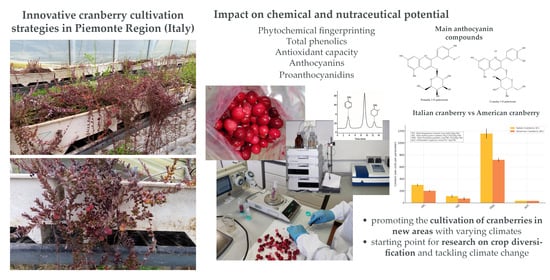
Impact on the Health-Promoting Potential of Cranberries for Food Applications Through Soilless Cultivation Practices in Piemonte Region (Italy): A Sustainable Opportunity for Nutraceutical Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Qualitative Analysis
2.3. Reagents
2.4. Extraction of Bioactive Compounds
2.5. Total Anthocyanin Content
2.6. Total Polyphenolic Content
2.7. Ferric Reducing Antioxidant Power
2.8. Total PACs Analysis
2.9. Phytochemicals and Nutritional Compounds
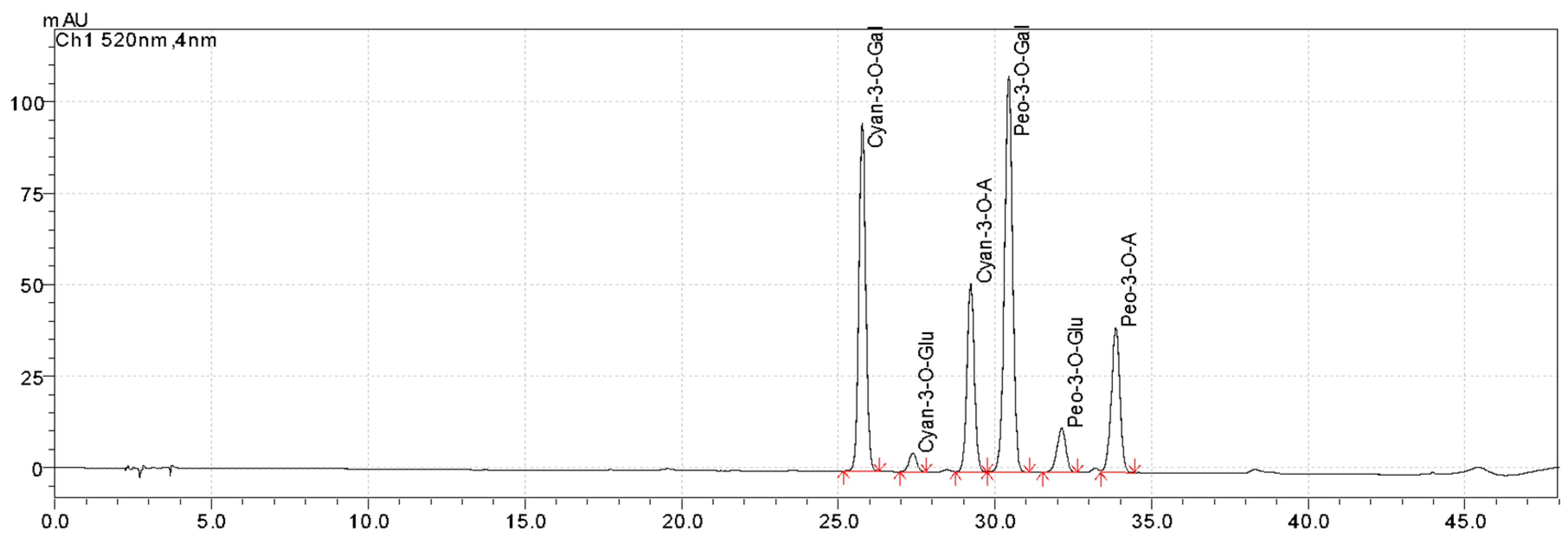
2.10. Anthocyanins Profiling
2.11. A-Type and B-Type PACs Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fruit Quality Analysis
3.2. Total Phenolic, Anthocyanin and Proanthocyanidin Content and Antioxidant Activity
3.3. Bioactive Compounds and Nutritional Substances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Česonienė, L.; Daubaras, R. Phytochemical Composition of the Large Cranberry (Vaccinium macrocarpon) and the Small Cranberry (Vaccinium oxycoccos). In Nutritional Composition of Fruit Cultivars; Elsevier: Amsterdam, The Netherlands, 2016; pp. 173–194. [Google Scholar]
- Sandler, H.; DeMoranville, C. Cranberry Production: A Guide for Massachusetts-Summary Edition; University of Massachusetts: Wareham, MA, USA, 2008. [Google Scholar]
- Nemzer, B.V.; Al-Taher, F.; Yashin, A.; Revelsky, I.; Yashin, Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health: Overview. Molecules 2022, 27, 1503. [Google Scholar] [CrossRef]
- Vorsa, N.; Johnson-Cicalese, J. American Cranberry. In Fruit Breeding; Springer: Boston, MA, USA, 2012; pp. 191–223. [Google Scholar]
