Anther Ontogeny and Pollen Development in Southern Highbush Blueberry (Vaccinium corymbosum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Environmental Conditions
2.2. Plant Material
2.3. Floral Bud and Flower Staging
2.4. Sample Collection and Preparation
2.5. Microscopy Analyses
2.5.1. Stereomicroscopy (SM)
2.5.2. Light Microscopy (LM)
2.5.3. Confocal Laser Scanning Microscopy (CLSM)
2.5.4. Scanning Electron Microscopy (SEM)
3. Results
3.1. Floral Staging and General Flower Morphology
3.1.1. Inflorescence and Flower Overview
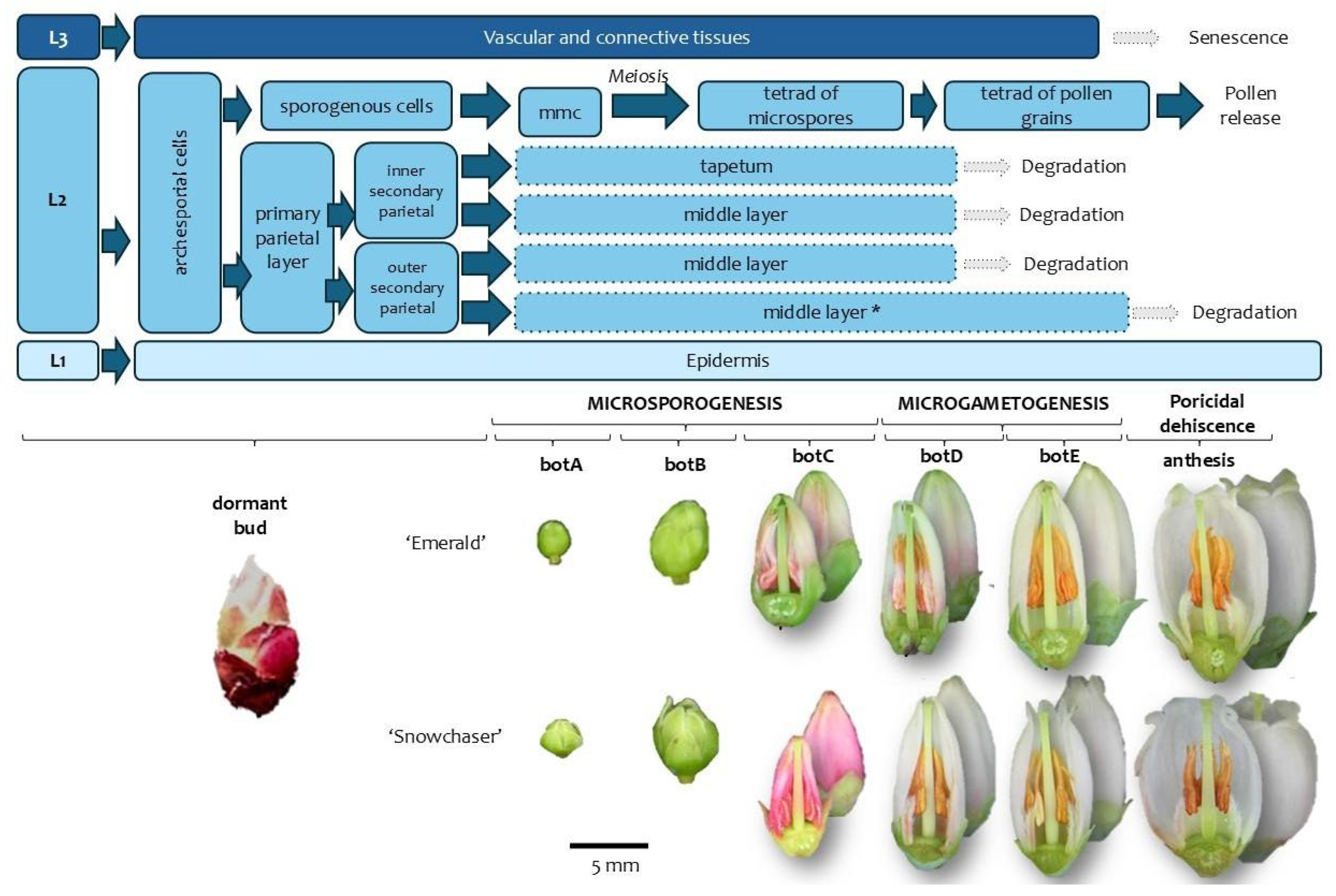
3.1.2. Floral Staging
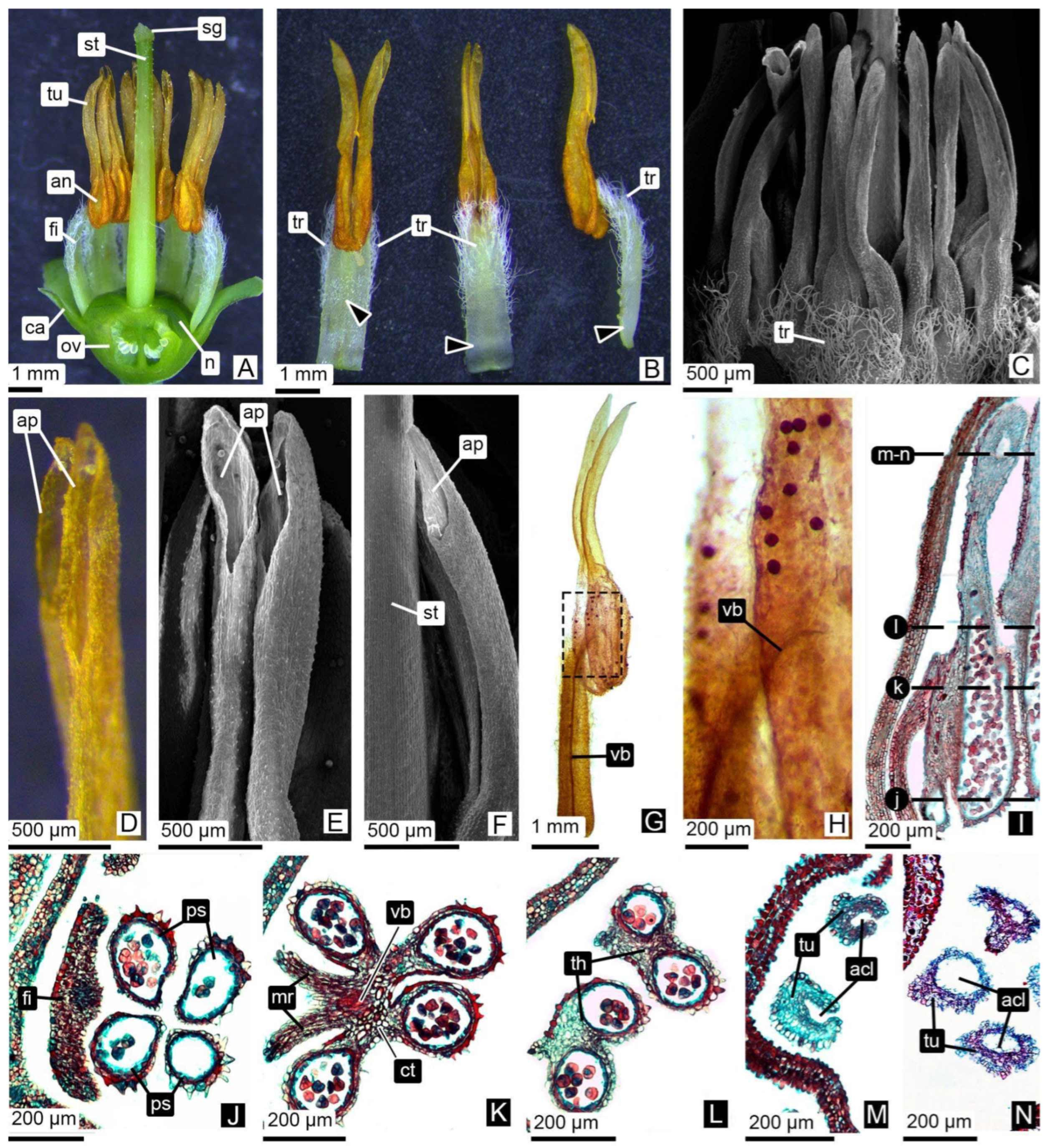
3.2. Androecium Ontogeny: Morphological and Anatomical Insights
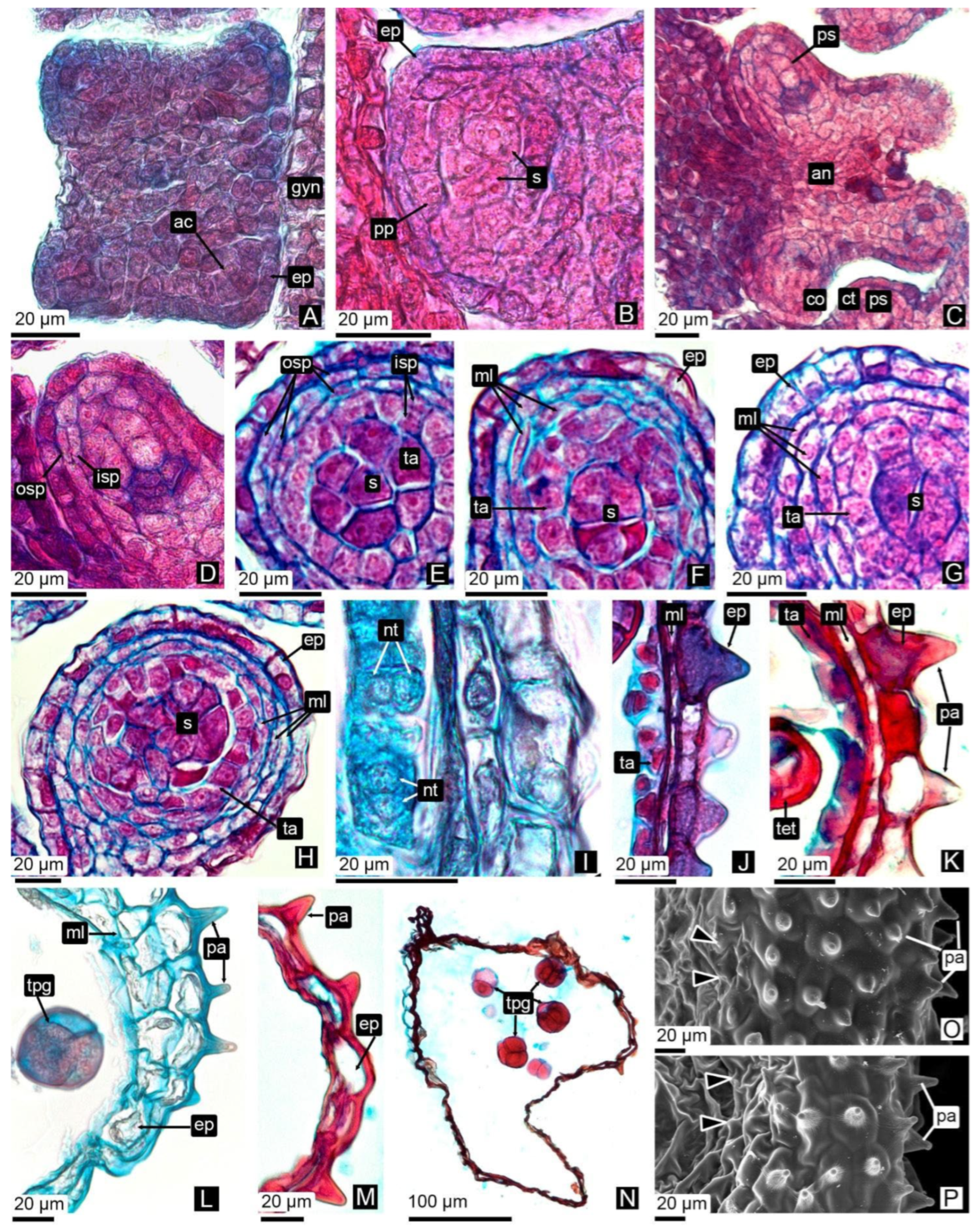
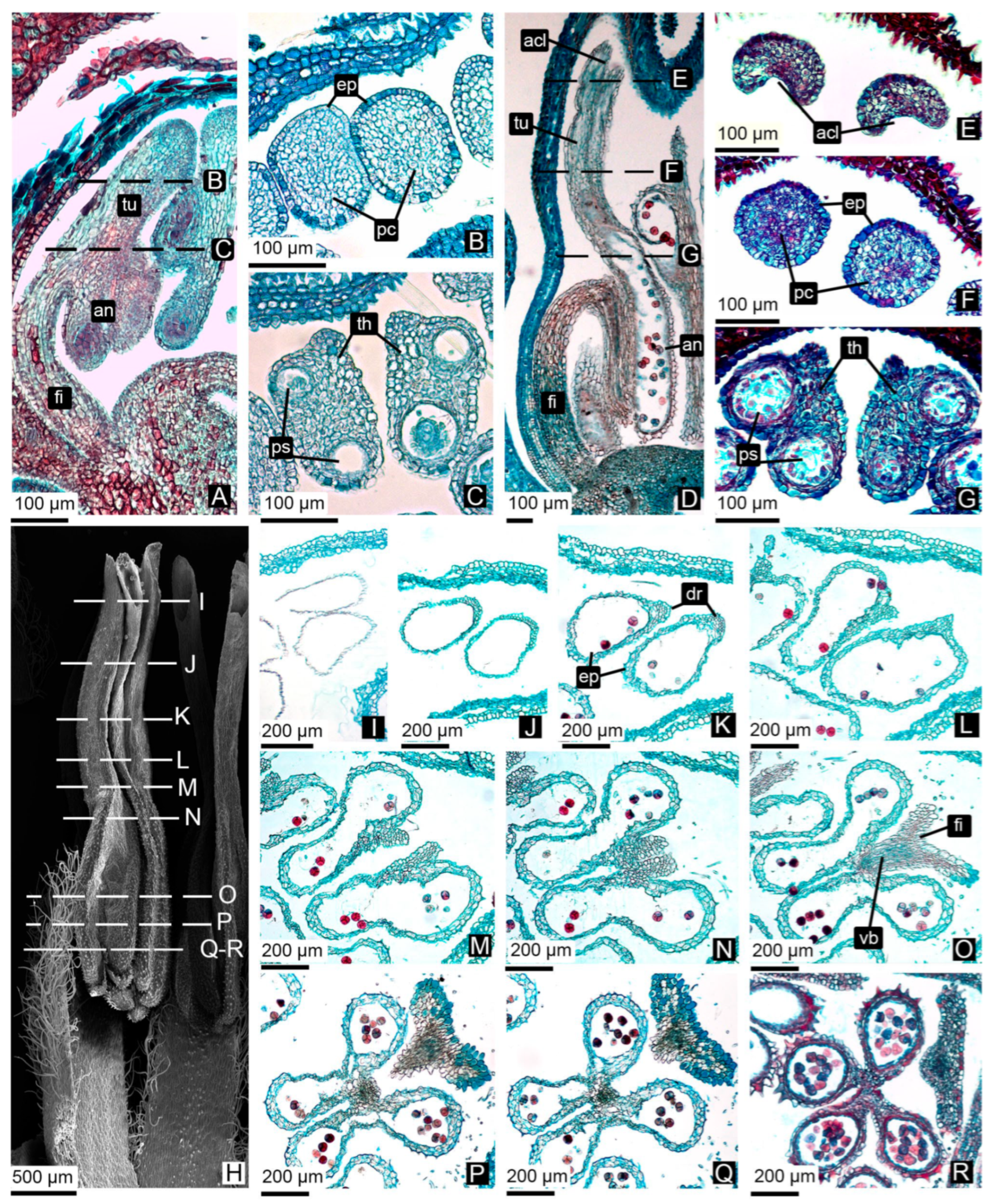
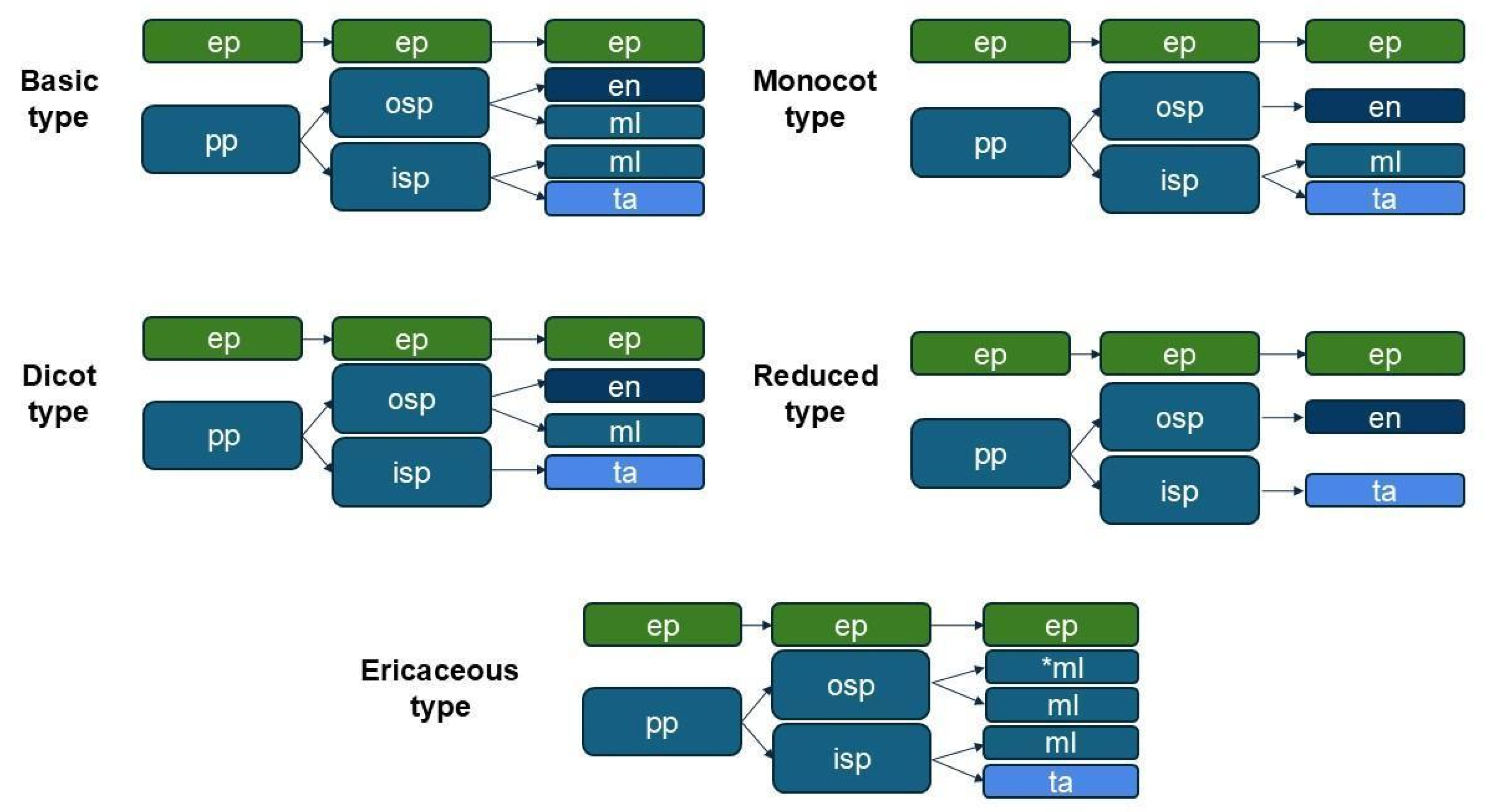
3.2.1. Anther Wall Ontogeny
3.2.2. Pollen Grain Formation
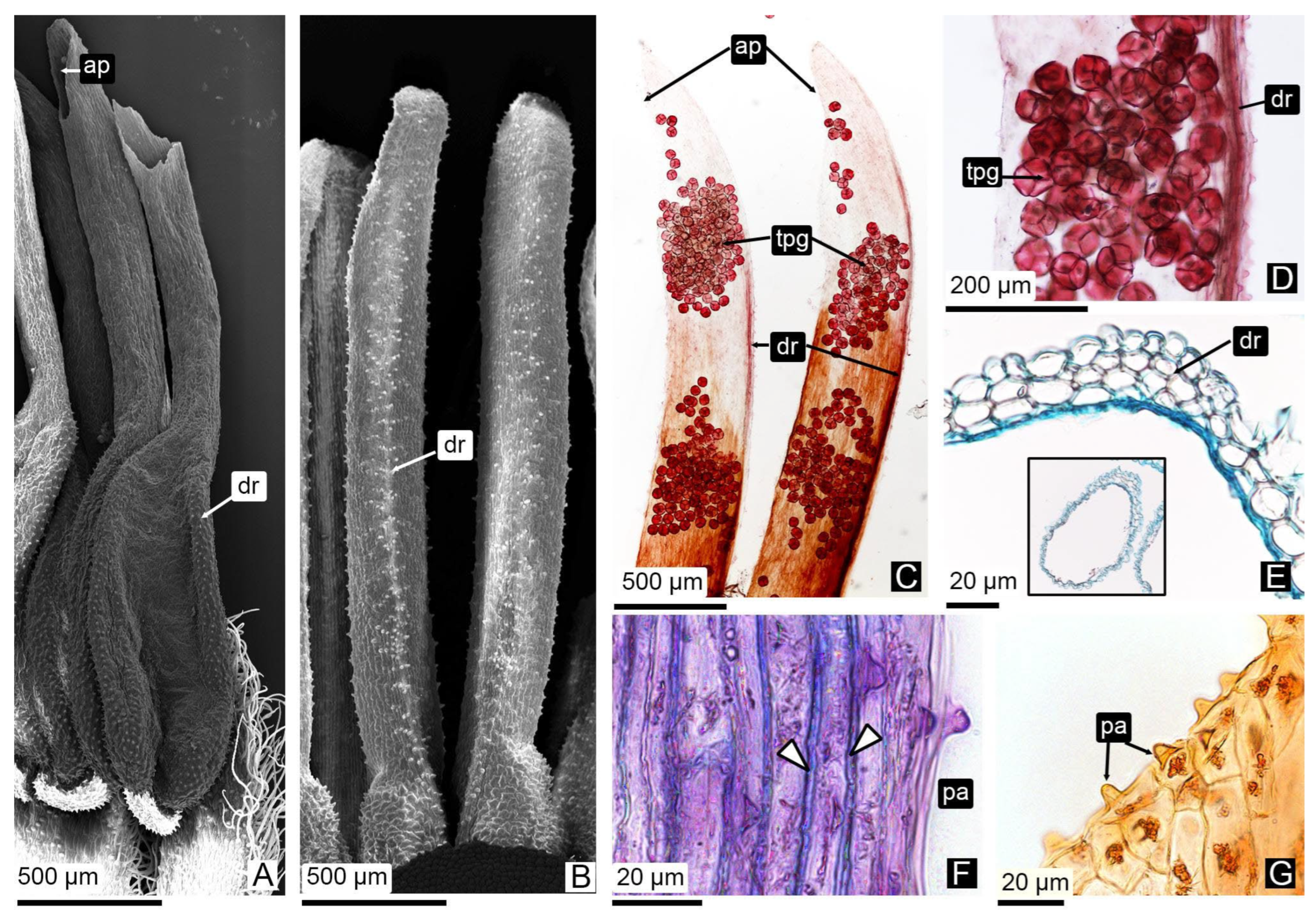
3.2.3. Tubule Ontogeny and Pollen Tetrad Release
4. Discussion
4.1. Floral Morphology and Anther Development
4.1.1. Anther Wall Ontogeny
4.1.2. Microsporogenesis and Pollen Development
4.1.3. Tubule Development and Persistent Pollen Tetrads Release
4.2. Floral Staging and Its Correlation with Androecium Development
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ac | Archesporial cells |
| acl | Apical cleft |
| an | Anther |
| ant | Flower at anthesis |
| ap | Apical pore |
| ca | Calyx |
| CLSM | Confocal laser scanning microscopy |
| co | Corolla |
| col | Colporus |
