LED Light and Plant Growth Regulators Affect Callus Induction, Shoot Organogenesis, dl-Tetrahydropalmatine Accumulation, and Activities of Antioxidant Enzymes in Corydalis turtschaninovii Besser
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Light Treatments
2.1.1. Explants, PGRs, and Culture Conditions for Callus Induction
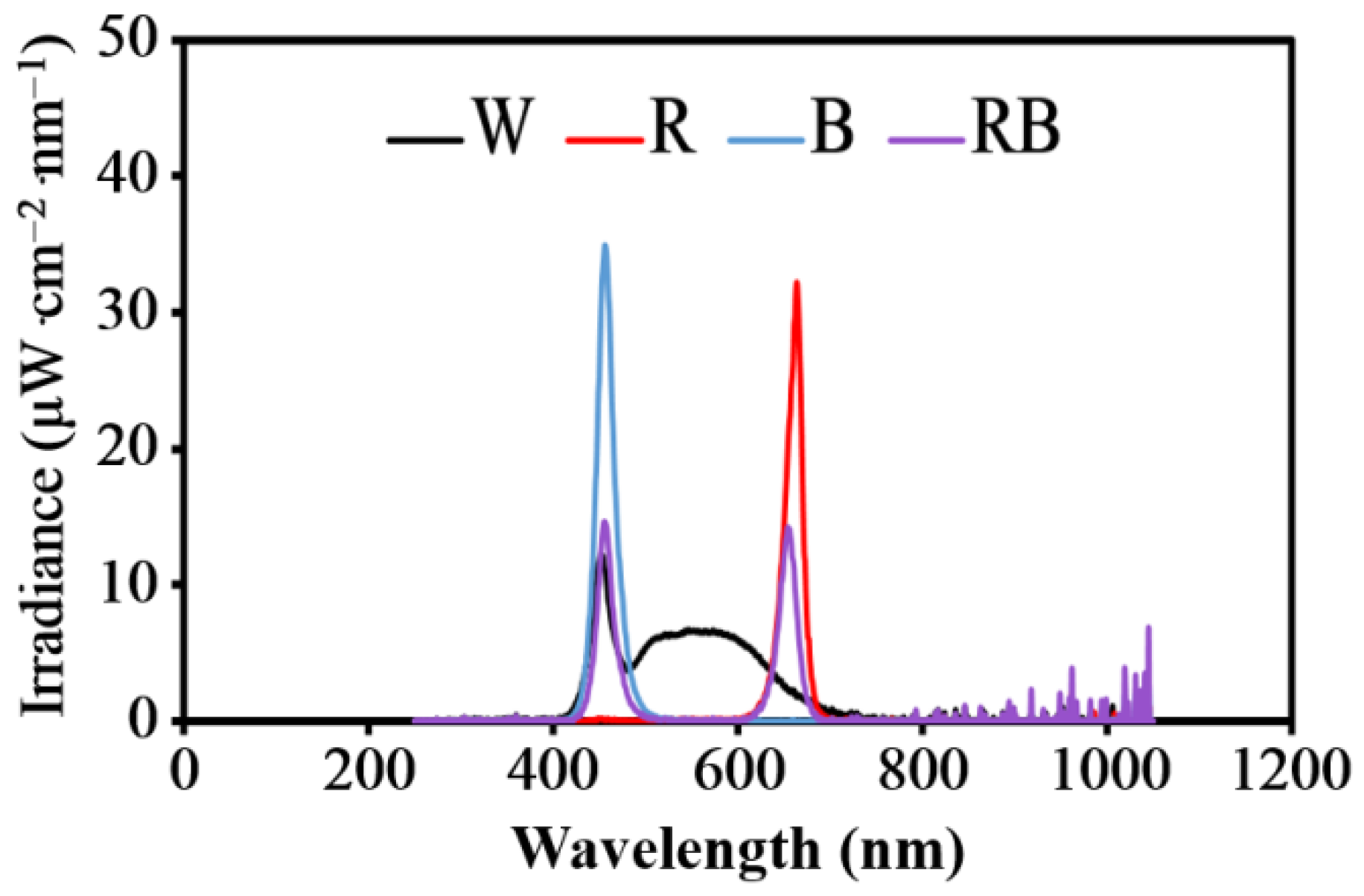
2.1.2. Effect of Light Intensity and Quality on Indirect Shoot Induction and dl-Tetrahydropalmatine Accumulation
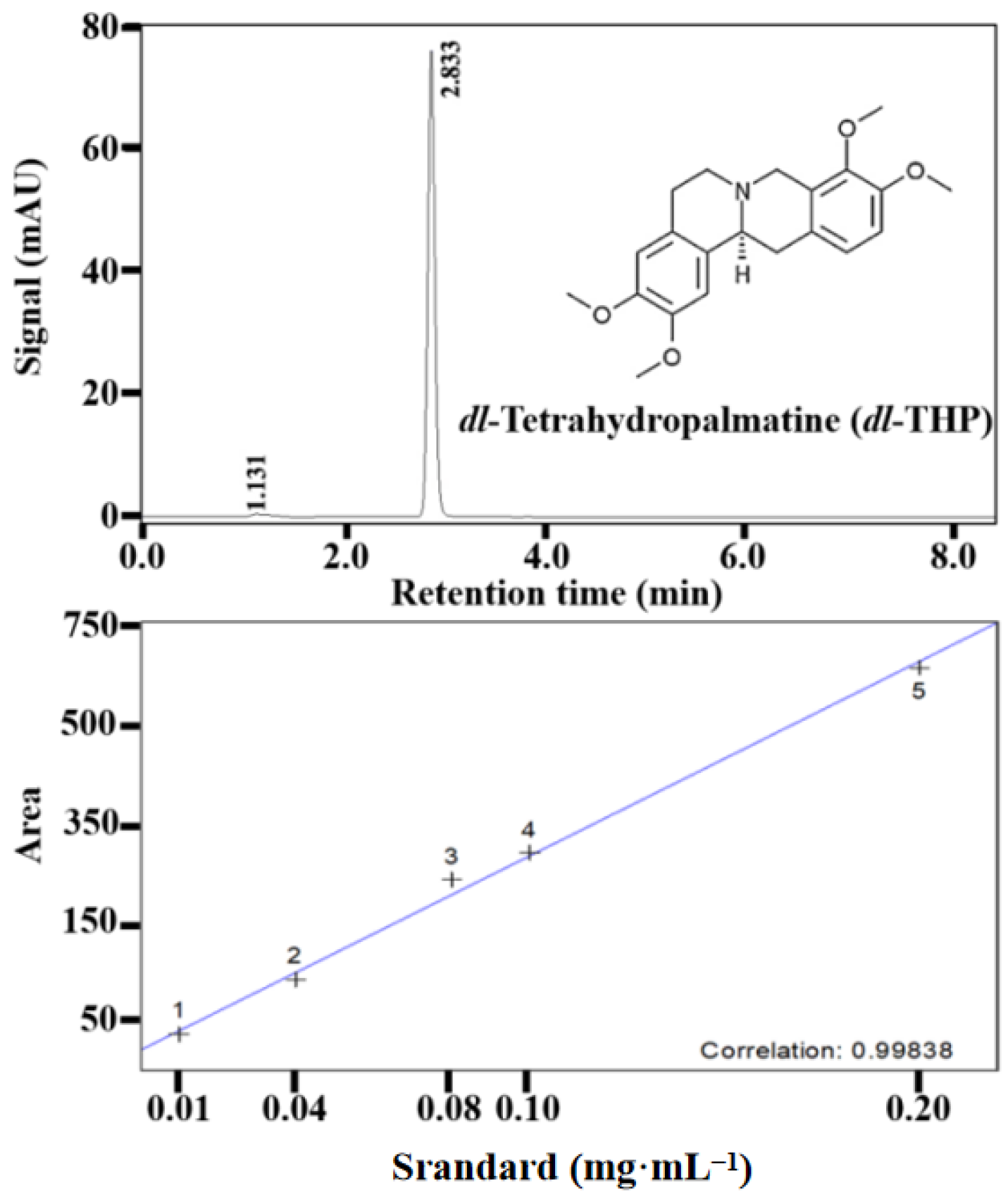
