1. Introduction
Rubber, a natural product derived from the rubber tree (
Hevea brasiliensis), is an indispensable strategic industrial material in China, playing a crucial role in the national economy and defense [
1]. In recent years, however, the rubber industry in Hainan Province, the country’s main rubber-producing region, has faced declining prices and reduced profitability [
2]. The traditional monoculture system has become increasingly unsustainable for local farmers [
3], prompting efforts to diversify land use through intercropping and under-tree cultivation [
4,
5]. Sixteen such models have been successfully established in China, including rubber tree–tea (
Camellia sinensis L.), rubber tree–cassava (
Manihot esculenta Crantz), rubber tree–coffee (
Coffea arabica L.), and rubber tree–edible mushroom systems [
6]. Among these, the rubber tree–edible mushroom model offers particularly high economic returns and can also improve soil fertility, thereby benefiting the rubber trees themselves [
7]. Moreover, cultivating mushrooms under rubber plantations in Hainan can alleviate summer vegetable shortages [
8] and stabilize mushroom supply during winter [
9].
Pleurotus giganteus (Berk.) Karunarathna & K.D. Hyde, formerly known as
Panus giganteus or
Lentinus giganteus, is an edible and medicinal mushroom traditionally consumed in Thailand, Sri Lanka, Malaysia, and China [
10]. In China, it is called “zhudugu” and valued for its crisp, tender texture and distinctive flavor [
11]. Nutritionally, it is rich in polysaccharides, proteins, amino acids, and trace elements [
12], while pharmacological studies have reported antitumor, antioxidant, antihypertensive, hepatoprotective, diuretic, and anti-inflammatory properties [
13,
14,
15,
16]. Notably,
P. giganteus is heat-tolerant and capable of completing its life cycle at high temperatures [
17]. Our group’s large-scale cultivation over five consecutive years in Hainan has revealed its strong adaptability and capacity to utilize diverse substrates, making it highly suitable for cultivation under local rubber plantations [
9]. This model requires low investment, yields rapid returns, and provides an important new source of income for rubber farmers. However, the main cultivation substrate used in Hainan remains rubber sawdust. Continued reliance on this substrate will increase competition for forestry resources and threaten the long-term sustainability of both the rubber and mushroom industries [
18]. Hence, identifying alternative raw materials is urgently needed.
As a major agricultural nation, China generates abundant straw resources [
19]. Annual production exceeds one billion tons [
20], much of which is underutilized and often burned or landfilled, leading to resource wastage and environmental pollution [
21,
22]. Because edible mushrooms obtain nutrients by degrading lignocellulose in substrates [
23], agricultural straws rich in cellulose and lignin represent promising renewable resources for mushroom cultivation [
24]. In 1986, the “replacing wood with grass” technology was introduced, advocating the use of herbaceous crop residues for mushroom cultivation, fungal feed, and organic fertilizers [
18]. This approach has since been successfully applied to
Auricularia heimuer F. Wu, B.K. Cui & Y.C. Dai, [
25],
Pleurotus pulmonarius (Fr.) Quél. [
26],
Ganoderma lucidum (Curtis) P. Karst [
27], and
Lepista sordida (Schumach.) Singer [
28], securing a stable raw-material supply while contributing to forest conservation [
24].
Optimizing substrate formulations for mushroom cultivation typically involves adjusting parameters such as the carbon-to-nitrogen (C:N) ratio, trace elements, porosity, and pretreatment [
29,
30,
31]. Traditional single-factor methods, however, are time-consuming, costly, and inadequate for capturing interactions among components [
32]. Mixture-design experiments, developed for multi-component systems, overcome these limitations and have been widely applied in the food, pharmaceutical, and chemical industries [
33,
34,
35]. Among these, the simplex-lattice design provides superior experimental coverage, statistical efficiency, and regression modeling [
36,
37,
38]. Recently, this approach has been used to optimize mushroom substrates, improving yield and nutritional quality in
Hericium erinaceus (Bull.) Pers. [
18],
Grifola frondosa (Dicks.) Gray [
39], and
Pleurotus pulmonarius (Fr.) Quél. [
40].
In this study, we aimed to develop sustainable straw-based substrates for P. giganteus cultivation under rubber plantations in Hainan province using the simplex-lattice mixture design. Agricultural straws were first screened for suitability based on mycelial growth under laboratory conditions. Selected straws were then combined with rubber sawdust in mixture designs, and their effects on mycelial growth, enzyme activity, and cultivation traits under plantation conditions were evaluated. Correlation analyses were performed to determine the contributions of individual components and their interactions. Finally, the optimal formulation was predicted and validated. This work provides a new technical pathway for the sustainable cultivation of P. giganteus in Hainan, supports the development of under-forest economies in the tropics, and contributes to rural revitalization efforts in China.
2. Materials and Methods
2.1. Tested Strain and Straw Collection
The tested strain, PG46, is a high-temperature-tolerant strain of P. giganteus Capable of forming fruiting bodies at 28–35 °C, making it suitable for summer cultivation in hot regions. Its biological transformation rate is 92.8%, and it exhibits excellent commercial traits, including uniform fruiting and suitability for facility-based cultivation. This strain was selected by our team after five consecutive years of large-scale cultivation in Hainan, China, and is currently preserved at the Environment and Plant Protection Institute, Chinese Academy of Tropical Agricultural Sciences.