| ct | Connective tissue |
| db | Dormant bud |
| dr | Dorsal reinforcement |
| ep | Epidermis |
| en | Endothecium |
| fi | Filament |
| gyn | Gynoecium |
| isp | Inner secondary parietal layer |
| L1–L3 | Histogenic layers of the floral meristem |
| LM | Light microscopy |
| lo | Locule |
| LS | Longitudinal section |
| ml | Middle layers |
| mmc/MMC | Microspore mother cell |
| MSU | Michigan State University |
| n | Nectary |
| NHB | Northern highbush blueberries |
| nm | Nectarostome |
| nt | Tapetal cell nucleus |
| osp | Outer secondary parietal layer |
| ov | Ovary |
| pa | Papilla |
| pc | Parenchymatous cells |
| pn | Pollen nuclei |
| pp | Primary parietal layer/stratum |
| ps | Pollen sac |
| s | Sporogenous cell |
| SEM | Scanning electron microscopy |
| sg | Stigma |
| SHB | Southern highbush blueberries |
| SM | Stereomicroscopy |
| st | Style |
| ta | Tapetum |
| tet | Tetrad of microspores |
| th | Theca |
| tpg | Tetrad of pollen grains |
| tr | Trichome |
| TS | Transversal section |
| tu | Tubule |
| vb | Vascular bundle |
References
- Brazelton, C.; Fain, C.; Ogg, M.; Riquelme, C.; Rodriguez, V.; Ilyas, S. Global State of the Blueberry Industry Report 2024; International Blueberry Organization: El Dorado Hills, CA, USA, 2024; pp. 1–239. [Google Scholar]
- Edger, P.P.; Iorizzo, M.; Bassil, N.V.; Benevenuto, J.; Ferrão, L.F.V.; Giongo, L.; Hummer, K.; Lawas, L.M.F.; Leisner, C.P.; Li, C.; et al. There and Back Again; Historical Perspective and Future Directions for Vaccinium Breeding and Research Studies. Hortic. Res. 2022, 9, uhac083. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries; CABI: Wallingford, UK, 2018; ISBN 9781780647265. [Google Scholar]