- Gareau, B.J.; Huang, X.; Gareau, T.P. Social and ecological conditions of cranberry production and climate change attitudes in New England. PLoS ONE 2018, 13, e0207237. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Brown, P.N.; Murch, S.J.; Shipley, P. Phytochemical Diversity of Cranberry (Vaccinium macrocarpon Aiton) Cultivars by Anthocyanin Determination and Metabolomic Profiling with Chemometric Analysis. J. Agric. Food Chem. 2012, 60, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Ehala, S.; Vaher, M.; Kaljurand, M. Characterization of phenolic profiles of Northern European berries by capillary electrophoresis and determination of their antioxidant activity. J. Agric. Food Chem. 2005, 53, 6484–6490. [Google Scholar] [CrossRef]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and Their Bioactive Constituents in Human Health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- Nie, Y.; Stürzenbaum, S.R. Proanthocyanidins of natural origin: Molecular mechanisms and implications for lipid disorder and aging-associated diseases. Adv. Nutr. 2019, 10, 464–478. [Google Scholar] [CrossRef]
- Borowska, E.J.; Mazur, B.; Gadza, R. Polyphenol, Anthocyanin and Resveratrol Mass Fractions and Antioxidant Properties of Cranberry Cultivars. Food Technol. Biotechnol. 2009, 47, 56. [Google Scholar]
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry (Vaccinium Macrocarpon L): Phytochemicals in cultivars of cranberry. J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Narwojsz, A.; Tańska, M.; Mazur, B.; Borowska, E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus). Plant Food Hum. Nutr. 2019, 74, 300–306. [Google Scholar] [CrossRef]
- Xia, J.-Y.; Yang, C.; Xu, D.-F.; Xia, H.; Yang, L.-G.; Sun, G.-J. Consumption of cranberry as adjuvant therapy for urinary tract infections in susceptible populations: A systematic review and meta-analysis with trial sequential analysis. PLoS ONE 2021, 16, e0256992. [Google Scholar] [CrossRef]
- Agricultural Marketing Resource Center. Cranberries. Available online: https://www.agmrc.org/commodities-products/fruits/cranberries (accessed on 25 July 2025).
