Seed Priming with Cold Plasma and Vacuum Increases the Amounts of Phenolic Compounds and Antioxidant Activity in Lavender Herb
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Pre-Sowing Plasma Treatment
2.3. Analysis of the Emergence Kinetics, Cultivation, and Morphometric Analysis of Seedlings
2.4. SEM Analysis of Seed Surface and Leaf Trichomes
2.5. Preparation of Plant Extracts
2.6. Analysis of Leaf Biochemical Parameters
2.7. HPLC Analysis of Hydroxycinnamic Acids
2.8. Statistical Analysis
3. Results
3.1. The Effect on Seed Surface Structure
3.2. The Effect on Seedling Emergence
3.3. The Effects on Seedling Growth
3.4. The Effect on the Density of Trichomes in Lavender Leaves
3.5. The Effects on the Leaf Content of Photosynthetic Pigments
3.6. The Effects on the Total Phenolic Compound Content and Antioxidant Activity
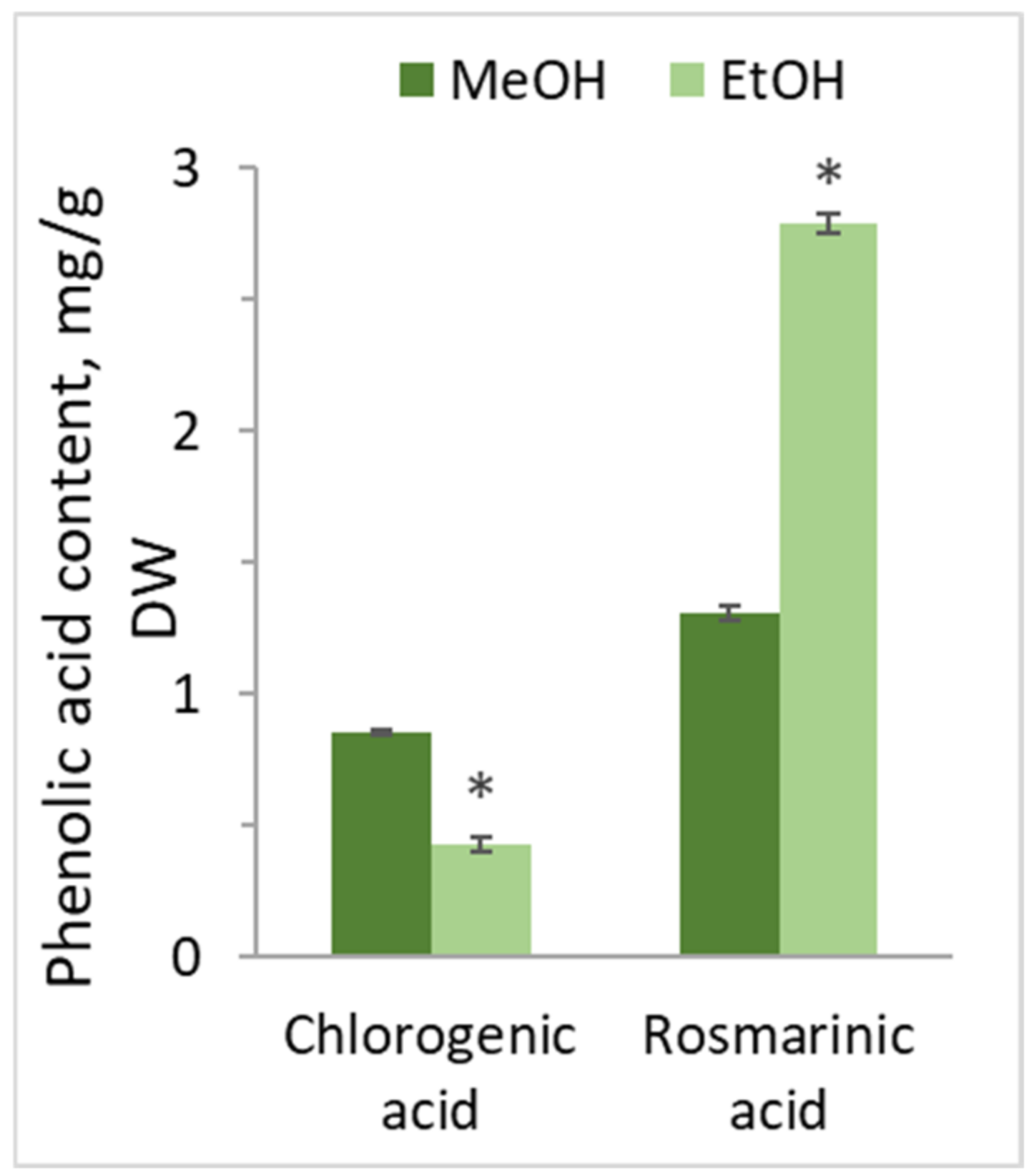
3.7. The Effects on the Rosmarinic and Clorogenic Acid Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPLC | High-Performance Liquid Chromatography |
| CP | Cold Plasma |
| LPC | Low-Pressure Cold Plasma |
| DBD | Dielectric Barrier Discharge |
| V | Vacuum |
| DW | Dry weight |
| TPC | Total Phenolic Compounds |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
References
- State Pharmacopoeia of Ukraine, 2nd ed.; State Enterprise Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines: Kharkiv, Ukraine, 2023; 424p.