- Lobos, G.A.; Hancock, J.F. Breeding Blueberries for a Changing Global Environment: A Review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef]
- Rivadeneira, M.F. Variedades de Arándanos Disponibles en Argentina. Available online: https://www.aianer.com.ar/web/download/202010inta_concordia_variedades_de_arandanos.pdf (accessed on 10 September 2025).
- Cromie, J.; Ternest, J.J.; Komatz, A.P.; Adunola, P.M.; Azevedo, C.; Mallinger, R.E.; Muñoz, P.R. Genotypic Variation in Blueberry Flower Morphology and Nectar Reward Content Affects Pollinator Attraction in a Diverse Breeding Population. BMC Plant Biol. 2024, 24, 814. [Google Scholar] [CrossRef]
- Hermann, P.M.; Palser, B.F. Stamen Development in the Ericaceae. I. Anther Wall, Microsporogenesis, Inversion, and Appendages. Am. J. Bot. 2000, 87, 934–957. [Google Scholar] [CrossRef]
- Kriebel, R.; Rose, J.P.; Bastide, P.; Jolles, D.; Reginato, M.; Sytsma, K.J. The Evolution of Ericaceae Flowers and their Pollination Syndromes at a Global Scale. Am. J. Bot. 2023, 110, e16220. [Google Scholar] [CrossRef] [PubMed]
- Ehlenfeldt, M.K.; Polashock, J.J. Highly Fertile Intersectional Blueberry Hybrids of Vaccinium padifolium Section Hemimyrtillus and V. Corymbosum Section Cyanococcus. J. Am. Soc. Hort. Sci. 2014, 139, 30–38. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Luteyn, J.L. Fertile Intersectional F1 Hybrids of 4x Vaccinium meridionale (Section Pyxothamnus) and Highbush Blueberry, V. corymbosum (Section Cyanococcus). HortScience 2021, 56, 318–323. [Google Scholar] [CrossRef]