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of Selected Cranberry Genotypes (Vaccinium macrocarpon Ait.) and Their Antioxidant Efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Mulberry: An ornamental tree that gives bioactive compounds for human health. In Proceedings of the IV International Symposium on Woody Ornamentals of the Temperate Zone, Torino, Italy, 3–4 March 2021; pp. 205–214. [Google Scholar]
- Donno, D.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Advances in Ribes x nidigrolaria Rud. Bauer and A. Bauer fruits as potential source of natural molecules: A preliminary study on physico-chemical traits of an underutilized berry. Sci. Hortic. 2018, 237, 20–27. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [PubMed]
- Upton, R.; Brendler, T. Cranberry Fruit, Vaccinium macrocarpon Aiton Revision: Standards of Analysis, Quality Control, and Therapeutics; American Herbal Pharmacopoeia: Soquel, CA, USA, 2016. [Google Scholar]
- Donno, D.; Neirotti, G.; Fioccardi, A.; Razafindrakoto, Z.R.; Tombozara, N.; Mellano, M.G.; Beccaro, G.L.; Gamba, G. Freeze-Drying for the Reduction of Fruit and Vegetable Chain Losses: A Sustainable Solution to Produce Potential Health-Promoting Food Applications. Plants 2025, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Nieves, J.M.; Ayala-Garay, O.J.; Serra, V.; Dumont, D.; Vercambre, G.; Génard, M.; Gautier, H. The effects of diurnal temperature rise on tomato fruit quality. Can the management of the greenhouse climate mitigate such effects? Sci. Hortic. 2021, 278, 109836. [Google Scholar] [CrossRef]
- Salama, A.-M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Illés, A.; Holb, I.J. Temperate fruit trees under climate change: Challenges for dormancy and chilling requirements in warm winter regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Metz 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef]
- Patil, J.R.; Mhatre, K.J.; Yadav, K.; Yadav, L.S.; Srivastava, S.; Nikalje, G.C. Flavonoids in plant-environment interactions and stress responses. Discov. Plants 2024, 1, 68. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18 Pt B, 820–897. [Google Scholar] [CrossRef]