- The European Pharmacopoeia, 11th ed.; European Commission: Strasbourg, France, 2023.
- Batiha, G.E.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A review of the bioactive components and pharmacological properties of Lavandula species. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Shamabadi, A.; Hasanzadeh, A.; Ahmadzade, A.; Ghadimi, H.; Gholami, M.; Akhondzadeh, S. The anxiolytic effects of Lavandula angustifolia (lavender): An overview of systematic reviews. J. Herb. Med. 2023, 40, 100672. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Hurina, V.; Herbina, N.; Maslii, Y.; Ivanauskas, L.; Vladymyrova, I.; Lytkin, D.; Gudžinskas, Z.; Severina, H.; Ruban, O.; et al. Phenolic compounds and pharmacological potential of Lavandula angustifolia extracts for the treatment of neurodegenerative diseases. Plants 2025, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Najafian, S.; Afshar, M.; Radi, M. Annual Phytochemical Variations and Antioxidant Activity within the Aerial Parts of Lavandula angustifolia, an Evergreen Medicinal Plant. Chem. Biodivers. 2022, 19, e202200536. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef]
- Lu, H.; Li, H.; Lu, H.; Li, X.; Zhou, A. Chemical composition of lavender essential oil and its antioxidant activity and inhibition against rhinitis-related bacteria. Afr. J. Microbiol. Res. 2010, 4, 309–313. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender essential oil: A review. Aust. Infect. Contr. 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Bogatyrova, O.; Hurina, V.; Naboka, O.; Filimonova, N.; Dzhoraieva, S.; Mykhailenko, O.; Georgiyants, V. Lavandula angustifolia Mill. of Ukrainian origin: A comparative study of the chemical composition and antimicrobial potential of herb extracts. Sci. Pharm. Sci. 2024, 5, 4–14. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K. Composition, biological properties and therapeutic effects of lavender (Lavandula angustifolia L). A review. Herba Pol. 2014, 60. [Google Scholar] [CrossRef]
- Pryvedeniuk, N.; Hlushchenko, L.; Kutsyk, T.; Shatkovskyi, A.; Shatkovska, K.; Shevchenko, T. Influence of mineral fertilizers and planting density on the growth, development and yield of narrow-leaved lavender (Lavandula angustifolia Mill.). Agric. For. 2023, 69, 165–180. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Chetvernya, S.; Bezruk, I.; Buydin, Y.; Dhurenko, N.; Palamarchuk, O.; Ivanauskas, L.; Georgiyants, V. Bioactive constituents of Iris hybrida (Iridaceae): Processing effect. Biomed. Chromatogr. 2022, 36, 5369. [Google Scholar] [CrossRef]
- Asadu, C.O.; Ezema, C.A.; Ekwueme, B.N.; Onu, C.E.; Onoh, I.M.; Adejoh, T.; Ezeorba, T.P.C.; Ogbonna, C.C.; Otuh, P.I.; Okoye, J.O.; et al. Enhanced efficiency fertilizers: Overview of production methods, materials used, nutrients release mechanisms, benefits and considerations. Environ. Pollut. Manag. 2024, 1, 32–48. [Google Scholar] [CrossRef]