- Chu, Y.; Lyrene, P.M. Artificial Induction of Polyploidy in Blueberry Breeding: A Review. HortScience 2025, 60, 100–110. [Google Scholar] [CrossRef]
- De Storme, N.; Geelen, D. Sexual Polyploidization in Plants—Cytological Mechanisms and Molecular Regulation. New Phytol. 2013, 198, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Drummond, F. Reproductive Biology of Wild Blueberry (Vaccinium angustifolium Aiton). Agriculture 2019, 9, 69. [Google Scholar] [CrossRef]
- Quiroga-Arcila, A.M.; McCune, F.; Fournier, V.; Giovenazzo, P. Bee-Ing a Pollinator: Constraints, Concerns, and Challenges of Lowbush Blueberry Pollination. Int. J. Fruit Sci. 2025, 25, 28–63. [Google Scholar] [CrossRef]
- Bieniasz, M.E.; Konieczny, A.M. Opportunities to Improve Effectiveness of Pollination of Blueberry CV. ‘Bluecrop’. Agriculture 2022, 12, 2126. [Google Scholar] [CrossRef]
- Leposavić, A.; Glišić, I.; Đorđević, M.; Jevremović, D.; Zejak, D.; Cerović, R. The Effect of Pollination Variant and Temperature on Reproductive Behaviour of Some Highbush Blueberry (Vaccinium corimbosum L.) Cultivars. Appl. Fruit Sci. 2024, 66, 2077–2089. [Google Scholar] [CrossRef]
- Bieniasz, M. The Differentiation of Highbush Blueberry Flower Buds. Acta Hortic. 2012, 932, 117–122. [Google Scholar] [CrossRef]