- Lu, Y.; Pekerti, B.N.; Toh, Z.S.; Broom, F.; Savage, G.; Liu, S.Q.; Huang, D. Physico-chemical parameters and proanthocyanidin profiles of cranberries cultivated in New Zealand. J. Food Compos. Anal. 2017, 63, 1–7. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.E.; Autio, W.R. Early-season air temperature affects phenolic production in ‘Early Black’cranberry fruit. Hortscience 2008, 43, 1737–1741. [Google Scholar] [CrossRef]
- Šedbarė, R.; Pašakinskienė, I.; Janulis, V. Changes in the composition of biologically active compounds during the ripening period in fruit of different large cranberry (Vaccinium macrocarpon Aiton) cultivars grown in the lithuanian collection. Plants 2023, 12, 202. [Google Scholar] [CrossRef]
- Urbstaite, R.; Raudone, L.; Janulis, V. Phytogenotypic anthocyanin profiles and antioxidant activity variation in fruit samples of the American cranberry (Vaccinium macrocarpon Aiton). Antioxidants 2022, 11, 250. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018, 244, 705–719. [Google Scholar] [CrossRef]
- Fioccardi, A.; Donno, D.; Razafindrakoto, Z.R.; Tombozara, N.; Henintsoa, S.; Mahitasoa, E.; Torti, V.; Solofoniaina, M.; Rosso, L.; Gamba, G.; et al. Assessing a “Least-Concern” Red List Tree Species from Madagascar Used in Traditional Medicine: Morella spathulata (Myricaceae) Phyto-Compounds and Anti-Inflammatory Properties. Plants 2024, 13, 2899. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L.J.F. Analytical strategies for fingerprinting of antioxidants, nutritional substances, and bioactive compounds in foodstuffs based on high performance liquid chromatography–mass spectrometry: An overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait.) and their Press Cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Šedbarė, R.; Jakštāne, G.; Janulis, V. Phytochemical composition of the fruit of large cranberry (Vaccinium macrocarpon Aiton) cultivars grown in the collection of the national botanic garden of Latvia. Plants 2023, 12, 771. [Google Scholar] [CrossRef] [PubMed]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in Disease Prevention and Cure: An Overview. Ind. J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
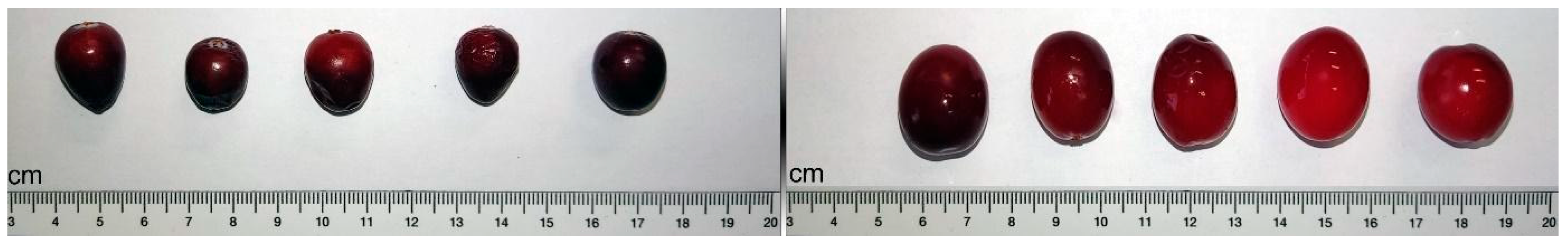
| Feature | Italian Cranberry (IC) | Canadian Cranberry (CC) |
|---|---|---|
| Weight (g) | 0.763 ± 0.361 a | 1.771 ± 0.532 b |
| Dry matter (g 100 g−1) | 12.5 ± 0.10 a | 11.7± 0.12 b |
| Width (mm) | 10.52 ± 1.91 a | 14.47 ± 1.79 b |
| Length (mm) | 11.59 ± 2.53 a | 16.25 ± 2.36 b |
