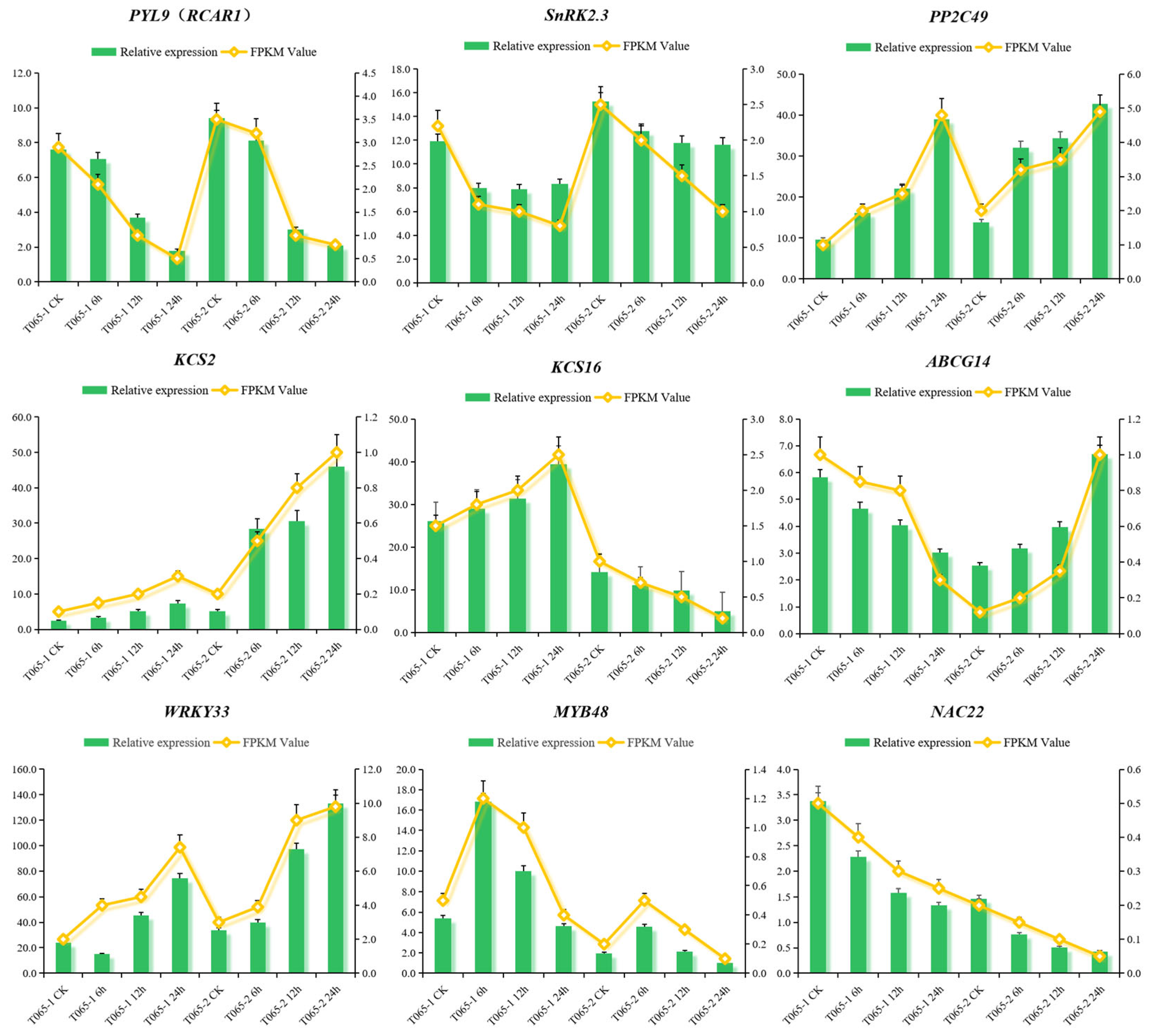
3.6. qRT-PCR Validation of DEGs
4. Discussion
4.1. Biological Mechanism of Cuticular Wax Response to Drought Stress
4.2. Molecular Mechanism of ABA Signaling of Chinese Cabbage Wax NILs in Response to Drought Stress
4.3. Functional Analysis of Wax Synthesis/Transport Genes and Transcription Factors in Response to Drought Stress in Waxy NILs of Chinese Cabbage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic Acid |
| CAT | Catalase |
| DEG | Differentially Expressed Gene |
| DW | Dry Weight |
| FDR | False Discovery Rate |
| FW | Fresh Weight |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| RWC | Relative Water Content |
| SOD | Superoxide Dismutase |
| TFs | Transcription Factors |
| MDA | Malondialdehyde |
| NILs | Near-Isogenic Lines |
| PEG | Polyethylene Glycol |
| POD | Peroxidase |
| Pro | Proline |
| qRT-PCR | Quantitative Real-time PCR |
| TW | Turgid Weight |
| WDL1 | Wilted Dwarf and Lethal 1 |
References
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizob acteria. Resour. Environ. Sustain. 2021, 5, 6267–6282. [Google Scholar] [CrossRef]
- Illouz-Eliaz, N.; Yu, J.; Swift, J.; Lande, K.; Jow, B.; Partida-Garcia, L.; Tuang, Z.K.; Lee, T.A.; Yaaran, A.; Gomez-Castanon, R.; et al. Drought recovery in plants triggers a cell-state-specific immune activation. Nat. Commun. 2025, 16, 8095. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Kumari, A.; Jagadesh, M.; Singh, S.K.; Bhatt, R.; Singh, M.; Seth, C.S.; Li, Y.R. Climate change adaptation: Challenges for agricultural sustainability. Plant Cell Environ. 2025, 48, 2522–2533. [Google Scholar] [CrossRef]
- Shah, W.U.H.; Lu, Y.; Liu, J.; Rehman, A.; Yasmeen, R. The impact of climate change and production technology heterogeneity on China’s agricultural total factor productivity and production efficiency. Sci. Total Environ. 2024, 907, 168027. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gao, J.; Sun, S.; Zhang, Z.; Yu, B.; Li, J.; Xie, C.; Li, G.; Wang, P.; Song, C.P.; et al. BONZAI proteins control global osmotic stress responses in plants. Curr. Biol. 2020, 30, 4815–4825. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Cao-Delgado, A.I. The physiology of plant responses to drought. Science. 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Kong, L.; Liu, Y.; Zhi, P.; Wang, X.; Xu, B.; Gong, Z.; Chang, C. Origins and evolution of cuticle biosynthetic machinery in land plants. Plant Physiol. 2020, 184, 1998–2010. [Google Scholar] [CrossRef]
- Lian, X.Y.; Gao, H.N.; Jiang, H.; Liu, C.; Li, Y.Y. MdKCS2 increased plant drought resistance by regulating wax biosynthesis. Plant Cell Rep. 2021, 40, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Oshima, Y.; Ishikawa, T.; Kajino, T.; Sakamoto, S.; Sato, M.; Toyooka, K.; Fujita, M.; Kawai-Yamada, M.; Taji, T.; et al. Arabidopsis DREB26/ERF12 and its close relatives regulate cuticular wax biosynthesis under drought stress condition. Plant J. 2024, 120, 2057–2075. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sack, L.; Li, Y.; Zhang, J.; Yu, K.; Zhang, Q.; He, N.; Yu, G. Relationships of stomatal morphology to the environment across plant communities. Nat. Commun. 2023, 14, 6629. [Google Scholar] [CrossRef]