- Gough, R.E.; Shutak, V.G.; Hauke, R.L. Growth and Development of Highbush Blueberry II. Reproductive Growth, Histological Studies. J. Am. Soc. Hortic. Sci. 1978, 103, 476–479. [Google Scholar] [CrossRef]
- Michigan State University. Growth Stages—Blueberries. Available online: https://www.canr.msu.edu/blueberries/growing_blueberries/growth-stages (accessed on 15 June 2025).
- Wichura, M.A.; Koschnick, F.; Jung, J.; Bauer, S.; Wichura, A. Phenological Growth Stages of Highbush Blueberries (Vaccinium Spp.): Codification and Description According to the BBCH Scale. Botany 2024, 102, 428–437. [Google Scholar] [CrossRef]
- Ramos, S.; De Ruyver, R.; Gattinoni, N.; Garin, R.; Garrán, S. Estación Agrometeorológica del INTA Concordia. 50 Años de Servicio a la Comunidad; INTA: Ciudad de Buenos Aires, Argentina, 2018; pp. 1–53. [Google Scholar]
- Tasi, H.A.; Bedendo, D.J.; Pausich, G.M.; López, L.O.; Schulz, G.; Garran, S.M.; Garin, O.R. Carta de Suelos de La Estación Experimental Agropecuaria Concordia. Departamento Concordia, Provincia de Entre Ríos. Escala 1:5000; INTA: Ciudad de Buenos Aires, Argentina, 2011; pp. 1–107. [Google Scholar]
- Maidana, M.M.; Contreras, F.I.; Vasek, O.M. La Variabilidad Climática como condicionante de presencia de Bacterias Lácticas Autóctonas, Noroeste de La Provincia de Corrientes. Argentina. Geográfica Digit. 2021, 17, 81. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Gonzalez, A.M. ImageJ: Una herramienta indispensable para medir el mundo Biológico. Folium 2018, 1, 1–17. [Google Scholar]