Eight agricultural straws commonly available in southern China (e.g., Jiangsu, Anhui, Sichuan, Guangxi and Hainan) were collected: wheat, rice, soybean, corn, sugarcane, chili, banana, and peanut straws. To ensure consistent quality, the straws were sourced from a provincial agricultural research institute based on local availability. All materials were freshly harvested, sun-dried to a constant weight (approximately 15% moisture content), and stored under dry conditions to prevent microbial contamination. Before use, the straws were crushed and sieved to a particle size of 3–5 mm to minimize variation in bulk density and bag height among substrate formulations.
2.2. Straw Screening for Substrate Formulation (Wood-to-Grass Replacement)
The collected straws were tested as replacements for rubber sawdust in a control formulation (CF: 45% rubber sawdust, 40% cottonseed hulls, 10% wheat bran, and 5% lime; moisture content adjusted to 60%). Five substitution ratios (0%, 25%, 50%, 75%, and 100%) of straw for rubber sawdust were evaluated, with all other components held constant, resulting in 33 experimental formulations (three replicates each).
After mixing, 35 ± 0.2 g of substrate was placed into 9 cm Petri dishes, sterilized at 121 °C for 60 min, and cooled to room temperature. A 6 mm mycelial plug from a 7-day-old culture grown on MYG medium (10 g maltose, 5 g glucose, 5 g yeast extract, 20 g agar per L, Dingguo Biotechnology, Beijing, China) was inoculated at the center of each dish. Plates were incubated in the dark at 28 °C. Radial mycelial growth was recorded daily along cross lines on the plate underside until full colonization [
41]. Based on growth rate and colony morphology, straws suitable for supporting PG46 growth were screened for subsequent experiments.
2.3. Mixture Design of Substrate Formulations
A simplex-lattice mixture design was developed using Design-Expert v8.0.6.1 [
38,
41], with the control formulation (CF) as the baseline. The screened straws were incorporated as the primary component (
X), replacing rubber sawdust, while the auxiliary components remained constant. Substitution levels were denoted as X
1…X
n, subject to X
n ≥ 0 and X
1 + X
2 + … + X
n = 100%. Replacement percentages were calculated relative to the 45% rubber sawdust content in CF.
2.4. Cultivation Experiment and Trait Measurement
Circular mycelial plugs (6 mm) of PG46 were inoculated into each straw formulation and the CF was incubated at 28 °C in the dark until full colonization, and assessed daily for growth rate and colony morphology.
For spawn preparation, colonized agar plugs were transferred to MYG liquid medium (10 g maltose, 5 g glucose, 5 g yeast extract per L) and incubated at 28 °C with shaking at 180 rpm (LABTECH, LSI-5002M, Namyangju-si, Republic of Korea) for approximately 7 days until uniform mycelial pellets formed. Spawn was inoculated into cultivation bags (1.2 kg substrate per bag; 24 bags per formulation) and incubated in the dark at 28 °C. After full colonization, bags were post-ripened for one week at low temperature to promote mycelial consolidation, then transferred to a simple greenhouse under a rubber plantation for soil covering cultivation. Before casing, the surface mycelium was scraped, and a 5 cm casing layer (moisture content 18%–20%, pH 6.0) was applied. During casing colonization, humidity was maintained at 80%–85% with diffuse light (300–500 lux) to induce primordia, then increased to 85%–90% until harvest.
Measured parameters included mycelial growth rate, colony morphology, lignocellulolytic enzyme activity (laccase, cellulase, neutral xylanase), growth cycle (from inoculation to maturation), yield of the first flush, biological efficiency () × 100%), contamination rate (() × 100%), and cap-to-stipe ratio. Each treatment had three biological replicates. Contamination rate was calculated from 24 bags per treatment, while growth cycle, yield, biological efficiency, and cap-to-stipe ratio were based on the average of 20 uncontaminated bags.
For enzyme assays, fully colonized substrates were sampled using sterile forceps, rapidly frozen in liquid nitrogen, and ground into powder. Approximately 0.1 g of ground material was homogenized in 0.1 mol L−1 phosphate buffer (pH 7.4) and centrifuged at 12,000× g for 4 min at 4 °C. Supernatants were analyzed using commercial enzyme assay kits (COMIN, Suzhou, China). Laccase (LAC) activity was defined as the amount of enzyme required to oxidize 1 µg of ABTS per minute per gram of sample; cellulase (CL) activity as the enzyme amount producing 1 µg of glucose min−1 g−1; and neutral xylanase (NEX) activity as that generating 1 µg of reducing sugar min−1 g−1.
For nutritional analysis, fruiting bodies were dried at 45 °C, ground, and sieved (100-mesh). Total polysaccharides were quantified using the phenol–sulfuric acid method [
42]; crude protein by the Kjeldahl method [
43], crude fat by Soxhlet extraction [
44], crude fiber by acid–base hydrolysis [
45]; and amino acids using an automatic amino acid analyzer [
46]. Each treatment included three biological replicates.
2.5. Statistical Analyses and Model Verification
Design-Expert v8.0.6.1 [
47] was used for regression, ANOVA, and contour analyses to examine relationships between substrate components and agronomic traits. Independent variables (substrate components) and dependent variables (traits) were defined, and data from 10 formulation points (three replicates each) were analyzed. Stepwise regression (
p < 0.05) was applied to identify significant terms. Models were evaluated by coefficient of determination (R
2 > 0.8) and lack-of-fit tests (
p > 0.05).
Predicted response values were calculated from the regression equations and compared with observed data to evaluate model accuracy. Contour plots were generated using the “Model Graphs” module to visualize response surfaces. The contour color gradient indicated response magnitude, and contour density reflected the rate of change. The direction and convergence of contours were used to identify maxima or minima in response values.