- Silva, S.M.; Luz, J.M.Q.; Nogueira, P.A.M.; Blank, A.F.; Sampaio, T.S.; Pinto, J.A.O.; Wisniewski Junior, A. Organo-mineral fertilization effects on biomass and essential oil of lavender (Lavandula dentata L.). Ind. Crop Prod. 2017, 103, 133–140. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Park, G. The effects of plasma on plant growth, development, and sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and Physiological Plant Processes Affected by Seed Treatment with Non-Thermal Plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Pańka, D.; Jeske, M.; Łukanowski, A.; Baturo-Cieśniewska, A.; Prus, P.; Maitah, M.; Maitah, K.; Malec, K.; Rymarz, D.; Muhire, J.D.D.; et al. Can cold plasma be used for boosting plant growth and plant protection in sustainable plant production? Agronomy 2022, 12, 841. [Google Scholar] [CrossRef]
- Bilea, F.; Garcia-Vaquero, M.; Magureanu, M.; Mihaila, I.; Mildažienė, V.; Mozetič, M.; Pawlat, J.; Primc, G.; Puac, N.; Robert, E.; et al. Non-Thermal Plasma as Environmentally-Friendly Technology for Agriculture: A Review and Roadmap. Crit. Rev. Plant Sci. 2024, 43, 428–486. [Google Scholar] [CrossRef]
- Mildaziene, V.; Paužaitė, G.; Nauciene, Z.; Zukiene, R.; Malakauskiene, A.; Norkevičienė, E.; Slepetiene, A.; Stukonis, V.; Olsauskaite, V.; Padarauskas, A.; et al. Effect of seed treatment with cold plasma and electromagnetic field on red clover germination, growth and content of major isoflavones. J. Phys. D Appl. Phys. 2020, 53, 264001. [Google Scholar] [CrossRef]
- Mildaziene, V.; Paužaitė, G.; Nauciene, Z.; Malakauskiene, A.; Zukiene, R.; Januskaitiene, I.; Jakstas, V.; Ivanauskas, L.; Filatova, I.; Lyuskevich, V. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process. Polym. 2017, 15, 1700059. [Google Scholar] [CrossRef]
- Ji, S.H.; Kim, T.; Panngom, K.; Hong, Y.J.; Pengkit, A.; Park, D.H.; Kang, M.H.; Lee, S.H.; Im, J.S.; Kim, J.S.; et al. Assessment of the effects of nitrogen plasma and plasma-generated nitric oxide on early development of Coriandum sativum. Plasma Process. Polym. 2015, 12, 1164–1173. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef]
- Judickaitė, A.; Lyushkevich, V.; Filatova, I.; Mildažienė, V.; Žūkienė, R. The potential of cold plasma and electromagnetic field as stimulators of natural sweeteners biosynthesis in Stevia rebaudiana Bertoni. Plants 2022, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wang, Y.; Zhou, X.; Wei, S.; Zhang, D. Cold plasma pretreatment technology for enhancing the extraction of bioactive ingredients from plant materials: A review. Ind. Crop Prod. 2024, 209, 117963. [Google Scholar] [CrossRef]
- Molina, R.; López-Santos, C.; Balestrasse, K.; Gómez-Ramírez, A.; Sauló, J. Enhancing essential oil extraction from Lavandin Grosso flowers via plasma treatment. Int. J. Mol. Sci. 2024, 25, 2383. [Google Scholar] [CrossRef] [PubMed]
- Hadri, A.; Benmimoun, Y.; Miloudi, K.; Bouhadda, Y.; Elsayed, S.; Abderrahmane, H. Effect of pulsed electric field treatment on the extraction of essential oil from lavender (Lavandula angustifolia Mill.). Int. J. Biol. Biotechnol. 2023, 20, 37–46. [Google Scholar]
- Judickaitė, A.; Venckus, J.; Koga, K.; Shiratani, M.; Mildažienė, V.; Žūkienė, R. Cold Plasma-Induced Changes in Stevia rebaudiana Morphometric and Biochemical Parameter Correlations. Plants 2023, 12, 1585. [Google Scholar] [CrossRef]