- González, A.M.; Cristóbal, C.L. Anatomía y Ontogenia de Semillas de Helicteres lhotzkyana (Sterculiaceae). Bonplandia 1997, 9, 287–294. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Company, Incorporated: New York, NY, USA, 1940; ISBN 0070325405. [Google Scholar]
- Luque, R.; Sousa, H.C.d.; Kraus, J.E. Métodos de Coloração de Roeser (1972): Modificado—E Kropp (1972) Visando a substituição do Azul de Astra Por Azul de Alcião 8GS Ou 8GX. Acta Bot. Bras. 1996, 10, 199–212. [Google Scholar] [CrossRef]
- Vasco, A.; Thadeo, M.; Conover, M.; Daly, D.C. Preparation of Samples for Leaf Architecture Studies, a Method for Mounting Cleared Leaves. Appl. Plant Sci. 2014, 2, apps.1400038. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.M.; Carvalho, C.R. NOR-Associated Heterochromatin of Pepper Chromosomes Stained with Acridine Orange. Caryologia 2004, 57, 172–176. [Google Scholar] [CrossRef]
- Matthews, J.R.; Knox, E.M. The Comparative Morphology of the stamen in the Ericaceae. Trans. Bot. Soc. Edinb. 1927, 29, 243–281. [Google Scholar] [CrossRef]
- Stevens, P.F. A Classification of the Ericaceae: Subfamilies and Tribes. Bot. J. Linn. Soc. 1971, 64, 1–53. [Google Scholar] [CrossRef]
- Venkateswarlu, J.; Maheswari Devi, H. An Embryological Approach to the Taxonomic Status of Vacciniaceae. Proc. Indian Acad. Sci. 1973, 78, 282–291. [Google Scholar] [CrossRef]
- Palser, B.F. Studies of Floral Morphology in the Ericales. V. Organography and Vascular Anatomy in Several United States Species of the Vacciniaceae. Bot. Gaz. 1961, 123, 79–111. [Google Scholar] [CrossRef]