Regression coefficients (K-values) represented the marginal effects of each component (X1, X2, X3) and their interactions on mycelial growth rate (Y). R2 indicated the proportion of variability explained by the model, while p-values assessed overall model significance (p ≤ 0.05) and lack-of-fit (p > 0.05). The optimization module applied a desirability function (D) to integrate multiple response variables, balancing objectives such as yield and enzyme activity to predict the optimal substrate formulation.
Verification of models was performed by comparing the predicted optimal formulation with the CF under both laboratory and cultivation conditions. Comparative analysis included mycelial growth rate, enzyme activity, growth cycle, yield, and nutritional composition.
2.6. Data Analysis
Data for mycelial growth rate, enzyme activity, growth cycle, yield, and nutritional component were analyzed by one-way ANOVA (α = 0.05) using SPSS Statistics 27.0 (IBM, Armonk, NY, USA). Graphs were generated using Origin 2018. The Scheffé quartic polynomial regression model in Design-Expert v8.0.6.1 was used to process mixture data. Partial correlation analysis was employed to evaluate the independent effects of each component on mycelial growth, enzyme activity, and fruiting traits. In all regression analyses, significant terms (p < 0.05) were identified by stepwise regression, and model adequacy was confirmed by a non-significant lack-of-fit test (p > 0.05). Contour plots were generated by fixing non-target components at their mean values to visualize pairwise interactions.
3. Results
3.1. Straw Screening Tests
To minimize formulation bias, five substitution ratios of Agricultural straw for rubber sawdust in the control formulation (CF) were tested (0%, 25%, 50%, 75%, and 100%). PG46 mycelia were able to grow on all formulations; however, the growth rate and colony morphology differed markedly among substrates and substitution levels.
At the 25% substitution level (
Figure 1A and
Figure S1A), mycelial growth in the mixed formulation containing banana straw (banana straw + rubber sawdust) was comparable to that in the control formulation (0.35 cm·d
−1), and both were significantly faster than in formulations containing other straws. The colonies in these treatments were dense and uniform. Formulations with corn or soybean straw supported moderate growth, whereas the remaining straws performed poorly. At 50% substitution (
Figure 1B and
Figure S1B), the control formulation continued to exhibit the highest growth rate (0.35 cm·d
−1), significantly exceeding all straw treatments. Following the control were formulations containing chili straw, banana straw, and sugarcane straw, while other formulations supported only limited mycelial extension. At 75% substitution (
Figure 1C and
Figure S1C), the formulation with banana straw again matched the control in growth rate (0.35 cm·d
−1) and significantly outperformed the other straw formulations. Formulations containing rice straw and sugarcane straw followed, while the rest exhibited slower growth. At 100% substitution (
Figure 1D and
Figure S1D), formulations composed entirely of banana straw (0.39 cm·d
−1) or chili straw (0.37 cm·d
−1) supported the fastest mycelial growth, showing no significant difference between them. Both produced dense, white colonies. These were followed by the control and sugarcane straw formulation, whereas the others performed less effectively.
Overall, banana straw performed comparably to the control formulation at 25% and 75% substitution levels, while the remaining straws supported slower growth across 25–75% substitution. Interestingly, when rubber sawdust was completely replaced (100%) by agricultural straw, formulations containing banana or chili straw performed significantly better than the control. Therefore, banana straw and chili straw were identified as suitable primary substrates for
P. giganteus cultivation. The variation in mycelial performance across substitution ratios (
Supplementary Figure S2) further underscores the importance of optimizing straw composition and proportion in substrate formulation.
3.2. Mixture Design of Substrate Formulations and Agronomic Trait Evaluation
Based on the straw screening results, rubber sawdust (X
1), banana straw (X
2), and chili straw (X
3) were selected as the three major components for further optimization. Using Design-Expert v8.0.6.1 software, their substitution ratios were adjusted within defined boundaries, generating ten experimental formulations (
Table 1).
During the mycelial growth stage, PG46 exhibited the fastest radial growth in formulations 6 and 9 (0.40 cm·d
−1), both significantly higher than in the other formulations. These were followed by formulations 2, 4, 7, 8, and 10, while the slowest growth occurred in formulations 1, 3, and 5 (
Figure 2A). Enzyme activity assays revealed clear differences among formulations. Laccase activity was highest in formulation 6 (493.31 µg·min
−1·g
−1), followed by formulations 10 and 3, with all treatments significantly exceeding the CF (
Figure 2B). Neutral xylanase activity was also highest in formulation 6 (6053.62 µg·min
−1·g
−1), followed by formulations 9 and 3, while all but formulations 2, 7, and 8 were significantly higher than the CF (
Figure 2C). In contrast, cellulase activity was greatest in the CF (826.14 µg·min
−1·g
−1) but did not differ significantly from formulation 4; formulations 6 and 10 showed intermediate activities, whereas the remaining treatments had lower values (
Figure 2D).
PG46 successfully colonized and produced fruiting bodies in all formulations (
Table 2). The shortest full-bag colonization times were recorded for formulations 7 (31 days) and 10 (28 days), both significantly faster than the other. Formulations 9, 8, and 5 followed. The relationship between radial growth and colonization time was non-linear, likely reflecting differences in substrate structure: formulations with higher proportions of banana straw (e.g., 2, 4, 6) produced slightly taller bags, resulting in longer colonization, whereas those richer in chili straw (e.g., 5, 8) yielded shorter bags and faster colonization.
Primordia initiation occurred within one week in formulations 7, 9, 10, and 2, while other formulations required up to two weeks. With the exception of formulations 6, 7, and 9, maturation time did not differ significantly among treatments. Overall, the cultivation cycle (from inoculation to harvest) mirrored the colonization trend: formulations 7 and 10 had the shortest cycles (42 days), followed by 9, 8, 5, and 2 (≤55 days), while others required longer cycles.