- Degutytė-Fomins, L.; Paužaitė, G.; Žukienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Richards, F.J.A. Flexible Growth Function for Empirical Use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Ivanauskas, L.; Uminska, K.; Gudžinskas, Z.; Heinrich, M.; Georgiyants, V.; Kozurak, A.; Mykhailenko, O. Phenological variations in the content of polyphenols and triterpenoids in Epilobium angustifolium herb originating from Ukraine. Plants 2023, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, M.; Majd, A.; Iranbakhsh, A. Transcriptional responses following seed priming with cold plasma and electromagnetic field in Salvia nemorosa L. J. Theor. Appl. Phys. 2020, 14, 323–328. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oraghi Ardebili, N.; Oraghi Ardebili, Z.; Shafaati, M.; Ghoranneviss, M. Non-thermal plasma induced expression of heat shock factor A4A and improved wheat (Triticum aestivum L.) growth and resistance against salt stress. Plasma Chem. Plasma Process. 2018, 38, 29–44. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Oraghi Ardebili, N.; Ghoranneviss, M.; Safari, N. Cold plasma relieved toxicity signs of nano zinc oxide in cayenne via modifying growth, differentiation, and physiology. Acta Physiol. Plant. 2018, 40, 154. [Google Scholar] [CrossRef]
- Babajani, A.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Eslami, B. Seed priming with non-thermal plasma modified plant reactions to selenium or zinc oxide nanoparticles: Cold plasma as a novel emerging tool for plant science. Plasma Chem. Plasma Process. 2019, 39, 21–34. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar] [CrossRef]
- Colinas, M.; Goossens, A. Combinatorial Transcriptional Control of Plant Specialized Metabolism. Trends Plant Sci. 2018, 23, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Milani, F.; Spada, A.; Papini, A.; Flamini, G.; Fico, G. Insight into micromorphology and phytochemistry of Lavandula angustifolia Mill. from Italy. S. Afr. J. Bot. 2023, 153, 83–93. [Google Scholar] [CrossRef]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant Growth-Defense Trade-Offs: Molecular Processes Leading to Physiological Changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the Influence of Alcohol: The Effect of Ethanol and Methanol on Lipid Bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Hitl, M.; Kladar, N.; Gavari’c, N.; Božin, B. Rosmarinic acid–human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.S.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What is the best ethanol-water ratio for the extraction of antioxidants from rosemary? Impact of the solvent on yield, composition, and activity of the extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef]
- Kiss, A.; Papp, V.A.; Pál, A.; Prokisch, J.; Mirani, S.; Toth, B.E.; Alshaal, T. Comparative Study on Antioxidant Capacity of Diverse Food Matrices: Applicability, Suitability and Inter-Correlation of Multiple Assays to Assess Polyphenol and Antioxidant Status. Antioxidants 2025, 14, 317. [Google Scholar] [CrossRef] [PubMed]