- Stephens, D.T.; Levesque, D.E.; Davis, A.R. Pollen-Ovule Ratios in Seven Species of Vaccinium (Ericaceae) and Stamen Structure in Vaccinium myrtilloides and Vaccinium vitis-idaea. Botany 2012, 90, 599–614. [Google Scholar] [CrossRef]
- Artopoeus, V.A. Über Den Bau Und Die Öffnungsweise der antheren und die entwickelung der samen der Erikaceen. Flora Oder Allg. Bot. Ztg. 1903, 92, 309–345. [Google Scholar]
- D’arcy, W.G.; Keating, R.C.; Buchmann, S.L. The Calcium Oxalate Package or So-Called Resorption Tissue in Some Angiosperm Anthers. In The Anther: Form, Function and Phylogeny; Cambridge University Press: Cambridge, UK, 1996; pp. 159–191. ISBN 9780521480635. [Google Scholar]
- Davis, G.L. Systematic Embryology of the Angiosperms; John Wiley & Sons: New York, NY, USA, 1966; ISBN 9780471198871. [Google Scholar]
- Xue, J.-S.; Yao, C.; Xu, Q.-L.; Sui, C.-X.; Jia, X.-L.; Hu, W.-J.; Lv, Y.-L.; Feng, Y.-F.; Peng, Y.-J.; Shen, S.-Y.; et al. Development of the Middle Layer in the Anther of Arabidopsis. Front. Plant Sci. 2021, 12, 634114. [Google Scholar] [CrossRef]
- Biswas, R.; Chaudhuri, S. The Tale of Tapetum: From Anther Walls to Pollen Wall. Nucleus 2024, 67, 611–630. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Beals, T.P.; Sanders, P.M. Anther Development: Basic Principles and Practical Applications. Plant Cell 1993, 5, 1217–1229. [Google Scholar] [CrossRef]