The first-flush yield was significantly highest in formulation 9, which achieved a biological efficiency (BE) of 59.71%. Formulations 1, 7, and 2 followed (51.22–54.56%), all significantly higher than the remaining treatments (38.10–47.68%). Natural contamination rates were lowest in formulations 9 and 10 (0%), moderate in 3 and 7 (4.17%), and highest in 8 (12.5%).
Morphological observations showed substantial variation among formulations (
Figure 3). Fruiting bodies from formulations 1, 3, 4, and 9 developed larger caps; those from 7, 2, 6, and 10 had medium-sized caps; and those from 5 and 8 had the smallest caps. Formulation 4 produced thin caps with elongated stipes, resulting in a low cap-to-stipe ratio, whereas formulation 9 produced thick caps with moderately long stipes and a balanced ratio. Formulations 1 and 3 produced the thickest caps with short stipes and the largest cap-to-stipe ratios, which are desirable traits for commercial quality.
Nutritional analyses of the fruiting bodies from high-yielding formulations (1, 2, 7, 9, 10) revealed significant differences (
Supplementary Table S1). Total polysaccharide content was highest in formulations 9 and 10 (≥300 mg·g
−1), significantly surpassing other treatments. Crude protein and total amino acid contents were highest in formulation 10 (17.04% and 27.21 mg·g
−1, respectively). Crude fiber content was high across all formulations except 9, though differences were not significant, and crude fat content was lowest in formulations 6 and 9 without a significant difference between them. Taken together, formulation 9 consistently outperformed the other in mycelial growth, enzymatic activity, yield, contamination resistance, and nutritional quality, indicating that it is the most promising straw-based substrate and a sustainable alternative to the conventional rubber-sawdust formulation.
3.3. Correlation Analysis
3.3.1. Correlations Between Mycelial Growth Rate and Substrate Components
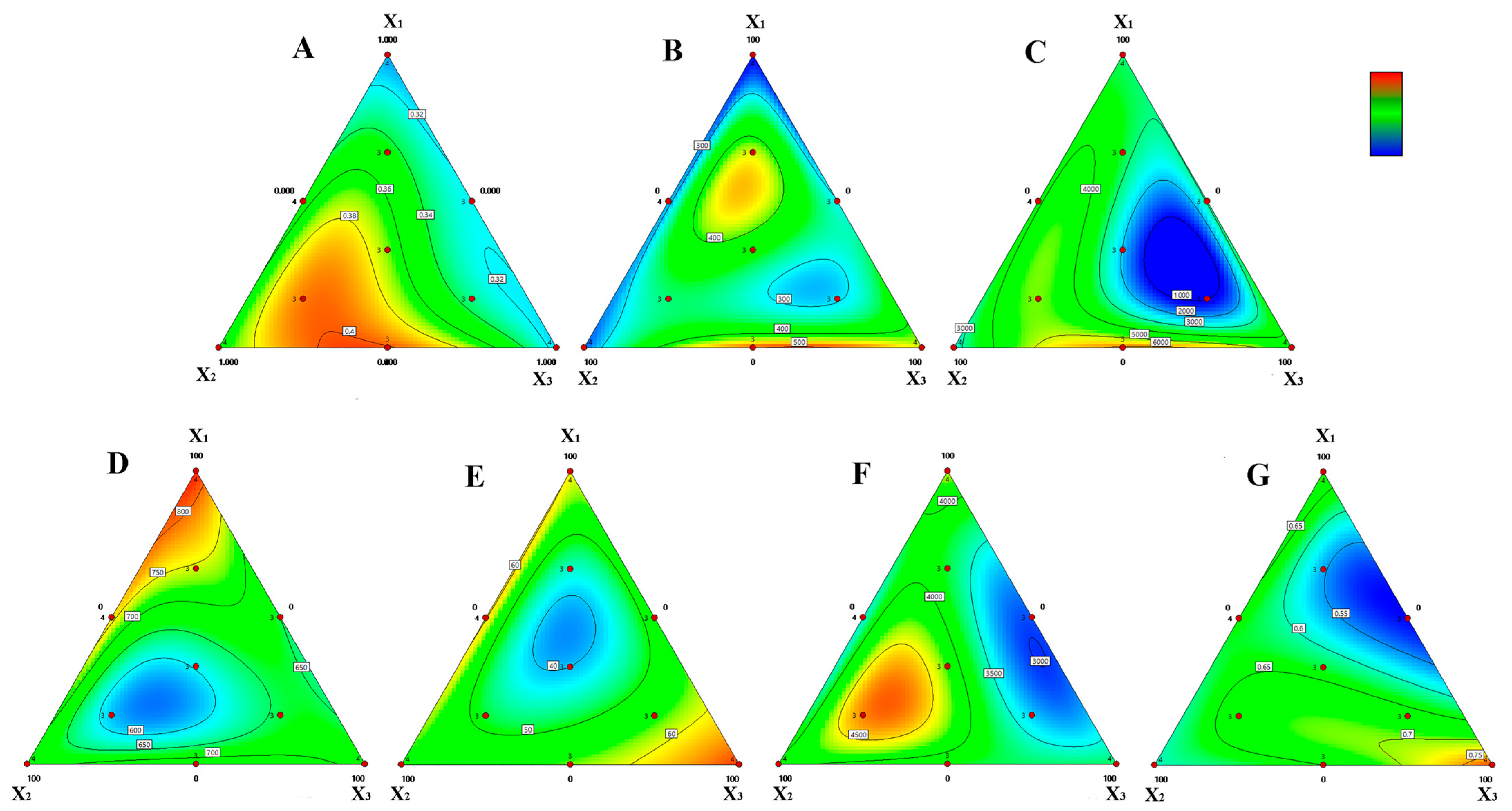
Statistical analysis of the mycelial growth rate in each formulation produced a regression equation describing the relationship between growth rate (Y) and the substrate components, rubber sawdust (X1), chili straw (X2), and banana straw (X3), as follows: Y = 0.307982 X1 + 0.360482 X2 + 0.312982 X3 + 0.0669283 X1X2 + 0.0432138 X1X3 + 0.258214 X2X3 + 0.961965X12X2X3 + 1.59197 X1X22X3 − 2.4696 X1X2X32, with a correlation coefficient (R2) of 0.9151.