- Liu, L.; Ashraf, M.A.; Morrow, T.; Facette, M. Stomatal closure in maize is mediated by subsidiary cells and the PAN2 receptor. New Phytol. 2024, 241, 1130–1143. [Google Scholar] [CrossRef]
- Sun, X.; Xiong, H.; Jiang, C.; Zhang, D.; Yang, Z.; Huang, Y.; Zhu, W.; Ma, S.; Duan, J.; Wang, X.; et al. Natural variation of DROT1 confers drought adaptation in upland rice. Nat. Commun. 2022, 13, 4265. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Li, Y.; Zhu, R.; Gu, Y.; Li, J.; Guo, H.; Ye, W.; Nabi, H.G.; Yang, T.; et al. The OsNAC41-RoLe1-OsAGAP module promotes root development and drought resistance in upland rice. Mol. Plant. 2024, 17, 1573–1593. [Google Scholar] [CrossRef]
- Zhang, X.; Mi, Y.; Mao, H.; Liu, S.; Chen, L.; Qin, F. Genetic variation in ZmTIP1 contributes to root hair elongation and drought tolerance in maize. Plant Biotechnol. J. 2020, 18, 1271–1283. [Google Scholar] [CrossRef]
- Ying, S.; Scheible, W.R.; Lundquist, P.K. A stress-inducible protein regulates drought tolerance and flowering time in Brachypodium and Arabidopsis. Plant Physiol. 2023, 191, 643–659. [Google Scholar] [CrossRef]
- Shavrukov, Y. Pathway to the molecular origins of drought escape and early flowering illuminated via the phosphorylation of SnRK2-Substrate 1 in Arabidopsis. Plant Cell Physiol. 2024, 65, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Du, H.; Ning, J.; Ye, H.; Xiong, L. Characterization of Glossy1-homologous genes in rice involved in leaf wax accumulation and drought resistance. Plant Mol. Biol. 2009, 70, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubès, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell. 2012, 24, 3106–3118. [Google Scholar] [CrossRef]
- Rowland, O.; Zheng, H.; Hepworth, S.R.; Lam, P.; Jetter, R.; Kunst, L. CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol. 2006, 142, 866–877. [Google Scholar] [CrossRef]
- Lei, P.; Pan, M.; Kang, S.; Zeng, P.; Ma, Y.; Peng, Y.; Ma, X.; Chen, W.; He, L.; Yang, H.; et al. A premature termination codon mutation in the onion AcCER2 gene is associated with both glossy leaves and thrip resistance. Hortic. Res. 2025, 12, uhaf006. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, X.; Huang, H.; Yin, M.; Jenks, M.A.; Kosma, D.K.; Yang, P.; Yang, X.; Zhang, H.; Lu, S. Deciphering the core shunt mechanism in Arabidopsis cuticular wax biosynthesis and its role in plant environmental adaptation. Nat. Plants. 2025, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Kerstiens, G. Water transport in plant cuticles: An update. J. Exp. Bot. 2006, 57, 2493–2499. [Google Scholar] [CrossRef]