2.2. Quantification of dl-Tetrahydropalmatine (dl-THP) by HPLC
2.3. Analysis of Activities of Antioxidant Enzymes
2.4. Preparation of Plant Extracts and Determination of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
2.5. Data Collection and Statistical Analysis
3. Results
3.1. Effect of Explant and PGR on Callus Induction
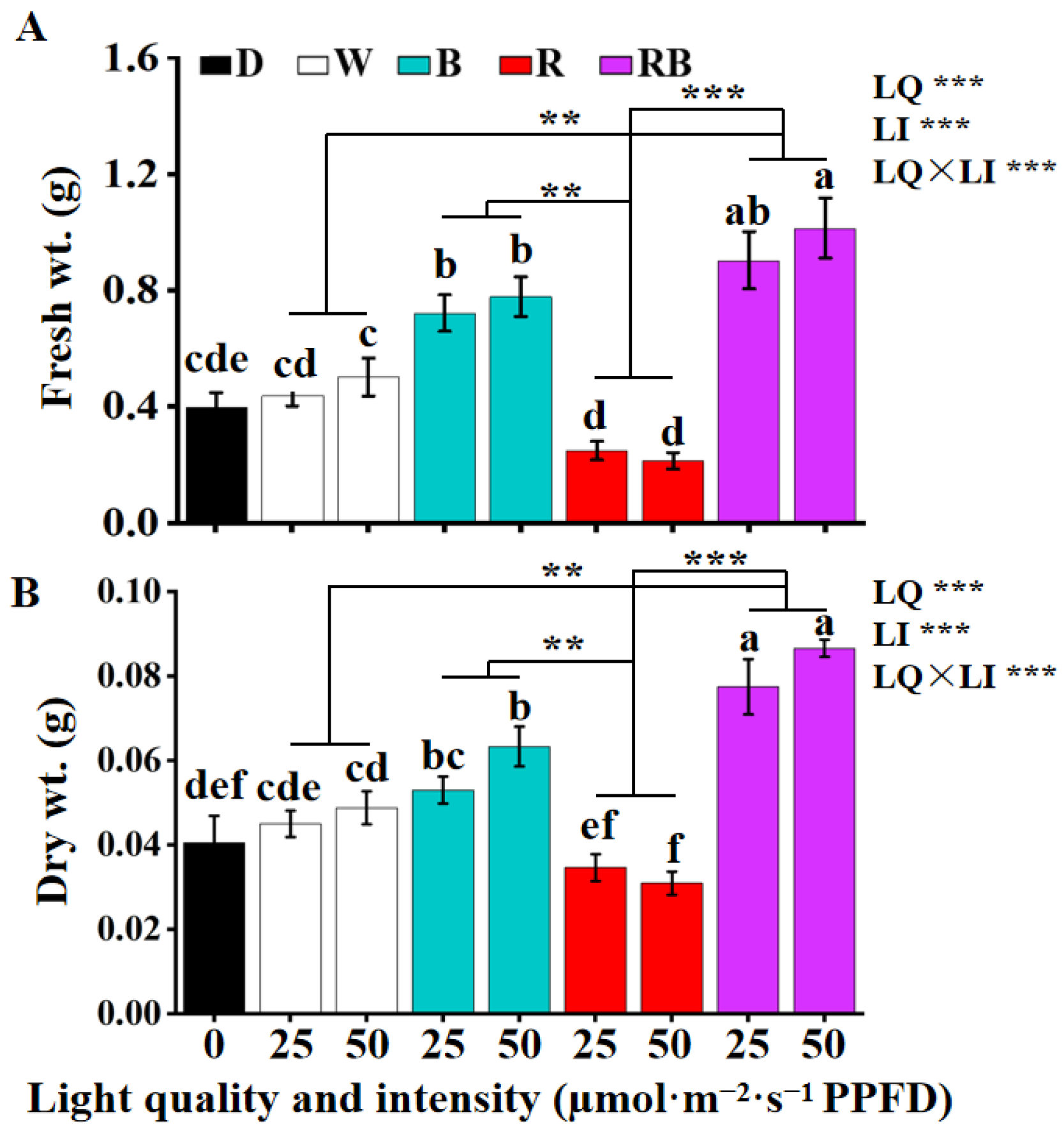
3.2. The Effects of the Light Quality and Intensity on the Fresh and Dry Weights of Callus
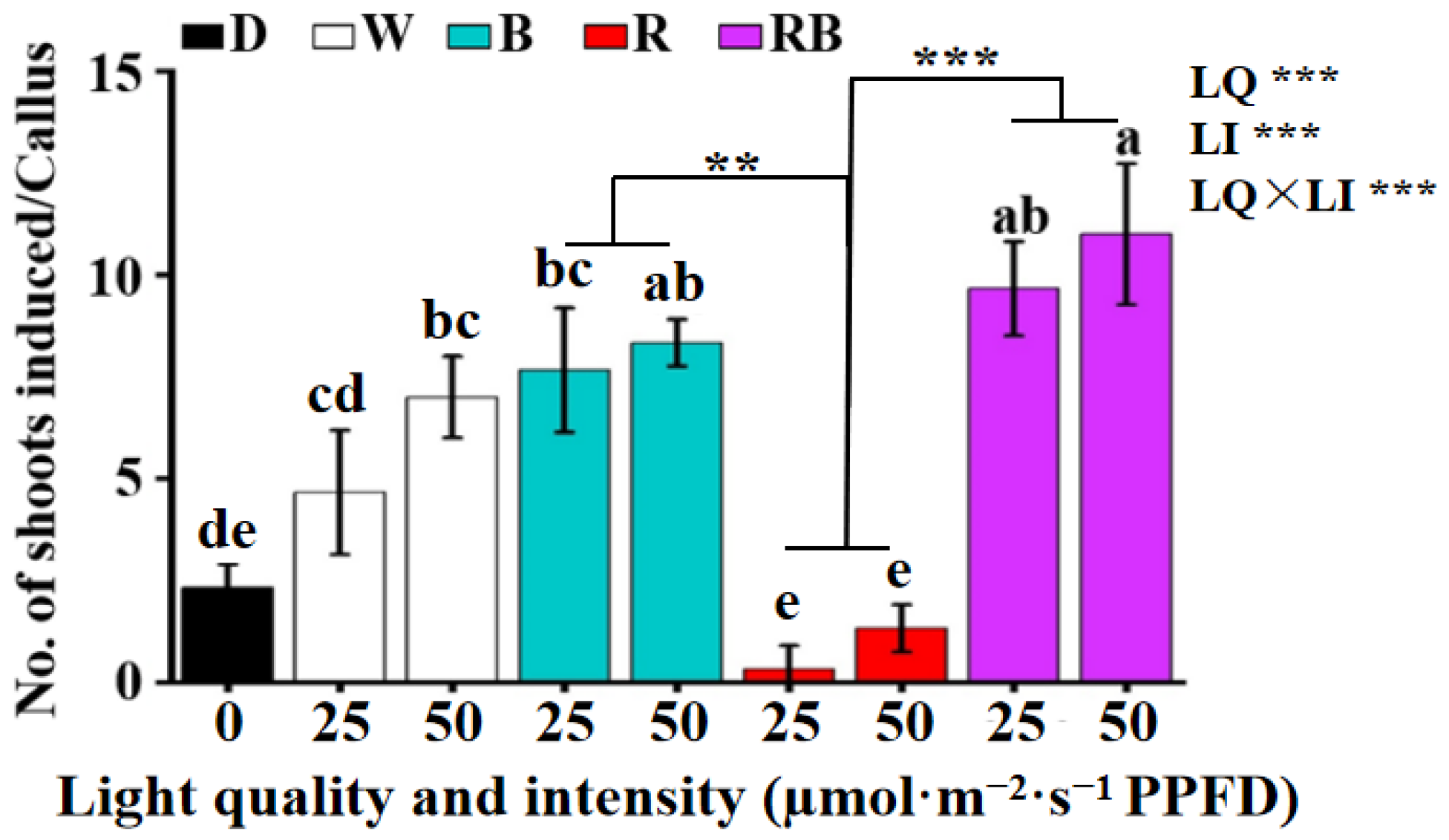