Analysis of variance (ANOVA) for the special quartic polynomial regression model (
Table 3) showed that the
p-values for both the quartic polynomial and linear mixture model were less than 0.0001, confirming that both effectively capture the relationship between substrate components and mycelial growth rate. The correlation plot between predicted and observed values (
Supplementary Figure S3A) further supported the model’s robustness, as data points were closely clustered along the fitted line. From the regression coefficients (K values), the relative influence of the three ingredients on mycelial growth rate followed the order: banana straw (K
X2 = 0.360482) > chili straw (K
X3 = 0.312982) > rubber sawdust (K
X1 = 0.307982). Variance analysis (
Table 3) indicated that the interaction terms X
2X
3 and X
1X
2X
32 were highly significant (
p ≤ 0.01), suggesting that these interactions strongly influenced PG46 mycelial growth. Combined interpretation of the regression equation and contour plot (
Figure 4A) showed that the X
2X
3 interaction (K = 0.258214, red) promoted mycelial growth, whereas the X
1X
2X
32 interaction (K = −2.4696, blue) had a suppressive effect. These results highlight that optimal combinations of banana and chili straw can synergistically enhance the mycelial growth rate of
P. giganteus.
3.3.2. Correlation Between Laccase Activity and Substrate Components
Regression analysis of laccase activity across the ten formulations yields the following equation describing the relationship between enzyme activity (Y) and substrate composition: Y = 238.025 X
1 + 261.548 X
2 + 424.458 X
3 + 124.746 X
1X
2 − 111.563 X
1X
3 + 609.619 X
2X
3 + 14,988.7 X
12X
2X
3 − 2599.81 X
1X
22X
3 − 13,517.5 X
1X
2X
32, with a correlation coefficient (R
2) of 0.9749. According to the ANOVA of the special quartic polynomial model for laccase activity (
Table 4), the
p-values for both the linear and quartic models were less than 0.0001, indicating that the models effectively described the relationship between substrate components and laccase activity. The correlation between observed and predicted values (
Supplementary Figure S3B) confirmed the model’s accuracy, with data points clustering tightly around the regression line.
Based on the regression coefficients (K values), the relative influence of substrate components on laccase activity was chili straw (K
X3 = 424.458) > banana straw (K
X2 = 261.548) > rubber sawdust (K
X1 = 238.025). Variance analysis (
Table 4) identified several highly significant interaction terms (
p ≤ 0.01), including X
1X
2, X
2X
3, X
12X
2X
3, X
1X
22X
3 and X
1X
2X
32, suggesting that complex component interactions substantially influenced enzyme production. From the regression model and contour plots (
Figure 4B), the interactions X
1X
2 (K = 124.746, red), X
2X
3 (K = 609.619) and X
12X
2X
3 (K = 14,988.7, red) were associated with increased laccase activity, whereas X
1X
22X
3 (K = −2599.81, blue) and X
1X
2X
32 (K = −13,517.5, blue) were associated with decreased activity. Overall, the combination of chili and banana straw had the most positive influence on laccase production, reflecting the enhanced ligninolytic potential of PG46 on these straw-based substrates.
3.3.3. Correlation Between Neutral Xylanase Activity and Substrate Components
Statistical analysis of neutral xylanase activity across the ten formulations produced the following regression equation describing the relationship between enzyme activity (Y) and substrate composition: Y = 3172.06 X
1 + 2701.71 X
2 + 4144.25 X
3 + 3362.97 X
1X
2 − 2080.76 X
1X
3 + 10,789.8 X
2X
3 + 29,438.5 X
12X
2X
3 + 88,355.8 X
1X
22X
3 − 338,750 X
1X
2X
32, with a correlation coefficient (R
2) of 0.9025. Analysis of variance (
Table 5) indicated that the
p-values of both the special quartic polynomial model and the linear mixture model were less than 0.05, confirming the reliability of the fitted equation. The correlation between observed and predicted values (
Supplementary Figure S3C) further supported the robustness of the model, as all data points clustered tightly along the regression line. From the regression coefficients (K values), the relative influence of substrate components on neutral xylanase activity followed the order: chili straw (K
X3 = 4144.25) > rubber sawdust (K
X1 = 3172.06) > banana straw (K
X2 = 2701.71). Variance analysis (
Table 5) showed that the interaction terms X
1X
2, X
2X
3, X
1X
22X
3 and X
1X
2X
32 were highly significant (
p ≤ 0.01), indicating that these interactions strongly influenced the neutral xylanase activity of PG46.