- Park, J.J.; Jin, P.; Yoon, J.; Yang, J.; Jeong, H.J.; Ranathunge, K.; Schreiber, L.; Franke, R.; Lee, I.; An, G. Mutation in Wilted Dwarf and Lethal 1 (WDL1) causes abnormal cuticle formation and rapid water loss in rice. Plant Mol. Biol. 2010, 74, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, D.; Liu, C.; Shen, W.; He, J.; Yue, Q.; Niu, C.; Yang, F.; Li, X.; Shen, X.; et al. MdGH3.6 is targeted by MdMYB94 and plays a negative role in apple water-deficit stress tolerance. Plant J. 2022, 109, 1271–1289. [Google Scholar] [CrossRef]
- Cao, F.; Qian, Q.; Li, Z.; Wang, J.; Liu, Z.; Zhang, Z.; Niu, C.; Xie, Y.; Ma, F.; Guan, Q. Natural variation in an HD-ZIP factor identifies its role in controlling apple leaf cuticular wax deposition. Dev. Cell. 2025, 60, 949–964. [Google Scholar] [CrossRef]
- Patwari, P.; Salewski, V.; Gutbrod, K.; Kreszies, T.; Dresen-Scholz, B.; Peisker, H.; Steiner, U.; Meyer, A.J.; Schreiber, L.; Dörmann, P. Surface wax esters contribute to drought tolerance in Arabidopsis. Plant J. 2019, 98, 727–744. [Google Scholar] [CrossRef]
- He, J.; Li, C.; Hu, N.; Zhu, Y.; He, Z.; Sun, Y.; Wang, Z.; Wang, Y. ECERIFERUM1-6A is required for the synthesis of cuticular wax alkanes and promotes drought tolerance in wheat. Plant Physiol. 2022, 190, 1640–1657. [Google Scholar] [CrossRef]
- Wang, R.H.; Wang, S.B.; Liu, S.T.; Li, Q.Y.; Zhang, Z.G.; Wang, L.H.; Zhao, Z.Z. Transcriptome analysis of waxy near-isogenic lines in Chinese cabbage floral axis. Acta Hortic. Sinica. 2022, 49, 62–72. [Google Scholar]
- Zhang, X.; Liu, Z.; Wang, P.; Wang, Q.; Yang, S.; Feng, H. Fine mapping of BrWax1, a gene controlling cuticular wax biosynthesis in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Mol. Breeding. 2013, 32, 867–874. [Google Scholar] [CrossRef]
- Yang, S.; Liu, H.; Wei, X.; Zhao, Y.; Wang, Z.; Su, H.; Zhao, X.; Tian, B.; Zhang, X.; Yuan, Y. BrWAX2 plays an essential role in cuticular wax biosynthesis in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Theor. Appl. Genet. 2022, 135, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, H.; Wei, X.; Zhao, Y.; Wang, Z.; Su, H.; Niu, L.; Yuan, Y.; Zhang, X. BrWAX3, encoding a beta-ketoacyl-CoA synthase, plays an essential role in cuticular wax biosynthesis in Chinese cabbage. Int. J. Mol. Sci. 2022, 23, 10938. [Google Scholar] [CrossRef]
- Li, B.; Yue, Z.; Ding, X.; Zhao, Y.; Lei, J.; Zang, Y.; Hu, Q.; Tao, P. A BrLINE1-RUP insertion in BrCER2 alters cuticular wax biosynthesis in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Front. Plant Sci. 2023, 14, 1212528. [Google Scholar] [CrossRef]
- Liu, C.; Yu, L.; Yang, L.; Tan, C.; Shi, F.; Ye, X.; Liu, Z. Identification of a new allele of BraA09g066480.3C controlling the wax-less phenotype of Chinese cabbage. BMC Plant Biol. 2023, 23, 408. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Dong, S.; Liu, C.; Zou, J.; Ren, J.; Feng, H. BrKCS6 mutation conferred a bright glossy phenotype to Chinese cabbage. Theor. Appl. Genet. 2023, 136, 216. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.F.; Treccarichi, S.; Nicotra, R.; Branca, F.; Pulvirenti, A.; Piero, A.R.L.; Sicilia, A. Comparative transcriptome analysis of B. oleracea L. var. italica and B. macrocarpa Guss. genotypes under drought stress: De novo vs reference genome assembly. Plant Stress. 2024, 14, 100657. [Google Scholar] [CrossRef]