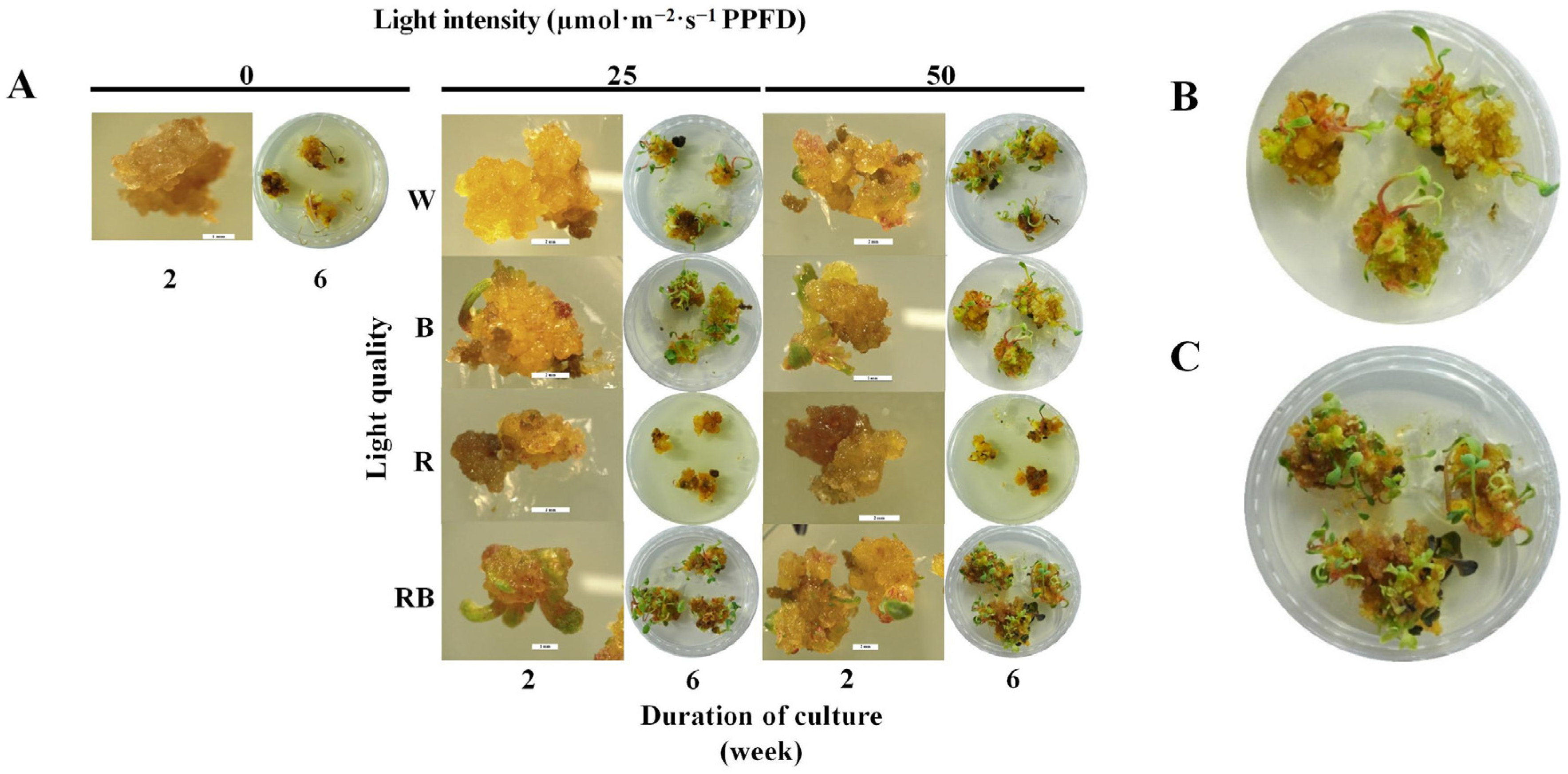
3.3. Effect of the Light Quality and Intensity on the Shoot Induction from Callus
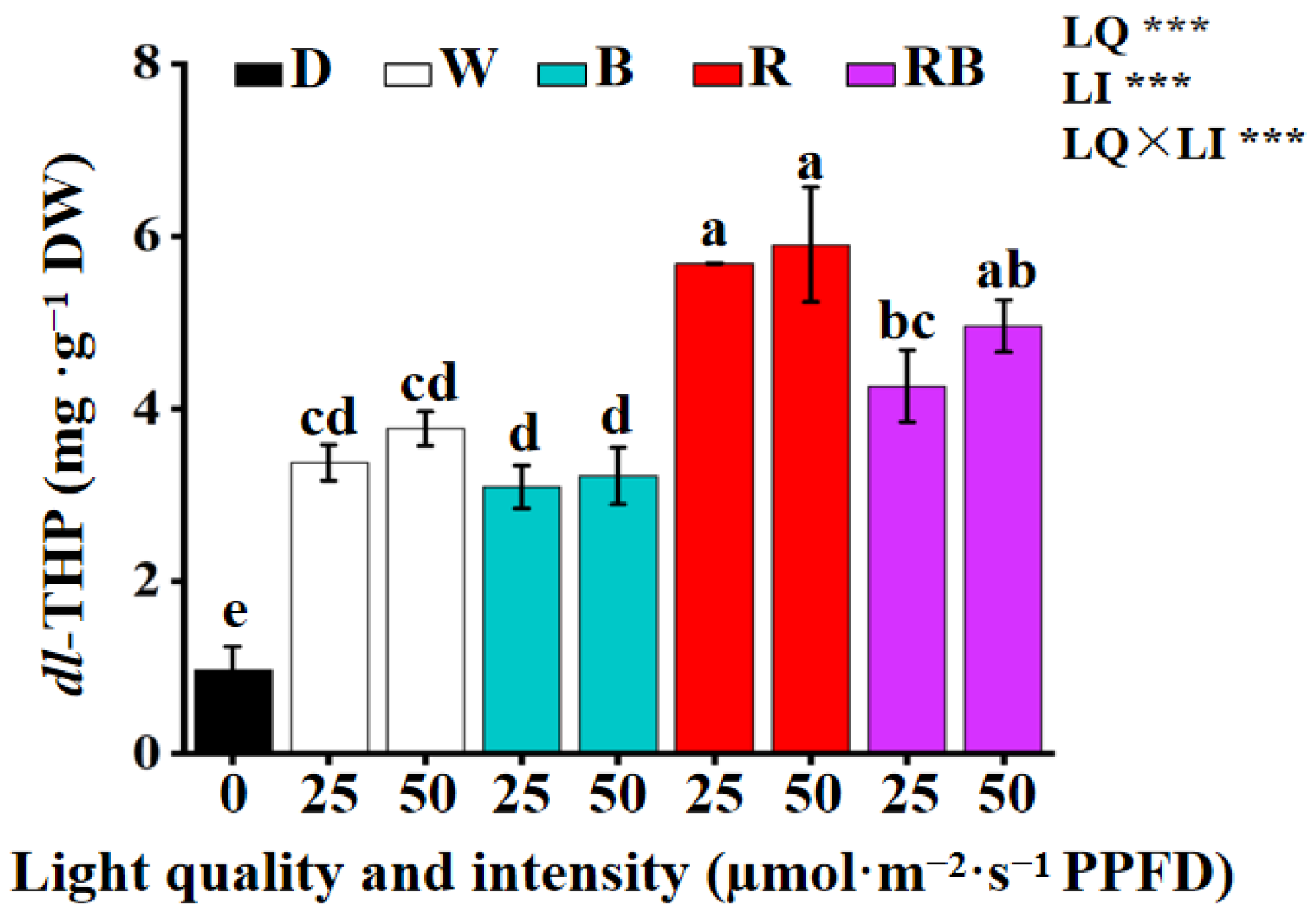
3.4. Effect of the Light Quality and Intensity on the Accumulation of dl-THP in Callus
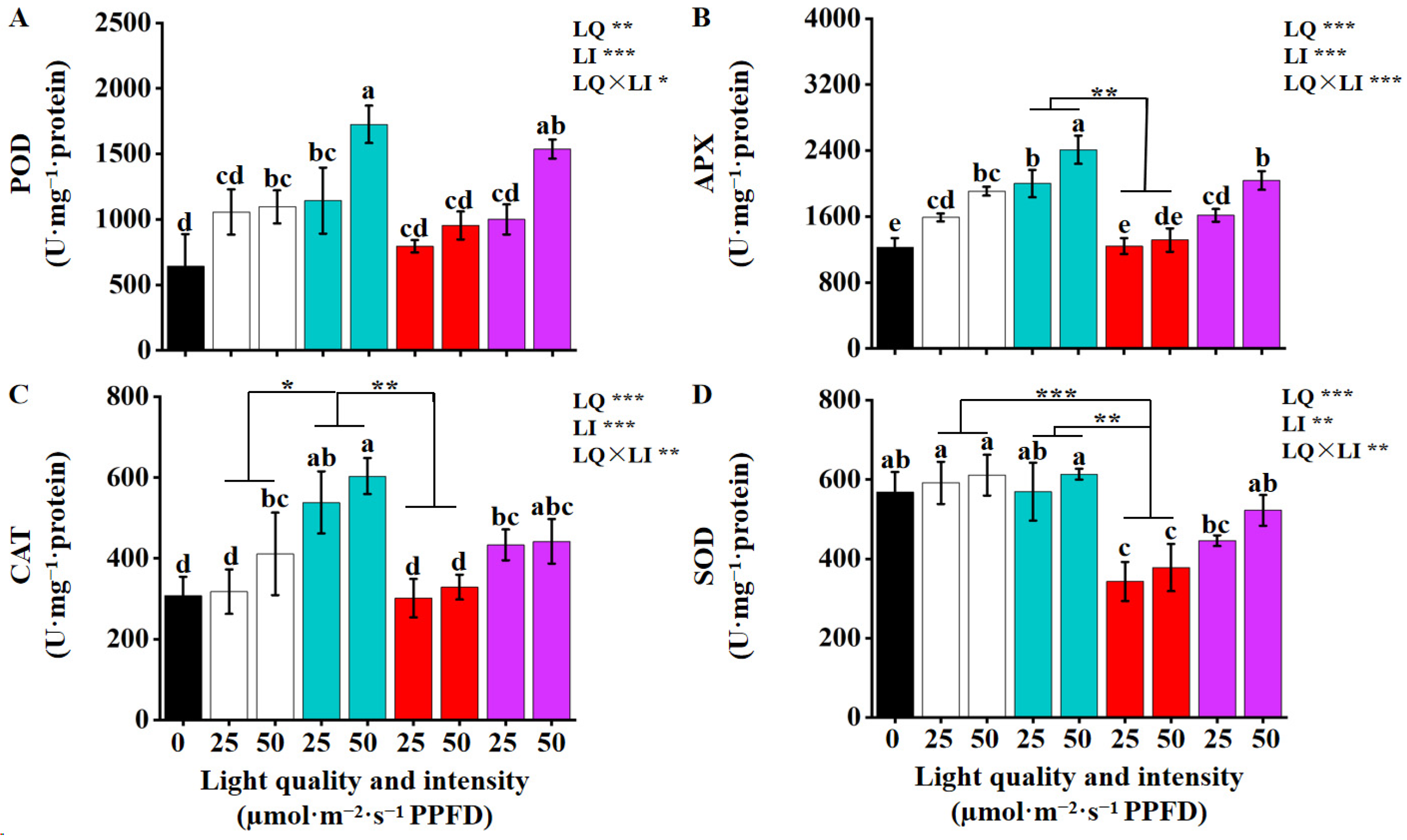
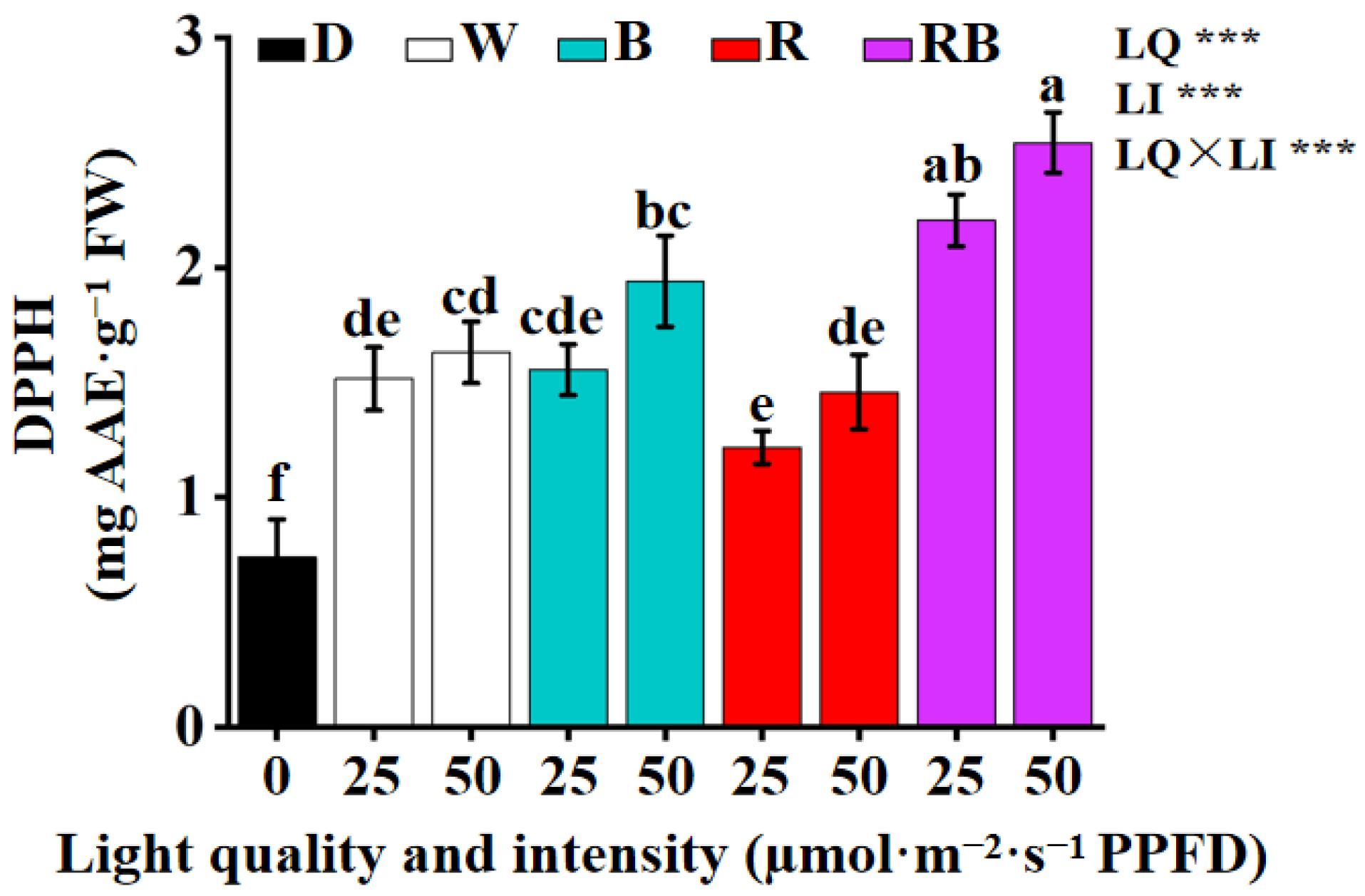
3.5. The Effects of the Light Quality and Intensity on the Activities of Antioxidant Enzyme
4. Discussion
4.1. Establish Technical System for Callus Induction
4.2. Effects of Light Quality and Intensity on Growth and Organogenesis
4.3. Light Regulation of Secondary Metabolite Biosynthesis
4.4. Antioxidant Defense System Under Different Light Treatments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.L.; Su, Z.Y.; Magnus, L. Corydalis. In Flora of China; Wu, Z.Y., Peter, H.R., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2008; Volume 7, pp. 295–428. [Google Scholar]
- Ministry of Food and Drug Safety. The Korean Pharmacopoeia, 10th ed.; Ministry of Food and Drug Safety: Osong, Republic of Korea, 2012; p. 1284.