Combined interaction of the regression model and contour plot (
Figure 4C) revealed that the interaction X
1X
2 (K = 3362.97, red), X
2X
3 (K = 10,789.8, red), and X
1X
22X
3 (K = 88,355.8, red) positively affected the neutral xylanase activity, whereas the interaction X
1X
2X
32 (K = −338,750, blue) negatively affected it. These results indicate that balanced proportions of chili straw and banana straw in the formulation can synergistically enhance xylanase activity in
P. giganteus, reflecting improved lignocellulose degradation efficiency on straw-based substrates.
3.3.4. Correlation Between Cellulase Activity and Substrate Components
Statistical analysis of cellulase activity across the ten formulations yields the following regression equation describing the relationship between enzyme activity (Y) and substrate composition: Y = 824.332 X
1 + 672.565 X
2 + 685.302 X
3 + 69.1842 X
1X
2 − 420.658 X
1X
3 + 215.064 X
2X
3 + 3082.76 X
12X
2X
3 − 11,694.1 X
1X
22X
3 − 150.578 X
1X
2X
32, with a correlation coefficient (R
2) of 0.8655. Analysis of variance for the regression model (
Table 6) showed that the
p-values of both the special quartic polynomial model and the linear mixture model were less than 0.0001, confirming the reliability of the fitted equation. The correlation between observed and predicted values (
Supplementary Figure S3D) further validated the model’s robustness, as data points clustered closely along the regression line.
Based on the regression coefficients (K values), the relative influence of each substrate composition on cellulase activity followed the order: rubber sawdust (K
X1 = 824.332) > chili straw (K
X3 = 685.302) > banana straw (K
X2 = 672.565). Variance analysis (
Table 6) revealed that the interaction terms X
1X
3 and X
1X
22X
3 were highly significant (
p ≤ 0.01), indicating that these interactions had a strong effect on cellulase activity. According to the regression model and contour plot (
Figure 4D), the interactions X
1X
3 (K = −420.658, blue) and X
1X
22X
3 (K = −11,694.1, blue) were associated with decreased cellulase activity. These results indicate that excessive proportions of chili straw in combination with rubber sawdust may reduce cellulase activity in
P. giganteus, possibly reflecting substrate-specific limitations in cellulose degradation efficiency.
3.3.5. Correlation Between Growth Cycle and Substrate Components
Regression analysis of the growth cycle produced the following equation relating growth cycle (Y) to substrate component: Y = 59.5875 X
1 + 54.8375 X
2 + 67.5875 X
3 + 13.8498 X
1X
2 − 40.0836 X
1X
3 − 15.917 X
2X
3 − 988.624 X
12X
2X
3 − 445.624 X
1X
22X
3 + 143.277 X
1X
2X
32, with a correlation coefficient (R
2) of 0.9052. Analysis of variance (
Table 7) showed that the
p-values of both the special quartic polynomial and linear mixture models were less than 0.0001, confirming the reliability of the fitted model. The correlation between observed and predicted values (
Supplementary Figure S3E) also demonstrated high predictive accuracy, with points clustering tightly around the regression line. From the regression coefficients (K values), the relative influence of each substrate component on the growth cycle followed the order: chili straw (K
X3 = 67.5875) > rubber sawdust (K
X1 = 59.5875) > banana straw (K
X2 = 54.8375). Variance analysis (
Table 7) identified the interaction terms X
1X
3 and X
12X
2X
3 as highly significant (
p ≤ 0.01), indicating that these interactions significantly affected the growth cycle of PG46. According to the regression model and contour plot (
Figure 4E), the interaction X
1X
3 (K = −40.0836, blue) and X
12X
2X
3 (K = −988.624, blue) were associated with a reduction in growth cycle length. These results suggest that appropriate proportions of chili straw in combination with rubber sawdust can shorten the cultivation period of
P. giganteus, likely by improving substrate aeration and nutrient balance.
3.3.6. Correlation Between Yield and Substrate Components
Statistical analysis of the first-flush yield in each formulation produced the following regression equation describing the relationship between yield (Y) and substrate composition: Y = 4370.86 X
1 + 4101.93 X
2 + 3757.38 X
3 − 2690.57 X
1X
2 − 4001.02 X
1X
3 − 596.319 X
2X
3 + 7388.91 X
12X
2X
3 + 95,197.7 X
1X
22X
3 − 26,650.4 X
1X
2X
32, with a correlation coefficient (R
2) of 0.8952. Analysis of variance for the special quartic polynomial model (
Table 8) showed that the
p-values of both the quartic and linear mixture models were less than 0.0001, confirming the reliability of the fitted equation. The correlation between observed and predicted values (
Supplementary Figure S3F) further supported the model’s robustness, as data points clustered closely along the regression line.
Based on the regression coefficients (K values), the relative influence of the compositions on yield followed the order: rubber sawdust (K
X1 = 4370.86) > banana straw (K
X2 = 4101.93) > chili straw (K
X3 = 3757.38). Variance analysis (
Table 8) identified the interaction terms X
1X
2, X
1X
3 and X
1X
22X
3 as highly significant (
p ≤ 0.01), indicating that these combinations substantially affected the yield. According to the regression model and contour plot (
Figure 4F), the interaction X
1X
22X
3 (K = 95,197.7, red) contributed positively to yield, whereas X
1X
2 (K= −2690.57, blue) and X
1X
3 (K = −4001.02, blue) had negative effects. These findings suggest that an appropriate balance between rubber sawdust and banana straw can enhance yield, while excessive proportions of either component in combination with chili straw may reduce productivity in