| TSS (°Brix) | 10.3 ± 0.1 a | 8.0 ± 0.7 b |
| pH (pH units) | 2.54 ± 0.04 a | 2.51 ± 0.01 a |
| TA (meq L−1) | 159 ± 1.4 a | 170 ± 5.7 a |
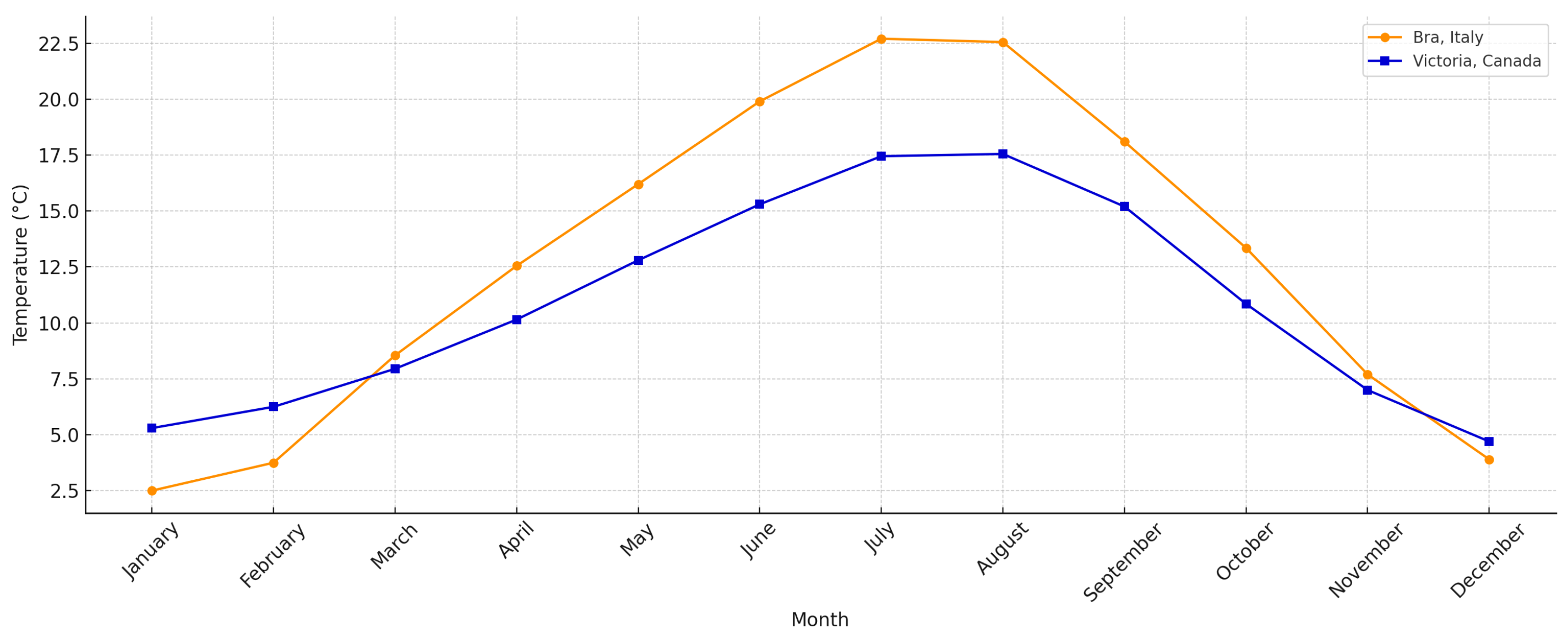
| Parameter | Bra, Piemonte, Italy | Victoria, Columbia Britannica, Canada |
|---|---|---|
| Mean Annual Temperature | About 12.4 °C | About 9.9 °C |
| Mean Annual Precipitation | About 1052 mm | About 961 mm |
| Warmest Month | July (about 29.1 °C) | July (about 22.1 °C) |
| Coldest Month | January (about −0.5 °C) | January (about 3.4 °C) |
| Peak Sunshine | July (about 11.6 h/day) | July (about 11 h/day) |
| Lowest Sunshine | November (about 3.5 h/day) | January (about 2.5 h/day) |
| Snowfall | Occasional | Minimal (about 33 cm/year) |
| Italian Cranberry (IC) | Canadian Cranberry (CC) | ||
|---|---|---|---|
| TPC | Total polyphenolic content (mgGAE 100 g−1 FW) | 299.13 ± 22.02 a | 201.73 ± 12.09 b |
| TAC | Total anthocyanin content (mgC3G 100 g−1 FW) | 112.05 ± 25.50 a | 70.72 ± 25.72 b |
| TPAC | Total PACs content (mg PAC A2 100 g−1 FW) | 1156.05 ± 79.87 a | 717.85 ± 30.89 b |
| AOC | Antioxidant capacity (mmol Fe2+ kg−1 FW) | 35.51 ± 1.12 a | 32.86 ± 1.39 b |
| Bioactive Class | Compound | Italian Cranberry (IC) | Canadian Cranberry (CC) |
|---|---|---|---|
| Cinnamic acids | Caffeic acid | <0.10 | <0.10 |
| Chlorogenic acid | 0.99 ± 0.39 | <0.31 | |
| Coumaric acid | 0.91 ± 0.11 | <0.97 | |
| Ferulic acid | 3.75 ± 1.31 a | 6.95 ± 7.72 a | |
| Flavonols | Hyperoside | <0.34 | <0.34 |
| Isoquercitrin | <0.03 | <0.03 | |
| Quercetin | <0.41 | <0.41 | |
| Quercitrin | <0.55 | <0.55 | |
| Rutin | <0.29 | <0.29 | |
| Benzoic acids | Ellagic acid | 0.49 ± 0.44 a | 0.35 ± 0.12 b |
| Gallic acid | <0.15 | <0.04 | |
| Catechins | Catechin | 2.13 ± 1.62 a | 5.12 ± 0.24 b |
| Epicatechin | 13.82 ± 3.50 a | 14.74 ± 1.24 a | |
| Vitamin C | Ascorbic acid | 5.27 ± 0.15 a | 5.54 ± 0.16 b |
| Dehydroascorbic acid | 10.92 ± 4.89 a | 19.04 ± 3.27 b | |
| Organic acids | Citric acid | <1.88 | <1.88 |
| Malic acid | 50.50 ± 6.65 a | 64.01 ± 23.80 a | |
| Oxalic acid | 47.89 ± 4.70 a | 43.70 ± 5.36 a | |
| Quinic acid | <2.61 | <2.61 | |
| Succinic acid | <0.71 | <0.71 | |
| Tartaric acid | 49.43 ± 5.85 a | 30.73 ± 7.01 b | |
| Anthocyanins (relative to TAC) | Cyanidin-3-O-Gal | 32.01± 1.98 a | 20.14 ± 1.29 b |
| Cyanidin-3-O-Glu | 1.91± 0.15 a | 0.52 ± 0.09 b | |