- Guo, Z.; Man, Y.; Wang, X.; Jin, H.; Sun, X.; Su, X.; Hao, J.; Mi, W. Levo-tetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci. Rep. 2014, 4, 3905. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Chen, C.; Zhang, Q.; Tan, C.N.; Hu, Y.J.; Li, P.; Wan, J.B.; Feng, G.; Xia, Z.N.; Yang, F.Q. Differential proteomic analysis of platelets suggested target-related proteins in rabbit platelets treated with Rhizoma Corydalis. Pharm. Biol. 2017, 55, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, E.; Babich, O.; Prosekov, A.; Asyakina, L.; Gureev, M.; Krasavin, M. Plants of the Russian Federation pharmacopeia: An unexhausted natural products research opportunity? Nat. Prod. Res. 2021, 35, 3525–3527. [Google Scholar] [CrossRef] [PubMed]
- Pakseresht, G.; Kahrizi, D.; Mansouri, M.; Ghorbani, T.; Kazemi, N. Study of callus induction and cell culture to secondary metabolite production in Hyssopus officinalis L. JRPS 2016, 5, 104–111. [Google Scholar]
- Salma, U.; Rahman, M.; Islam, S.; Haque, N.; Jubair, T.; Haque, A.; Mukti, I. The influence of different hormone concentration and combination on callus induction and regeneration of Rauwolfia serpentina L. Benth. Pak. J. Biol. Sci. PJBS 2008, 11, 1638–1641. [Google Scholar] [CrossRef]
- Rad, S.Z.K.; Rameshrad, M.; Hosseinzadeh, H. Toxicology effects of Berberis vulgaris (barberry) and its active constituent, berberine: A review. Iran. J. Basic Med. Sci. 2017, 20, 516. [Google Scholar] [CrossRef]
- Maheshwari, P.; Songara, B.; Kumar, S.; Jain, P.; Srivastava, K.; Kumar, A. Alkaloid production in Vernonia cinerea: Callus, cell suspension and root cultures. Biotechnol. J. Healthcare Nutr. Technol. 2007, 2, 1026–1032. [Google Scholar] [CrossRef]
- Ikuta, A.; Syōno, K.; Furuya, T. Alkaloids of callus tissues and redifferentiated plantlets in the Papaveraceae. Phytochemistry 1974, 13, 2175–2179. [Google Scholar] [CrossRef]
- Sagare, A.; Lee, Y.; Lin, T.; Chen, C.; Tsay, H. Cytokinin-induced somatic embryogenesis and plant regeneration in Corydalis yanhusuo (Fumariaceae)—A medicinal plant. Plant Sci. 2000, 160, 139–147. [Google Scholar] [CrossRef]
- Chang, J.I.; Shin, S.W.; Chi, H.J. Production of Corydalis alkaloids by plant cell culture (I). Kor. J. Pharmacogn. 1995, 26, 419–425. [Google Scholar]
- Pang, J.; Xiong, Y.; Zeng, Y.; Chen, X.; Zhang, X.; Li, Y.; Wu, K.; Zeng, S.; Teixeira da Silva, J.A.; Ma, G. Shoot organogenesis and plant regeneration from leaf and petiole explants of Corydalis saxicola Bunting. Vitr. Cell Dev. Biol. Plant 2023, 59, 121–128. [Google Scholar] [CrossRef]
- Zhao, J.; Verpoorte, R. Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: From biochemical processing to metabolic engineering. Phytochem. Rev. 2007, 6, 435–457. [Google Scholar] [CrossRef]
- Hsu, B.; Kin, K. Pharmacological study of tetrahydropalmatine and its analogs. A new type of central depressants. Arch. Int. Pharmacodyn. Ther. 1962, 139, 318–327. [Google Scholar]
- Tian, B.; Tian, M.; Huang, S.M. Advances in phytochemical and modern pharmacological research of Rhizoma Corydalis. Pharm. Biol. 2020, 58, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.K.; Lin, M.T. DL-Tetrahydropalmatine may act through inhibition of amygdaloid release of dopamine to inhibit an epileptic attack in rats. Neurosci. Lett. 2001, 307, 163–166. [Google Scholar] [CrossRef]
- Lin, T.Y.; Chen, I.Y.; Lee, M.Y.; Lu, C.W.; Chiu, K.M.; Wang, S.J. Inhibition of glutamate release from rat cortical nerve terminals by dehydrocorydaline, an alkaloid from Corydalis yanhusuo. Molecules 2022, 27, 960. [Google Scholar] [CrossRef]
- Nalawade, S.M.; Sagare, A.; Kuo, C.; Lo, S.; Lee, C.; Lee, Y.; Tsay, H. Tissue culture of Corydalis yanhusuo (Fumariaceae) and its medicinal compound production from somatic embryo-derived plants. In Liquid Culture Systems for in Vitro Plant Propagation; Springer: Berlin/Heidelberg, Germany, 2005; pp. 557–565. [Google Scholar]
- Zhang, D.X.; Li, X.F.; Shao, S.L. Callus culture and conditions for dl-tetrahydropalmatine formationin Corydalis yanhuso from northeast of China. Zhiwu Yanjiu 2003, 23, 302–307. [Google Scholar]
- Baker, C.J.; Orlandi, E.W. Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 1995, 33, 299–321. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.R.; Sivanesan, I. Direct adventitious shoot regeneration, in vitro flowering, fruiting, secondary metabolite content and antioxidant activity of Scrophularia takesimensis Nakai. Plant Cell Tissue Organ Cult. 2015, 123, 607–618. [Google Scholar] [CrossRef]
- Sivanesan, I.; Park, S.W. Optimizing factors affecting adventitious shoot regeneration, in vitro flowering and fruiting of Withania somnifera (L.) Dunal. Ind. Crops Prod. 2015, 76, 323–328. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z. The effects of different light qualities on rapeseed (Brassica napus L.) plantlet growth and morphogenesis in vitro. Sci. Hortic. 2013, 150, 117–124. [Google Scholar] [CrossRef]
- Dutta Gupta, S.; Sahoo, T. Light emitting diode (LED)-induced alteration of oxidative events during in vitro shoot organogenesis of Curculigo orchioides Gaertn. Acta Physiol. Plant. 2015, 37, 233. [Google Scholar] [CrossRef]
- Zhao, J.; Thi, L.T.; Park, Y.G.; Jeong, B.R. Light quality affects growth and physiology of Carpesium triste Maxim. cultured in vitro. Agriculture 2020, 10, 258. [Google Scholar] [CrossRef]
- Gaafar, A.R.Z.; Qahtan, A.A.; Alatar, A.A. Unlocking the potential of green synthesis: Biogenic silver nanoparticles enhance biomass and bioactive chemicals in Ricinus communis Callus. Biotechnol. Biotechnol. Equip. 2024, 38, 2421996. [Google Scholar] [CrossRef]
- Kim, H.H.; Mizuno, K.; Lee, H.S.; Koo, J.G.; Kong, W.S. Distribution of indicator plant of climate change in major islands of the Korean peninsula. J. Environ. Sci. Int. 2021, 30, 29–43. [Google Scholar] [CrossRef]
- Sarker, R.; Mustafa, B.M. Regeneration and Agrobacterium-mediated genetic transformation of two indigenous potato varieties of Bangladesh. Plant Tissue Cult 2002, 12, 69–77. [Google Scholar]
- Wang, Q.; Zhang, F.; Smith, D.L. Application of GA3 and kinetin to improve corn and soybean seedling emergence at low temperature. Environ. Exp. Bot. 1996, 36, 377–383. [Google Scholar] [CrossRef]