| Treatment | Vi, % | Me, h | Qu, h |
|---|---|---|---|
| Control | 57.8 ± 2.0 | 123.4 ± 3.8 | 27.0 ± 2.2 |
| V2 | 56.7 ± 8.2 | 127.5 ± 5.3 | 28.9 ± 1.6 |
| LCP0.5 | 57.8 ± 2.0 | 129.9 ± 6.0 | 27.7 ± 3.5 |
| LCP1 | 60.6 ± 2.0 | 125.9 ± 0.9 | 25.0 ± 0.9 |
| DBD2 | 40.0 ± 4.4 * | 137.6 ± 4.6 * | 23.4 ± 3.9 |
| DBD3 | 69.2 ± 0.8 * | 132.7 ± 1.7 * | 22.8 ± 0.9 |
| Morphometric Parameter | Control | V2 | LCP0.5 | LCP1 | DBD2 | DBD3 |
|---|---|---|---|---|---|---|
| Seedling length, cm | 20.7 ± 0.4 | 20.8 ± 0.4 | 21.7 ± 0.4 * | 20.5 ± 0.4 | 21.6 ± 0.4 | 20.9 ± 0.4 |
| Seedling weight, mg | 834 ± 74 | 884 ± 54 | 972 ± 67 | 828 ± 69 | 848 ± 54 | 1013 ± 79 * |
| Root length, cm | 9.8 ± 0.3 | 9.6 ± 0.3 | 9.9 ± 0.2 | 10.2 ± 0.2 | 11.2 ± 0.2 * | 9.5 ± 0.2 |
| Root weight, mg | 81.3 ± 6.1 | 88.7 ± 6.1 | 108.0 ± 6.4 * | 103.7 ± 7.1 * | 106.0 ± 5.9 * | 98.3 ± 6.3 * |
| Number of leaves | 29.4 ± 1.6 | 28.9 ± 1.7 | 32.2 ± 2.1 | 31.6 ± 2.0 | 34.3 ± 2.5 * | 32.3 ± 1.7 |
| Leaf weight, mg | 559 ± 53 | 575 ± 38 | 644 ± 48 | 507 ± 50 | 551 ± 41 | 653 ± 56 |
| Trichome Type | Control | V2 | LCP0.5 | LCP1 | DBD2 | DBD3 |
|---|---|---|---|---|---|---|
| Glandular | 2.0 ± 0.6 | 4.7 ± 0.6 * | 4.7 ± 0.6 * | 3.3 ± 0.8 | 2.8 ± 1.1 | 1.1 ± 0.4 |
| Dendritic | 63.2 ± 4.3 | 76.7 ± 4.8 * | 60.8 ± 3.2 | 61.8 ± 3.4 | 52.7 ± 3.6 * | 61.8 ± 5.1 |
| Total | 65.2 ± 4.6 | 81.4 ± 5.8 * | 65.5 ± 4.1 | 65.1 ± 4.4 | 55.5 ± 4.6 | 62.9 ± 5.4 |
| Part of glandular trichomes, % | 3.1 ± 0.2 | 5.8 ± 0.5 * | 7.2 ± 1.3 * | 5.1 ± 0.8 * | 5.1 ± 0.4 * | 1.8 ± 0.4 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurina, V.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Tuckute, S.; Ivanauskas, L.; Marksa, M.; Georgiyants, V.; Mykhailenko, O.; Mildaziene, V. Seed Priming with Cold Plasma and Vacuum Increases the Amounts of Phenolic Compounds and Antioxidant Activity in Lavender Herb. Horticulturae 2025, 11, 1413. https://doi.org/10.3390/horticulturae11121413
Hurina V, Nauciene Z, Zukiene R, Degutyte-Fomins L, Tuckute S, Ivanauskas L, Marksa M, Georgiyants V, Mykhailenko O, Mildaziene V. Seed Priming with Cold Plasma and Vacuum Increases the Amounts of Phenolic Compounds and Antioxidant Activity in Lavender Herb. Horticulturae. 2025; 11(12):1413. https://doi.org/10.3390/horticulturae11121413
Chicago/Turabian StyleHurina, Viktoriia, Zita Nauciene, Rasa Zukiene, Laima Degutyte-Fomins, Simona Tuckute, Liudas Ivanauskas, Mindaugas Marksa, Victoriya Georgiyants, Olha Mykhailenko, and Vida Mildaziene. 2025. "Seed Priming with Cold Plasma and Vacuum Increases the Amounts of Phenolic Compounds and Antioxidant Activity in Lavender Herb" Horticulturae 11, no. 12: 1413. https://doi.org/10.3390/horticulturae11121413
APA StyleHurina, V., Nauciene, Z., Zukiene, R., Degutyte-Fomins, L., Tuckute, S., Ivanauskas, L., Marksa, M., Georgiyants, V., Mykhailenko, O., & Mildaziene, V. (2025). Seed Priming with Cold Plasma and Vacuum Increases the Amounts of Phenolic Compounds and Antioxidant Activity in Lavender Herb. Horticulturae, 11(12), 1413. https://doi.org/10.3390/horticulturae11121413