P. giganteus.
3.3.7. Correlation Between Cap-to-Stipe Ratio and Substrate Components
Regression analysis of the cap-to-stipe ratio produced the following equation relating morphological traits (Y) to substrate composition: Y = 0.690263 X
1 + 0.600263 X
2 + 0.777763 X
3 − 0.0289493 X
1X
2 − 0.879916 X
1X
3 − 0.153249 X
2X
3 − 4.82104 X
12X
2X
3 + 5.73896 X
1X
22X
3 + 3.81265 X
1X
2X
32, with a correlation coefficient (R
2) of 0.9892. Analysis of variance for the regression model (
Table 9) revealed that the
p-values of both the quartic polynomial and linear models were less than 0.0001, confirming that the model effectively described the relationship between substrate composition and cap-to-stipe ratio. The correlation between observed and predicted values (
Supplementary Figure S3G) further supported model robustness, with all data points closely following the regression line. From the regression coefficients (K values), the relative influence of the main ingredients on the cap-to-stipe ratio followed the order: chili straw (K
X3 = 0.777763) > rubber sawdust (K
X1 = 0.690263) > banana straw (K
X2 = 0.600263). Except for the X
1X
2 term, all other interaction terms were highly significant (
p ≤ 0.01), indicating strong interactive effects among components.
The regression model and contour plot (
Figure 4G) showed that the interactions X
1X
3 (K = −0.879916, blue), X
2X
3 (K = −0.153249, blue), and X
12X
2X
3 (K = −4.82104, blue) were associated with a lower cap-to-stipe ratio, whereas X
1X
22X
3 (K = 5.73896, red) and X
1X
2X
32 (K = 3.81265, red) increased the ratio. These results indicate that higher proportions of chili straw tend to promote desirable morphological traits, such as thicker caps and balanced stipes, enhancing the commercial quality of
P. giganteus fruiting bodies.
3.4. Calculating and Testing the Optimal Substrate Formulation
Based on the regression analyses described above, the expected response values for each evaluation index were integrated to determine the optimal substrate formulation for P. giganteus PG46 under the “replacing wood with grass” approach. The predicted optimal formulation consisted of 9.96% rubber sawdust, 24.33% banana straw, 10.70% chili straw, 40% cottonseed hulls, 10% wheat bran, and 5% lime.
To validate the predictive model, both mycelia culture and mushroom cultivation experiments were conducted using the optimized formulation and the control formulation (CF). The results (
Table 10) showed that the optimized formulation produced significantly higher growth rate, laccase activity, and neutral xylanase activity compared with the CF, while cellulase activity was slightly lower but not significantly different. In the cultivation experiment, marked improvements in agronomic traits were observed for the optimized formulation relative to the CF. The growth cycle was shortened by 12 days, and both yield and biological efficiency were significantly increased, although the cap-to-stipe ratio was slightly lower. Importantly, the contamination rate of the optimized formulation remained at 0% across three independent trials, demonstrating its stability and practical reliability.
Nutritional analysis of the fruiting bodies further supported these findings. The optimized formulation yielded mushrooms with significantly higher crude polysaccharide, crude protein and total amino acids contents compared to the CF. Notably, the crude protein content was approximately three times higher than that of the control. Although the levels of crude fiber and crude fat were lower in the optimized formulation, the differences were not statistically significant.
Collectively, these results confirm that the optimized substrate formulation can effectively replace the traditional rubber sawdust-based medium, offering a green and sustainable alternative for the tropical cultivation of P. giganteus.
4. Discussion
This study aimed to optimize sustainable substrate formulations for P. giganteus PG46 under the “replacing wood with grass” concept. We first screened agricultural residues as potential alternatives to rubber sawdust before screening the conventional substrate used in Hainan Province. A simplex-lattice mixture design was then applied to evaluate the interactions among the most promising residues (banana and chili straw) and rubber sawdust, followed by cultivation trials to assess agronomic traits, enzyme activities, and nutritional composition. Finally, regression-based correlation analyses were used to elucidate how individual components and their interactions influenced PG46 performance. The findings are discussed below in relation to previous studies, possible mechanisms, and their implications for sustainable mushroom cultivation.
4.1. Straw Screening and Feasibility of “Replacing Wood with Grass”
Hainan Province is characterized by high temperature and humidity [
48], conditions that limit large-scale cultivation of many commercial mushrooms in China, including
Agaricus bisporus (J.E. Lange) Imbach [
49],
Flammulina velutipes (Curtis) Singer [
50],
H.
erinaceus, and
P. eryngii [
51]. As a heat-tolerant species,
P. giganteus is particularly suited for tropical mushroom farming, especially under rubber plantations. However, reliance on rubber sawdust as a substrate raises long-term sustainability concerns. The “replacing wood with grass” strategy, which aligns with both ecological and economic objectives, reduces dependence on sawdust, lowers production costs, and increases farmer income [
24]. Our straw screening confirmed the feasibility of this strategy, as PG46 mycelia grew well in all tested residues. Banana and chili straws supported the fastest and densest mycelial growth, highlighting their compatibility with local resource availability, Hainan being both a major banana producer and a leading chili-growing region. However, mycelia growth varied across substitution ratios, suggesting that an optimal balance rather than complete replacement is required to achieve stable performance.
4.2. Mixture Design and Cultivation Outcomes
Traditional substrate optimization in mushroom cultivation often relies on single-factor experiments or empirical experience [
52,
53]. Such methods fail to account for the non-linear interactions among components. Mixture design, which constrains component proportions within a simplex space, offers a more robust and predictive approach. This technique has been successfully applied in food, chemical, and mushroom production systems [
18,
40,
54,
55,
56,
57,
58]. Our simplex-lattice design generated ten substrate formulations combining rubber sawdust, banana straw, and chili straw. Across these formulations, mycelial growth rate and laccase activity generally exceeded those of the control (rubber sawdust-based). Interestingly, enzyme activity did not always correlate with growth rate, consistent with previous observations [
40] that fungal development stages are regulated by different metabolic cues. Most formulations shortened the cultivation cycle relative to the control, in line with Atila et al. [
59], who reported that straw-based substrates accelerate crop cycles due to lower lignin and higher ash contents, which facilitate faster nutrient release [
60].