- Krishna, P. Brassinosteroid-mediated stress responses. J. Plant Growth Regul. 2003, 22, 289–297. [Google Scholar] [CrossRef]
- Wang, H.; Dai, B.; Shu, X.; Wang, H.; Ning, P. Effect of kinetin on physiological and biochemical properties of maize seedlings under arsenic stress. Adv. Mater. Sci. Eng. 2015, 2015, 714646. [Google Scholar] [CrossRef]
- Ikram, U.H. Callus proliferation and somatic embroyogenesis in cotton (Gossypium hirsutum L.). Afr. J. Biotechnol. 2005, 4, 206–209. [Google Scholar]
- Okubo, H.; Wada, K.; Uemoto, S. In vitro morphogenetic response and distribution of endogenous plant hormones in hypocotyl segments of snapdragon (Antirrhinum majus L.). Plant Cell Rep. 1991, 10, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.E.; Ordás, R.J.; Fernández, B.; Centeno, M.L. Relationships between hormonal contents and the organogenic response in Pinus pinea cotyledons. Plant Physiol. Biochem. 2001, 39, 377–384. [Google Scholar] [CrossRef]
- Sakpere, A.; Ajayi, S.; Adelusi, A. Effect of growth regulators and explant types on callus induction in Telfairia occidentalis Hook F. Afr. J. Biotechnol. 2014, 13, 2015–2021. [Google Scholar] [CrossRef]
- Ramazani, S.H.R.; Moshtaghi, R. Optimizing callus induction in Guar (Cyamopsis tetragonoloba L.): Impact of hormonal compositions and explant types. Agrotech. Ind. Crops 2025, 5, 104–110. [Google Scholar] [CrossRef]
- Seibert, M.; Wetherbee, P.J.; Job, D.D. The effects of light intensity and spectral quality on growth and shoot initiation in tobacco callus. Plant Physiol. 1975, 56, 130–139. [Google Scholar] [CrossRef]
- Dehestani-Ardakani, M.; Karimi Dorche, M.; Rahmati, M. Potential impact of different LED light spectra on callus induction, regeneration and plantlet growth of two cultivars of Caladium bicolor. J. Hortic. Postharvest Res. 2025, 8, 89–104. [Google Scholar] [CrossRef]
- Martin-Urdiroz, N.; Garrido-Gala, J.; Martin, J.; Barandiaran, X. Effect of light on the organogenic ability of garlic roots using a one-step in vitro system. Plant Cell Rep. 2004, 22, 721–724. [Google Scholar] [CrossRef]
- Tubić, L.; Anačkov, G.; Milojević, J.; Ghalawenji, N.; Mitić, N.; Igić, R.; Zdravković-Korać, S. High variability in the tissue culture response of root-tips of Allium ascalonicum individuals and optimization of the regeneration procedure. Plant Cell Tissue Organ Cult. 2014, 118, 101–110. [Google Scholar] [CrossRef]
- Ali, M.; Mujib, A.; Tonk, D.; Zafar, N. Plant regeneration through somatic embryogenesis and genome size analysis of Coriandrum sativum L. Protoplasma 2017, 254, 343–352. [Google Scholar] [CrossRef]
- Tariq, U.; Ali, M.; Abbasi, B.H. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J. Photochem. Photobiol. B 2014, 130, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nacheva, L.; Dimitrova, N.; Koleva-Valkova, L.; Stefanova, M.; Ganeva, T.; Nesheva, M.; Tarakanov, I.; Vassilev, A. In vitro multiplication and rooting of plum rootstock ‘Saint Julien’ (Prunus domestica subsp. insititia) under fluorescent light and different LED spectra. Plants 2023, 12, 2125. [Google Scholar] [CrossRef] [PubMed]
- Mogilevskaya, I.; Zholobova, O.; Tereshchenko, T.; Fomenko, N.; Grichik, E. How Light Spectrum and Light Intensity Using Mixed LEDs Affect the Growth of Populus F1-3 g14 Hybrid and Callus Morphology in vitro. J. Plant Growth Regul. 2025, 44, 4820–4836. [Google Scholar] [CrossRef]
- Miranda, N.A.; Xavier, A.; Otoni, W.C.; Gallo, R.; Gatti, K.C.; de Moura, L.C.; Souza, D.M.; Maggioni, J.H.; Santos, S.S.d.O. Quality and intensity of light in the in vitro development of microstumps of Eucalyptus urophylla in a photoautotrophic system. For. Sci. 2020, 66, 754–760. [Google Scholar] [CrossRef]
- Biswal, B.; Jena, B.; Giri, A.K.; Acharya, L. Monochromatic light elicited biomass accumulation, antioxidant activity, and secondary metabolite production in callus culture of Operculina turpethum (L.). Plant Cell Tissue Organ Cult. 2022, 149, 123–134. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Jafri, F.I.; Siddiqui, M.H.; Alamri, S.; Akhtar, M.S. Synergistic effects of selenium nanoparticles and LED light on enhancement of secondary metabolites in sandalwood (Santalum album) plants through in-vitro callus culturing technique. PeerJ 2024, 12, e18106. [Google Scholar] [CrossRef]
- Coelho, G.C.; Rachwal, M.F.; Dedecek, R.A.; Curcio, G.R.; Nietsche, K.; Schenkel, E.P. Effect of light intensity on methylxanthine contents of Ilex paraguariensis A. St. Hil. Biochem. Syst. Ecol. 2007, 35, 75–80. [Google Scholar] [CrossRef]
- Setiawati, T.; Ayalla, A.; Nurzaman, M.; Mutaqin, A. Influence of light intensity on leaf photosynthetic traits and alkaloid content of Kiasahan (Tetracera scandens L.). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Bogor, Indonesia, 1–2 November 2017; p. 012025. [Google Scholar]
- Shohael, A.; Ali, M.; Yu, K.; Hahn, E.; Islam, R.; Paek, K. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem. 2006, 41, 1179–1185. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Wahab, P.E.M.; Halim, M.R.A. Effect of different light intensities on total phenolics and flavonoids synthesis and anti-oxidant activities in young ginger varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897. [Google Scholar] [CrossRef]
- Nezafatian, E.; Farhadian, O.; Daneshvar, E.; Bhatnagar, A. Investigating the effects of salinity and light stresses on primary and secondary metabolites of Tetraselmis tetrathele: Total phenolic compounds, fatty acid profile, and biodiesel properties. Biomass Bioenergy 2024, 181, 107050. [Google Scholar] [CrossRef]
- Walter, M.H.; Strack, D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat. Prod. Rep. 2011, 28, 663–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Yu, C.L.; Liu, Y.J.; Kan, S.L.; Chen, M.; Cao, Y.N.; Wang, H.W.; Li, J.M.; Peng, D. An autotetraploid genome of Corydalis sheareri provides insight into the evolution and benzylisoquinoline alkaloids diversity of Corydalis. Genom. Commun. 2025, 2, e002. [Google Scholar] [CrossRef]