- Singh, K.P.; Kumari, P.; Yadava, D.K. Development of de-novo transcriptome assembly and SSRs in all hexaploid Brassica with functional annotations and identification of heat-shock proteins for thermotolerance. Front. Genet. 2022, 13, 958217. [Google Scholar] [CrossRef]
- Ahmad, J.; Ali, A.A.; Al-Huqail, A.A.; Qureshi, M.I. Triacontanol attenuates drought-induced oxidative stress in Brassica juncea L. by regulating lignification genes, calcium metabolism and the antioxidant system. Plant Physiol. Biochem. 2021, 166, 985–998. [Google Scholar] [CrossRef]
- Schonfeld, M.A.; Johnson, R.C.; Carver, B.F.; Mornhinweg, D.W. Water relations in winter wheat as drought resistance indicators. Crop Sci. 1998, 28, 526–531. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Guidi, L.; Bongi, G.; Ciompi, S.; Soldatini, G.F. In Vicia faba leaves photoinhibition from ozone fumigation in light precedes a decrease in quantum yield of functional PSII centres. J. Plant Physiol. 1999, 154, 167–172. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dewey, C.N.; Bo, L. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011, 12, 323. [Google Scholar]
- Wang, R.; Mei, Y.; Xu, L.; Zhu, X.; Wang, Y.; Guo, J.; Liu, L. Differential proteomic analysis reveals sequential heat stress-responsive regulatory network in radish (Raphanus sativus L.) taproot. Planta. 2018, 247, 1109–1122. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fei, N. Identification of Drought Resistant Oat Germplasm Resources and Its Antioxidant Defense Mechanisms in Response to Drought Stress. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2024. [Google Scholar]
- Jiang, C.; Li, X.; Zou, J.; Ren, J.; Jin, C.; Zhang, H.; Yu, H.; Jin, H. Comparative transcriptome analysis of genes involved in the drought stress response of two peanut (Arachis hypogaea L.) varieties. BMC Plant Biol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.; Yu, X.; Shen, H.; Li, H.; Cheng, D.; Liu, A.; Liu, J.; Liu, C.; Zhao, S.; et al. Cuticular wax accumulation is associated with drought tolerance in wheat near-isogenic lines. Front. Plant Sci. 2016, 7, 1809. [Google Scholar] [CrossRef]
- Lu, G.; Tian, Z.; Chen, P.; Liang, Z.; Zeng, X.; Zhao, Y.; Li, C.; Yan, T.; Hang, Q.; Jiang, L. Comprehensive morphological and molecular insights into drought tolerance variation at germination stage in Brassica napus accessions. Plants. 2024, 13, 3296. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.; Zhao, S.; Guo, Y. The PYR-PP2C-CKL2 module regulates ABA-mediated actin reorganization during stomatal closure. New Phytol. 2022, 233, 2168–2184. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.J.; Zhang, Y.L.; Liu, X.; Huang, H.; Zhou, X.E.; Wang, W.L.; Zeng, A.; Zhao, C.Z.; Si, T.; Du, J.; et al. Combining chemical and genetic approaches to increase drought resistance in plants. Nat. Commun. 2017, 30, 1183. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Zhao, Y.; Wang, X.; Fu, Y.; Chen, C.; Du, X.; Sheng, F. PYL2 regulates drought and blast (Magnaporthe oryzae) resistance in rice. Plant Sci. 2025, 360, 112690. [Google Scholar] [CrossRef]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.Y.; Márquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade a PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.Y.; Yoon, I.S.; Byun, M.O.; Kim, S.T.; Jung, K.H.; Kim, B.G. Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef]
- Kumar, V.V.S.; Yadav, S.K.; Verma, R.K.; Shrivastava, S.; Ghimire, O.; Pushkar, S.; Rao, M.V.; Kumar, T.S.; Chinnusamy, V. The abscisic acid receptor OsPYL6 confers drought tolerance to indica rice through dehydration avoidance and tolerance mechanisms. J. Exp. Bot. 2021, 72, 1411–1431. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, X.; Ma, W.; Wang, Z.; Luo, D.; Zhou, Q.; Liu, W.; Fang, L.; Jin, J.; Searle, I.R.; et al. MsPYL6 and MsPYL9 improves drought tolerance by regulating stomata in alfalfa (Medicago sativa). Plant J. 2025, 122, e70265. [Google Scholar] [CrossRef]
- Xue, T.; Wang, D.; Zhang, S.; Ehlting, J.; Ni, F.; Jakab, S.; Zheng, C.; Zhong, Y. Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genomics. 2008, 9, 550. [Google Scholar] [CrossRef]
- Hasan, M.M.; Liu, X.D.; Waseem, M.; Guang-Qian, Y.; Alabdallah, N.M.; Jahan, M.S.; Fang, X.W. ABA activated SnRK2 kinases: An emerging role in plant growth and physiology. Plant Signal Behav. 2022, 17, 2071024. [Google Scholar] [CrossRef]
- Liu, H.; Song, S.; Zhang, H.; Li, Y.; Niu, L.; Zhang, J.; Wang, W. Signaling transduction of ABA, ROS, and Ca2+ in plant stomatal closure in response to drought. Int. J. Mol. Sci. 2022, 23, 14824. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xiong, L. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc. Natl. Acad. Sci. USA 2013, 110, 17790–17795. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Man, Y.; Zhang, C.; Wang, Y.; You, C.; Li, Y. Apple sUMo E3 ligase MdSIZ1 regulates cuticular wax biosynthesis by SUMOylating transcription factor MdMYB30. Plant Physiol. 2023, 191, 1771–1788. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yang, L.; Hu, W.; Song, J.; Kuang, L.; Huang, Y.; Liu, D.; Liu, Y. The CsMYB44-csi-miR0008-CsCER1 module regulates cuticular wax biosynthesis and drought tolerance in citrus. New Phytol. 2025, 246, 1757–1779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Li, M.; Cheng, Z.; Song, X.; Shang, X.; Guo, W. Overexpression of a cotton nonspecific lipid transfer protein gene, GhITP4, enhances drought tolerance by remodeling lipid profiles, regulating abscisic acid homeostasis and improving tricarboxylic acid cycle in cotton. Environ. Exp. Bot. 2022, 201, 104991. [Google Scholar] [CrossRef]