| Cyanidin-3-O-A | 17.73 ± 0.07 a | 12.45 ± 0.36 b | |
| Peonidin-3-O-Gal | 40.1 ± 1.13 a | 26.19 ± 0.89 b | |
| Peonidin-3-O-Glu | 4.86 ± 0.29 a | 2.11 ± 0.1 b | |
| Peonidin-3-O-A | 15.44 ± 0.78 a | 9.31 ± 0.76 b | |
| Proanthocyanidins (relative to TPAC) | A-type dimer | 283.2 ± 7.18 a | 250.4 ± 0.15 a |
| A-type trimer | 352 ± 3.5 a | 180.5 ± 0.05 b | |
| B-type dimer | 487.9 ± 3.39 a | 269.6 ± 16.6 b | |
| B-type trimer | 32.9 ± 0.28 a | 17.4 ± 0.1 b | |
| Sugars | Fructose | 0.31 ± 0.03 a | 0.19 ± 0.03 b |
| Glucose | 3.24 ± 0.85 a | 7.00 ± 0.10 b | |
| Sucrose | 1.95 ± 0.54 a | 0.77 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobrero, T.; Asteggiano, A.; Donno, D.; Rosso, L.; Occhipinti, A.; Mellano, M.G.; Fioccardi, A.; Beccaro, G.L.; Gamba, G. Impact on the Health-Promoting Potential of Cranberries for Food Applications Through Soilless Cultivation Practices in Piemonte Region (Italy): A Sustainable Opportunity for Nutraceutical Production. Horticulturae 2025, 11, 1418. https://doi.org/10.3390/horticulturae11121418
Sobrero T, Asteggiano A, Donno D, Rosso L, Occhipinti A, Mellano MG, Fioccardi A, Beccaro GL, Gamba G. Impact on the Health-Promoting Potential of Cranberries for Food Applications Through Soilless Cultivation Practices in Piemonte Region (Italy): A Sustainable Opportunity for Nutraceutical Production. Horticulturae. 2025; 11(12):1418. https://doi.org/10.3390/horticulturae11121418
Chicago/Turabian StyleSobrero, Teresa, Alberto Asteggiano, Dario Donno, Lorenzo Rosso, Andrea Occhipinti, Maria Gabriella Mellano, Annachiara Fioccardi, Gabriele Loris Beccaro, and Giovanni Gamba. 2025. "Impact on the Health-Promoting Potential of Cranberries for Food Applications Through Soilless Cultivation Practices in Piemonte Region (Italy): A Sustainable Opportunity for Nutraceutical Production" Horticulturae 11, no. 12: 1418. https://doi.org/10.3390/horticulturae11121418
APA StyleSobrero, T., Asteggiano, A., Donno, D., Rosso, L., Occhipinti, A., Mellano, M. G., Fioccardi, A., Beccaro, G. L., & Gamba, G. (2025). Impact on the Health-Promoting Potential of Cranberries for Food Applications Through Soilless Cultivation Practices in Piemonte Region (Italy): A Sustainable Opportunity for Nutraceutical Production. Horticulturae, 11(12), 1418. https://doi.org/10.3390/horticulturae11121418