- Wang, Y.; Subrizi, F.; Carter, E.M.; Sheppard, T.D.; Ward, J.M.; Hailes, H.C. Enzymatic synthesis of benzylisoquinoline alkaloids using a parallel cascade strategy and tyrosinase variants. Nat. Commun. 2022, 13, 5436. [Google Scholar] [CrossRef]
- Khodorova, N.V.; Shavarda, A.L.; Lequart-Pillon, M.; Laberche, J.-C.; Voitsekhovskaja, O.V.; Boitel-Conti, M. Biosynthesis of benzylisoquinoline alkaloids in Corydalis bracteata: Compartmentation and seasonal dynamics. Phytochemistry 2013, 92, 60–70. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Chen, Y. Advances in the biosynthesis of naturally occurring benzylisoquinoline alkaloids. Front. Plant Sci. 2025, 16, 1548471. [Google Scholar] [CrossRef]
- Sun, H.; Song, H.; Deng, X.; Liu, J.; Yang, D.; Zhang, M.; Wang, Y.; Xin, J.; Chen, L.; Liu, Y. Transcriptome-wide characterization of alkaloids and chlorophyll biosynthesis in Lotus plumule. Front. Plant Sci. 2022, 13, 885503. [Google Scholar] [CrossRef]
- Timoneda, A.; Sheehan, H.; Feng, T.; Lopez-Nieves, S.; Maeda, H.A.; Brockington, S. Redirecting primary metabolism to boost production of tyrosine-derived specialised metabolites in planta. Sci. Rep. 2018, 8, 17256. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; An, H.; Zhang, X.; Zhou, B. Integrated transcriptome and metabolome analysis reveals the anthocyanin biosynthesis mechanisms in blueberry (Vaccinium corymbosum L.) leaves under different light qualities. Front. Plant Sci. 2022, 13, 1073332. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Huo, J.; Lin, Q.; Mo, L.; Zheng, L.; Meng, X.; Song, X.; Liang, J.; Chen, T. The influence of varying wavelengths of led light on the development, physiology response, and metabolism activities of micropropagated Dendrobium hybrid ‘shuijing’ plantlets. Horticulturae 2024, 10, 774. [Google Scholar] [CrossRef]
- Ali, A.; Mashwani, Z.-u.-R.; Raja, N.I.; Mohammad, S.; Luna-Arias, J.P.; Ahmad, A.; Kaushik, P. Phytomediated selenium nanoparticles and light regimes elicited in vitro callus cultures for biomass accumulation and secondary metabolite production in Caralluma tuberculata. Front. Plant Sci. 2023, 14, 1253193. [Google Scholar] [CrossRef]
- Gad, A.G.; Habiba; Zheng, X.; Miao, Y. Low light/darkness as stressors of multifactor-induced senescence in rice plants. Int. J. Mol. Sci. 2021, 22, 3936. [Google Scholar] [CrossRef] [PubMed]
- Santos-Tierno, R.; Garcia, R.; Fonseca, E.; Faleiro, F.; Moreira, D.; Pacheco, G.; Mansur, E. Light quality and explant type modulate growth, antioxidant properties and bioactive compounds production of calluses of Passiflora setacea cv BRS Pérola do Cerrado. Plant Cell Tissue Organ Cult. 2021, 147, 635–646. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Yu, C.Y.; Seong, E.S. Effect of light-emitting diode light sources on antioxidant activity and chemical composition variations of phenols, flavonoids, and terpenoids in Oplopanax elatus extracts. J. Appl. Biol. Chem. 2025, 68, 226–233. [Google Scholar] [CrossRef]
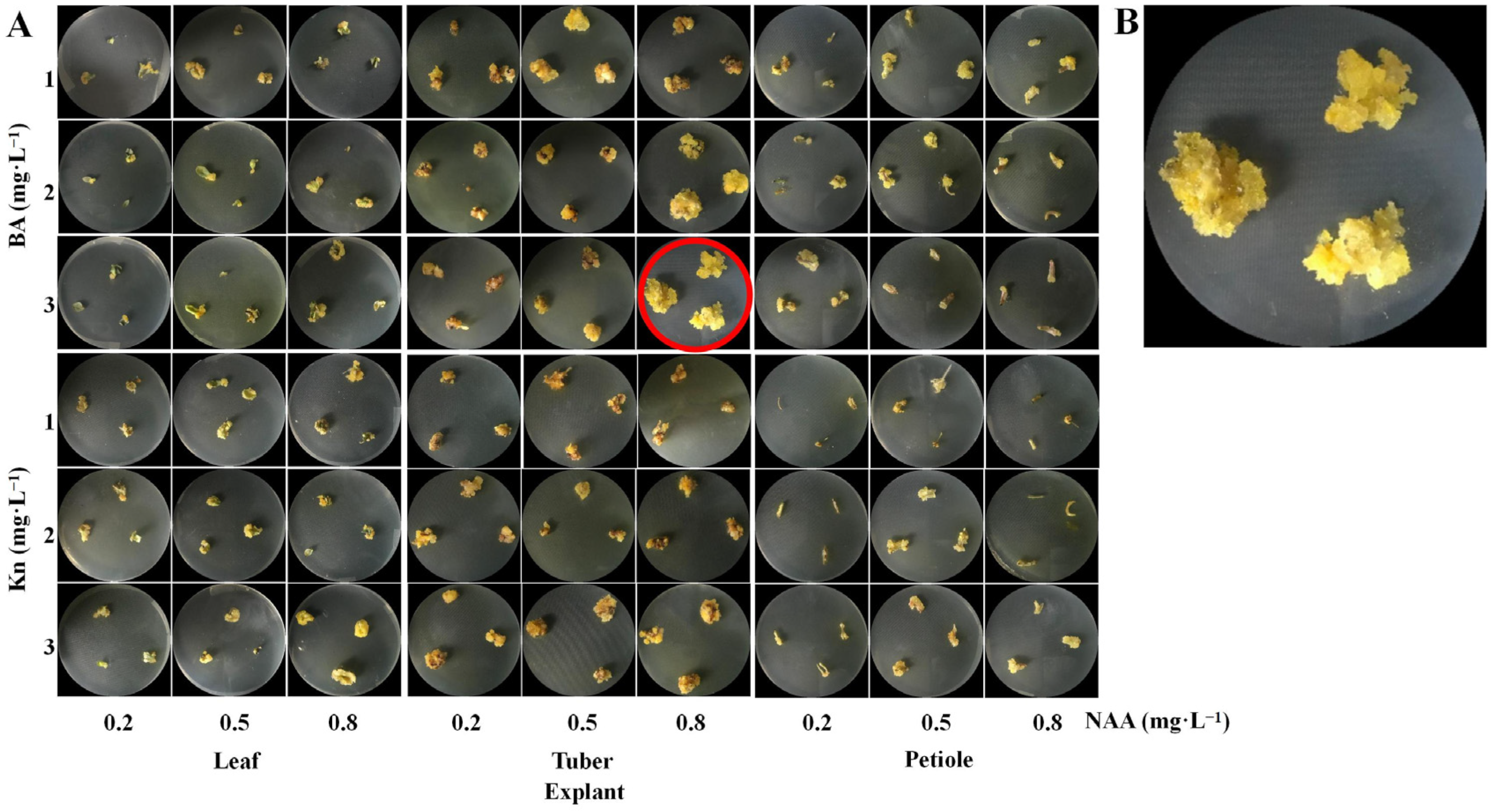
| Cytokinin (A) | Conc. (mg∙L−1) (B) | NAA Conc. (mg∙L−1) (C) | Callus Induction Frequency (%) | Fresh Wt. (g) | Phenotypic Description |
|---|---|---|---|---|---|
| BA z | 1 | 0.2 | 55.6 ± 5.6 bcd y | 0.11 ± 0.01 g | Compact, dark yellow |
| 0.5 | 88.9 ± 11.1 abc | 0.26 ± 0.02 cde | Compact, dark yellow | ||
| 0.8 | 72.2 ± 11.1 abcd | 0.22 ± 0.01 def | Compact, dark yellow, slight browning | ||
| 2 | 0.2 | 50.0 ± 9.6 cd | 0.15 ± 0.01 fg | Friable, light yellow | |
| 0.5 | 72.2 ± 11.1 abcd | 0.21 ± 0.01 def | Friable, light yellow | ||
| 0.8 | 94.4 ± 5.6 ab | 0.23 ± 0.02 def | Slight compact, yellow | ||
| 3 | 0.2 | 38.9 ± 5.6 d | 0.18 ± 0.01 efg | Friable, yellow | |
| 0.5 | 72.2 ± 5.6 abcd | 0.26 ± 0.01 cde | Friable, yellow, slight browning | ||
| 0.8 | 100.0 ± 0.0 a | 0.34 ± 0.02 bc | Friable, yellow | ||
| Kn | 1 | 0.2 | 61.1 ± 11.1 abcd | 0.08 ± 0.01 g | Compact with adventitious roots |
| 0.5 | 94.4 ± 5.6 ab | 0.21 ± 0.01 def | Compact with adventitious roots | ||
| 0.8 | 66.7 ± 9.6 abcd | 0.14 ± 0.01 fg | Compact with adventitious roots | ||
| 2 | 0.2 | 61.1 ± 5.6 abcd | 0.26 ± 0.02 cde | Compact, yellow | |
| 0.5 | 66.7 ± 9.6 abcd | 0.25 ± 0.03 cde | Compact, yellow | ||
| 0.8 | 77.8 ± 5.6 abcd | 0.28 ± 0.02 bcd | Compact, slight browning | ||
| 3 | 0.2 | 38.9 ± 5.6 d | 0.14 ± 0.01 fg | Compact, yellow | |
| 0.5 | 66.7 ± 0.0 abcd | 0.36 ± 0.02 b | Compact, yellow | ||
| 0.8 | 88.9 ± 5.6 abc | 0.60 ± 0.05 a | Friable, light yellow |
| Cytokinin (A) | Conc. (mg∙L−1) (B) | NAA Conc. (mg∙L−1) (C) | Callus Induction Frequency (%) | Fresh Wt. (g) | Phenotypic Description |
|---|---|---|---|---|---|
| BA z | 1 | 0.2 | 72.2 ± 5.6 b y | 0.36 ± 0.02 efg | Compact, dark yellow |
| 0.5 | 94.4 ± 5.6 ab | 0.70 ± 0.01 c | Slightly friable, yellow | ||
| 0.8 | 83.3 ± 0.0 ab | 0.46 ± 0.00 def | Compact, slight browning | ||
| 2 | 0.2 | 72.2 ± 5.6 b | 0.30 ± 0.02 g | Compact, dark yellow | |
| 0.5 | 88.9 ± 5.6 ab | 0.54 ± 0.03 d | Compact, dark yellow | ||
| 0.8 | 94.4 ± 5.6 ab | 1.06 ± 0.05 b | Friable, yellow | ||
| 3 | 0.2 | 83.3 ± 0.0 ab | 0.55 ± 0.01 cd | Compact, dark yellow | |
| 0.5 | 100.0 ± 0.0 a | 0.59 ± 0.02 cd | Slightly friable, yellow | ||
| 0.8 | 100.0 ± 0.0 a | 1.58 ± 0.06 a | Friable, light yellow | ||
| Kn | 1 | 0.2 | 72.2 ± 5.6 b | 0.25 ± 0.01 g | Compact, dark yellow |
| 0.5 | 94.4 ± 5.6 ab | 0.33 ± 0.02 efg | Compact with adventitious roots | ||
| 0.8 | 94.4 ± 5.6 ab | 0.26 ± 0.02 g | Compact with adventitious roots | ||
| 2 | 0.2 | 83.3 ± 9.6 ab | 0.29 ± 0.02 g | Slightly friable, yellow | |
| 0.5 | 94.4 ± 5.6 ab | 0.31 ± 0.02 fg | Slightly friable, yellow | ||
| 0.8 | 100 ± 0.0 a | 0.36 ± 0.01 efg | Compact, slight browning | ||
| 3 | 0.2 | 83.3 ± 0.0 ab | 0.49 ± 0.04 de | Compact, slight browning | |
| 0.5 | 94.4 ± 5.6 ab | 0.49 ± 0.04 de | Compact, slight browning | ||
| 0.8 | 100.0 ± 0.0 a | 0.57 ± 0.03 cd | Compact, yellow |
| Cytokinin (A) | Conc. (mg∙L−1) (B) | NAA Conc. (mg∙L−1) (C) | Callus Induction Frequency (%) | Fresh Wt. (g) | Phenotypic Description |
|---|---|---|---|---|---|
| BA z | 1 | 0.2 | 77.8 ± 5.6 abc y | 0.06 ± 0.01 f | Compact, dark yellow |
| 0.5 | 88.9 ± 5.6 abc | 0.26 ± 0.02 bc | Friable, light yellow | ||
| 0.8 | 83.3 ± 0.0 abc | 0.06 ± 0.01 f | Friable, light yellow | ||