- Debono, A.; Yeats, T.H.; Rose, J.K.; Bird, D.; Jetter, R.; Kunst, L.; Samuels, L. Arabidopsis LTPG is a glycosylphosphatidylinositol-anchored lipid transfer protein required for export of lipids to the plant surface. Plant Cell. 2009, 21, 1230–1238. [Google Scholar] [CrossRef]
- Kuromori, T.; Fujita, M.; Urano, K.; Tanabata, T.; Sugimoto, E.; Shinozaki, K. Overexpression of AtABCG25 enhances the abscisic acid signal in guard cells and improves plant water use efficiency. Plant Sci. 2016, 251, 75–81. [Google Scholar] [CrossRef]
- Liu, L.B.; Bai, W.P.; Li, H.J.; Tian, Y.; Yuan, H.J.; Garant, T.M.; Liu, H.S.; Zhang, J.; Bao, A.K.; Rowland, O.; et al. ZxABCG11 from the xerophyte Zygophyllum xanthoxylum enhances drought tolerance in Arabidopsis thaliana through modulating cuticular wax accumulation. Environ. Exp. Bot. 2021, 190, 104570. [Google Scholar] [CrossRef]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Liu, R.; Liu, H.; Yang, H.; Zhu, Z.; Xu, R.; Wang, P.; Deng, X.; Xue, S.; et al. CsMYB96 confers resistance to water loss in citrus fruit by simultaneous regulation of water transport and wax biosynthesis. J. Exp. Bot. 2022, 73, 953–966. [Google Scholar] [CrossRef]

| Gene | ID | Forward Sequence | Reverse Sequence | Fragment Length (bp) |
|---|---|---|---|---|
| PYL9 (RCAR1) | BraA10g000560 | TCGTCTCGTTTGGTCAGTGG | ACTTGTAGTTGCAGGGAGGC | 145 |
| PP2C49 | BraA09g053530 | TTCCAACAATCCGGTCAGGG | ACGTACGTTGCTGCTTCTGA | 178 |
| SNRK2.3 | BraA07g017590 | AATGCTGGACGGTTTAGCGA | GGTGCAGGACTTCCATCCAA | 140 |
| KCS2 | BraA10g002650 | AGCAGACGACAACGCCTTTA | CTTCTCCGGCGATAGCCATT | 107 |
| KCS16 | BraA03g059790 | CGGTCGATACTCTTCTCCGC | CTATAGCGATGACGCCAGCA | 189 |
| ABCG14 | BraA05g024710 | CATCAAGCGGAGAACAGGGT | AACTCGGTCCACATGCTCAG | 145 |
| WRK33 | BraA03g020120 | ACCATCGGTTGTCCAGTGAG | AGGGTCTTGTACCGGTCTGT | 147 |
| MYB48 | BraA06g021070 | GGAGATCGGCGATGGGATTT | AGCTCAGTGACAAGACGCTC | 146 |
| NAC22 | BraA05g035170 | AATCGGCGAGAGTAGGAGGT | TGCCCACAAGGCTACCATTT | 156 |
| G6PD | GGGTATGCCAGGACTAAGCTC | GAATCATAAGGGCCACTCACAT | 134 |
| Sample | Raw Reads (bp) | Clean Reads (bp) | Clean Base (Gb) | Q20(%) | Q30(%) | Total Reads (bp) | Reads Mapped (bp) |
|---|---|---|---|---|---|---|---|
| T065-1_CK-1 | 55,971,960 | 55,343,562 | 8.3 | 97.61 | 93.84 | 55,343,562 | 51,122,706 (92.37%) |
| T065-1_CK-2 | 48,334,946 | 47,690,188 | 7.15 | 98.18 | 95.02 | 47,690,188 | 44,393,154 (93.09%) |
| T065-1_CK-3 | 61,091,588 | 60,131,432 | 9.02 | 98.59 | 96.1 | 60,131,432 | 56,229,016 (93.51%) |
| T065-1_6h-1 | 49,610,578 | 48,946,874 | 7.34 | 98.05 | 94.74 | 48,946,874 | 45,535,098 (93.03%) |
| T065-1_6h-2 | 55,353,160 | 54,669,708 | 8.2 | 98.53 | 95.9 | 54,669,708 | 51,194,721 (93.64%) |
| T065-1_6h-3 | 56,204,542 | 55,500,438 | 8.33 | 98.16 | 94.99 | 55,500,438 | 51,750,521 (93.24%) |
| T065-1_12h-1 | 64,703,426 | 63,736,304 | 9.56 | 98.51 | 95.94 | 63,736,304 | 59,347,842 (93.11%) |