Formulation 9 achieved the highest first-flush yield and among the lowest contamination rates, likely owing to favorable substrate porosity and water retention, although these properties warrant further investigation. The low contamination levels observed in formulations 9 and 10 indicate a strong potential for open-field cultivation under rubber plantations. Morphologically, most mixtures produced cap-to-stipe ratios above 0.6, with formulation 3 reaching 0.78, a desirable feature since a larger cap proportion increases marketable yield. Given that cultivation management was uniform across treatments, the observed differences likely stem from substrate composition, consistent with earlier findings that nutrient balance (C/N ratio and trace elements) affects mushroom morphogenesis [
61]. Nutritionally, straw-based substrates enhanced polysaccharide, crude protein, and amino acid contents compared with the control, confirming that substrate composition strongly influences fruiting-body quality [
62]. Overall, the mixture design approach effectively produced balanced “wood-to-grass” formulations that improved agronomic traits and nutritional quality while reducing sawdust dependency. However, these results were derived from a single strain (PG46); validation across additional genotypes is needed. Moreover, this study evaluated only the first flush and standard nutritional indices; cumulative yield and performance under open-field conditions warrant further study.
4.3. Correlation Analysis
Regression-based correlation analyses clarified the contributions of each component and their interactions to Vegetative and reproductive performance. Banana straw most strongly promoted mycelial growth, likely due to its higher content of soluble sugars, cellulose, and potassium [
63], whereas rubber sawdust, richer in lignin, provided slower nutrient release [
64]. Synergistic effects were evident between banana and chili straws; however, excessive chili combined with sawdust reduced growth, possibly through altered packing density or imbalanced C/N ratios.
Enzyme activity patterns revealed trait-specific preferences. Chili straw strongly enhanced laccase and xylanase activities, while rubber sawdust favored cellulase production. Positive interactions between banana–sawdust and banana–chili combinations promoted laccase synthesis, but the magnitude and direction of effects varied by proportions. At higher chili or banana ratios, inhibition of laccase and xylanase was observed, possibly due to C/N imbalance, phenolic interference, or metabolic competition [
65,
66,
67]. Conversely, sawdust induced cellulase production, whereas its interactions with chili straw reduced activity, potentially due to limited enzyme–substrate contact or pH fluctuations [
68]. Excessive banana straw might also hinder cellulase activity through the formation of pectin–lignin complexes [
69].
For agronomic traits, Chili straw most effectively shortened the growth cycle, followed by sawdust and banana straw. Interactions between sawdust and chili further accelerated colonization, likely through complementary structural and nutritional effects [
70]. Yield was highest with sawdust, followed by banana straw and chili straw, underscoring the importance of maintaining structural stability and gradual nutrient release. Balanced mixtures, such as formulation 9, mitigated yield reductions and improved overall performance. Cap development was enhanced by chili straw, though certain binary interactions (sawdust × chili; banana × chili) negatively affected cap expansion, again highlighting the role of proportion balance.
Together, these findings confirm that banana and chili straw enhance mycelial growth and hemicellulolytic capacity, while rubber sawdust provides the structural and nutritional stability required for cellulolytic activity and yield. Optimal outcomes emerged from balanced mixtures that maximized synergistic effects while minimizing antagonistic interactions. Nevertheless, environmental variation and site conditions may influence these relationships. Future studies should thus include multi-season and multi-location validations, as well as physicochemical (porosity, density, soluble sugars, phenolics) and molecular (transcriptomic, metabolomic) analyses to elucidate mechanisms governing substrate interactions.
4.4. Implications for Sustainable Mushroom Cultivation
The study demonstrates that the “replacing wood with grass” concept is a viable strategy for P. giganteus cultivation in tropical regions. By partially substituting rubber sawdust with banana and chili straws, growers can reduce dependence on forest-derived materials, lower production costs, and enhance both productivity and nutritional quality. This strategy not only promotes the valorization of local agro-residues but also supports resource recycling and circular agriculture.
In Hainan, where rubber plantations coexist with extensive banana and chili cultivation, this model represents a practical approach to integrated agroforestry systems and sustainable under-forest economies. Beyond Hainan, the principles exhibited here can be adapted to other tropical production systems facing similar constraints, thereby expanding the global applicability of straw-based substrates for sustainable mushroom cultivation.
5. Conclusions
Using the “replacing wood with grass” approach, we successfully optimized a straw-based substrate formulation for P. giganteus cultivation through a simplex-lattice mixture design. The final formulation, comprising 9.97% rubber sawdust, 24.33% banana straw, 10.70% chili straw, 40% cottonseed hulls, 10% wheat bran, and 5% lime, showed clear advantages in mycelial growth, enzyme activity, yield, and nutritional quality over the control.
Correlation analyses revealed that both the main components and their interactions significantly influenced agronomic traits, with their effects dependent on relative proportions. These results provide a scientifically validated formulation for localized P. giganteus cultivation in tropical regions and a framework for future optimization and strain improvement. By reducing reliance on wood-based substrate and promoting the valorization of agricultural residues, this study contributes to the sustainable expansion of tropical mushroom cultivation and strengthens the under-forest economy. Future research should integrate long-term field trials and mechanistic studies to refine straw-based substrate formulations and ensure stable commercial-scale application.