| 2 | 0.2 | 61.1 ± 5.6 c | 0.13 ± 0.01 ef | Slight compact, yellow | |
| 0.5 | 100.0 ± 0.0 a | 0.34 ± 0.01 ab | Friable, light yellow | ||
| 0.8 | 94.4 ± 5.6 ab | 0.33 ± 0.01 ab | Slight compact, yellow | ||
| 3 | 0.2 | 66.7 ± 9.6 bc | 0.22 ± 0.01 cde | Friable, yellow | |
| 0.5 | 88.9 ± 5.6 abc | 0.29 ± 0.02 bc | Few induced callus tissue was observed | ||
| 0.8 | 94.4 ± 5.6 ab | 0.33 ± 0.01 ab | Few induced callus tissue was observed | ||
| Kn | 1 | 0.2 | 61.1 ± 9.6 c | 0.25 ± 0.03 bcd | Few induced callus tissue was observed |
| 0.5 | 94.4 ± 9.6 ab | 0.33 ± 0.03 ab | Compact with adventitious roots | ||
| 0.8 | 72.2 ± 9.6 abc | 0.27 ± 0.02 bc | Few and compact | ||
| 2 | 0.2 | 66.7 ± 9.6 bc | 0.14 ± 0.01 ef | Few induced callus tissue was observed | |
| 0.5 | 88.9 ± 5.6 abc | 0.39 ± 0.03 a | Slight compact, yellow | ||
| 0.8 | 77.8 ± 5.6 abc | 0.20 ± 0.02 cde | Few induced callus tissue was observed | ||
| 3 | 0.2 | 77.8 ± 5.6 abc | 0.05 ± 0.01 f | Few and compact | |
| 0.5 | 88.9 ± 5.6 abc | 0.16 ± 0.01 de | Slight compact, yellow | ||
| 0.8 | 94.4 ± 5.6 ab | 0.21 ± 0.01 cde | Friable, light yellow |
| Tested Parameters | Variation Source | Sum of Squares | F Value | p Value |
|---|---|---|---|---|
| Callus induction frequency (%) | Cytokinin type (A) | 82.288 | 0.471 | 0.497 ns |
| Cytokinin conc. (mg∙L−1) (B) | 277.778 | 0.794 | 0.460 ns | |
| NAA conc. (mg∙L−1) (C) | 10,586.247 | 30.266 | 0.000 *** | |
| A × B | 133.700 | 0.382 | 0.685 ns | |
| A × C | 627.576 | 1.794 | 0.181 ns | |
| B × C | 4969.42 | 7.104 | 0.000 *** | |
| A × B × C | 174.918 | 0.250 | 0.908 ns | |
| Fresh wt. (g) | Cytokinin type (A) | 0.0200 | 20.417 | 0.000 *** |
| Cytokinin conc. (mg∙L−1) (B) | 0.183 | 91.335 | 0.000 *** | |
| NAA conc. (mg∙L−1) (C) | 0.215 | 107.696 | 0.000 *** | |
| A × B | 0.061 | 30.606 | 0.000 *** | |
| A × C | 0.01 | 5.089 | 0.011 * | |
| B × C | 0.144 | 35.991 | 0.000 *** | |
| A × B × C | 0.065 | 16.294 | 0.000 *** |
| Tested Parameters | Variation Source | Sum of Squares | F Value | p Value |
|---|---|---|---|---|
| Callus induction frequency (%) | Cytokinin type (A) | 128.621 | 1.923 | 0.174 ns |
| Cytokinin conc. (mg∙L−1) (B) | 627.484 | 4.692 | 0.015 * | |
| NAA conc. (mg∙L−1) (C) | 3528.619 | 26.384 | 0.000 *** | |
| A × B | 195.508 | 1.462 | 0.245 ns | |
| A × C | 72.039 | 0.539 | 0.588 ns | |
| B × C | 236.615 | 0.885 | 0.483 ns | |
| A × B × C | 113.183 | 0.423 | 0.791 ns | |
| Fresh wt. (g) | Cytokinin type (A) | 1.3 | 508.136 | 0.000 *** |
| Cytokinin conc. (mg∙L−1) (B) | 0.975 | 190.422 | 0.000 *** | |
| NAA conc. (mg∙L−1) (C) | 1.095 | 213.996 | 0.000 *** | |
| A × B | 0.058 | 11.415 | 0.000 *** | |
| A × C | 0.789 | 154.234 | 0.000 *** | |
| B × C | 0.806 | 78.712 | 0.000 *** | |
| A × B × C | 0.495 | 48.329 | 0.000 *** |
| Tested Parameters | Variation Source | Sum of Squares | F Value | p Value |
|---|---|---|---|---|
| Callus induction frequency (%) | Cytokinin type (A) | 82.29 | 0.47 | 0.497 ns |
| Cytokinin conc. (mg∙L−1) (B) | 277.78 | 0.79 | 0.460 ns | |
| NAA conc. (mg∙L−1) (C) | 10,586.25 | 30.26 | 0.000 *** | |
| A × B | 133.70 | 0.38 | 0.685 ns | |
| A × C | 627.58 | 1.79 | 0.181 ns | |
| B × C | 4969.42 | 7.10 | 0.000 *** | |
| A × B × C | 174.92 | 0.25 | 0.908 ns | |
| Fresh wt. (g) | Cytokinin type (A) | 0.02 | 20.42 | 0.000 *** |
| Cytokinin conc. (mg∙L−1) (B) | 0.18 | 91.34 | 0.000 *** | |
| NAA conc. (mg∙L−1) (C) | 0.22 | 107.70 | 0.000 *** | |
| A × B | 0.06 | 30.61 | 0.000 *** | |
| A × C | 0.01 | 5.09 | 0.011 * | |
| B × C | 0.14 | 35.99 | 0.000 *** | |
| A × B × C | 0.07 | 16.29 | 0.000 *** |
| Treatment | Phenotypic Description | ||
|---|---|---|---|
| Light quality | Light intensity (µmol·m−2·s−1 PPFD) | 2 weeks of culture | 6 weeks of culture |
| Dark (D) 0 | Friable, browning, without adventitious shoot emergence | Browning with withered shoots | |
| White (W) | 25 | Slight compact, yellow, without adventitious shoot | Compact with adventitious shoot emergence, slight browning |
| 50 | Compact, yellow, with adventitious shoot emergence | Compact, adventitious shoot emergence in clusters, slight browning, some shoots withered | |
| Blue (B) | 25 | Compact, yellow, with adventitious shoot emergence | Compact with adventitious shoot emergence and friable green callus |
| 50 | Compact, light yellow, with adventitious shoot emergence | Compact with adventitious shoot emergence and green nodular callus | |
| Red (R) | 25 | Compact, dark yellow | Compact, dark yellow, severe browning |
| 50 | Compact, dark yellow | Compact, dark yellow, severe browning with few adventitious shoots | |
| 1:1 red/blue (RB) combination | 25 | Compact, dark yellow, with adventitious shoot emergence | Compact with adventitious shoot emergence and green nodular callus |
| 50 | Compact, dark yellow, with adventitious shoot emergence | Compact with adventitious shoot and somatic embryo emergence, as well as green nodular callus | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Jeong, B.R. LED Light and Plant Growth Regulators Affect Callus Induction, Shoot Organogenesis, dl-Tetrahydropalmatine Accumulation, and Activities of Antioxidant Enzymes in Corydalis turtschaninovii Besser. Horticulturae 2025, 11, 1420. https://doi.org/10.3390/horticulturae11121420
Zhao J, Jeong BR. LED Light and Plant Growth Regulators Affect Callus Induction, Shoot Organogenesis, dl-Tetrahydropalmatine Accumulation, and Activities of Antioxidant Enzymes in Corydalis turtschaninovii Besser. Horticulturae. 2025; 11(12):1420. https://doi.org/10.3390/horticulturae11121420
Chicago/Turabian StyleZhao, Jin, and Byoung Ryong Jeong. 2025. "LED Light and Plant Growth Regulators Affect Callus Induction, Shoot Organogenesis, dl-Tetrahydropalmatine Accumulation, and Activities of Antioxidant Enzymes in Corydalis turtschaninovii Besser" Horticulturae 11, no. 12: 1420. https://doi.org/10.3390/horticulturae11121420
APA StyleZhao, J., & Jeong, B. R. (2025). LED Light and Plant Growth Regulators Affect Callus Induction, Shoot Organogenesis, dl-Tetrahydropalmatine Accumulation, and Activities of Antioxidant Enzymes in Corydalis turtschaninovii Besser. Horticulturae, 11(12), 1420. https://doi.org/10.3390/horticulturae11121420