| T065-1_12h-2 | 55,833,164 | 55,184,828 | 8.28 | 98.19 | 95.04 | 55,184,828 | 51,379,555 (93.10%) |
| T065-1_12h-3 | 62,590,542 | 61,669,342 | 9.25 | 98.49 | 95.79 | 61,669,342 | 57,413,137 (93.10%) |
| T065-1_24h-1 | 50,352,048 | 49,548,960 | 7.43 | 98.16 | 95.02 | 49,548,960 | 45,997,192 (92.83%) |
| T065-1_24h-2 | 48,717,002 | 48,130,402 | 7.22 | 98.17 | 94.95 | 48,130,402 | 44,844,386 (93.17%) |
| T065-1_24h-3 | 60,572,812 | 59,697,122 | 8.95 | 98.41 | 95.66 | 59,697,122 | 55,657,624 (93.23%) |
| T065-2_CK-1 | 53,967,764 | 53,224,194 | 7.98 | 98.06 | 94.76 | 53,224,194 | 49,619,884 (93.23%) |
| T065-2_CK-2 | 49,883,586 | 49,286,986 | 7.39 | 98.24 | 95.15 | 49,286,986 | 46,031,445 (93.39%) |
| T065-2_CK-3 | 43,768,680 | 43,218,538 | 6.48 | 98.08 | 94.82 | 43,218,538 | 40,273,898 (93.19%) |
| T065-2_6h-1 | 42,287,230 | 41,700,960 | 6.26 | 98.11 | 94.85 | 41,700,960 | 38,910,365 (93.31%) |
| T065-2_6h-2 | 51,673,158 | 51,040,546 | 7.66 | 98.02 | 94.69 | 51,040,546 | 47,470,769 (93.01%) |
| T065-2_6h-3 | 55,846,988 | 55,095,294 | 8.26 | 98.34 | 95.41 | 55,095,294 | 51,362,435 (93.22%) |
| T065-2_12h-1 | 67,306,842 | 66,262,070 | 9.94 | 98.37 | 95.59 | 66,262,070 | 61,944,090 (93.48%) |
| T065-2_12h-2 | 52,821,232 | 52,081,868 | 7.81 | 97.98 | 94.59 | 52,081,868 | 48,484,670 (93.09%) |
| T065-2_12h-3 | 51,577,828 | 50,802,656 | 7.62 | 98.28 | 95.27 | 50,802,656 | 47,376,753 (93.26%) |
| T065-2_24h-1 | 48,824,910 | 48,147,622 | 7.22 | 98.2 | 95.1 | 48,147,622 | 44,913,833 (93.28%) |
| T065-2_24h-2 | 45,400,798 | 44,813,942 | 6.72 | 97.9 | 94.34 | 44,813,942 | 41,755,712 (93.18%) |
| T065-2_24h-3 | 55,644,802 | 54,856,316 | 8.23 | 98.37 | 95.52 | 54,856,316 | 51,296,769 (93.51%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, S.; Zhao, Z.; Xu, N.; Li, Q.; Zhang, Z.; Liu, S. Physiological Response and Transcriptome Analysis of Waxy Near-Isogenic Lines in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Under Drought Stress. Horticulturae 2025, 11, 1431. https://doi.org/10.3390/horticulturae11121431
Wang R, Wang S, Zhao Z, Xu N, Li Q, Zhang Z, Liu S. Physiological Response and Transcriptome Analysis of Waxy Near-Isogenic Lines in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Under Drought Stress. Horticulturae. 2025; 11(12):1431. https://doi.org/10.3390/horticulturae11121431
Chicago/Turabian StyleWang, Ronghua, Shubin Wang, Zhizhong Zhao, Nianfang Xu, Qiaoyun Li, Zhigang Zhang, and Shuantao Liu. 2025. "Physiological Response and Transcriptome Analysis of Waxy Near-Isogenic Lines in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Under Drought Stress" Horticulturae 11, no. 12: 1431. https://doi.org/10.3390/horticulturae11121431
APA StyleWang, R., Wang, S., Zhao, Z., Xu, N., Li, Q., Zhang, Z., & Liu, S. (2025). Physiological Response and Transcriptome Analysis of Waxy Near-Isogenic Lines in Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Under Drought Stress. Horticulturae, 11(12), 1431. https://doi.org/10.3390/horticulturae11121431