- Scott, R.J.; Spielman, M.; Dickinson, H.G. Stamen Structure and Function. Plant Cell Online 2004, 16, S46–S60. [Google Scholar] [CrossRef]
- Åstrand, J.; Knight, C.; Robson, J.; Talle, B.; Wilson, Z.A. Evolution and Diversity of the Angiosperm Anther: Trends in Function and Development. Plant Reprod. 2021, 34, 307–319. [Google Scholar] [CrossRef]
- Huang, Y.H.; Johnson, C.E.; Sundberg, M.D. Floral Morphology and Development of `Sharpblue’ Southern Highbush Blueberry in Louisiana. J. Am. Soc. Hortic. Sci. 1997, 122, 630–633. [Google Scholar] [CrossRef]
- Chamorro, F.J.; Nates-Parra, G. Floral and Reproductive Biology of Vaccinium meridionale (Ericaceae) in the Eastern Andes of Colombia. Rev. Biol. Trop. 2015, 63, 1197–1212. [Google Scholar] [CrossRef]
- Hermann, P.M.; Palser, B.F. Stamen Development in the Ericaceae. II. Granular Pouches and Dehiscence. Flora Morphol. Distrib. Funct. Ecol. Plants 2018, 239, 122–140. [Google Scholar] [CrossRef]
- Matthews, J.R.; Taylor, G. The Structure and development of the Stamen in Erica hirtiflora. Trans. Bot. Soc. Edinb. 1927, 29, 235–242. [Google Scholar] [CrossRef]
- Kron, K.A.; Judd, W.S.; Stevens, P.F.; Crayn, D.M.; Anderberg, A.A.; Gadek, P.A.; Quinn, C.J.; Luteyn, J.L. Phylogenetic Classification of Ericaceae: Molecular and Morphological Evidence. Bot. Rev. 2002, 68, 335–423. [Google Scholar] [CrossRef]
- Golam Sarwar, A.K.M.; Ito, T.; Takahashi, H. An Overview of Pollen Morphology and its Relevance to the Sectional Classification of Vaccinium L. (Ericaceae). Jpn. J. Palynol. 2006, 52, 15–34. [Google Scholar]
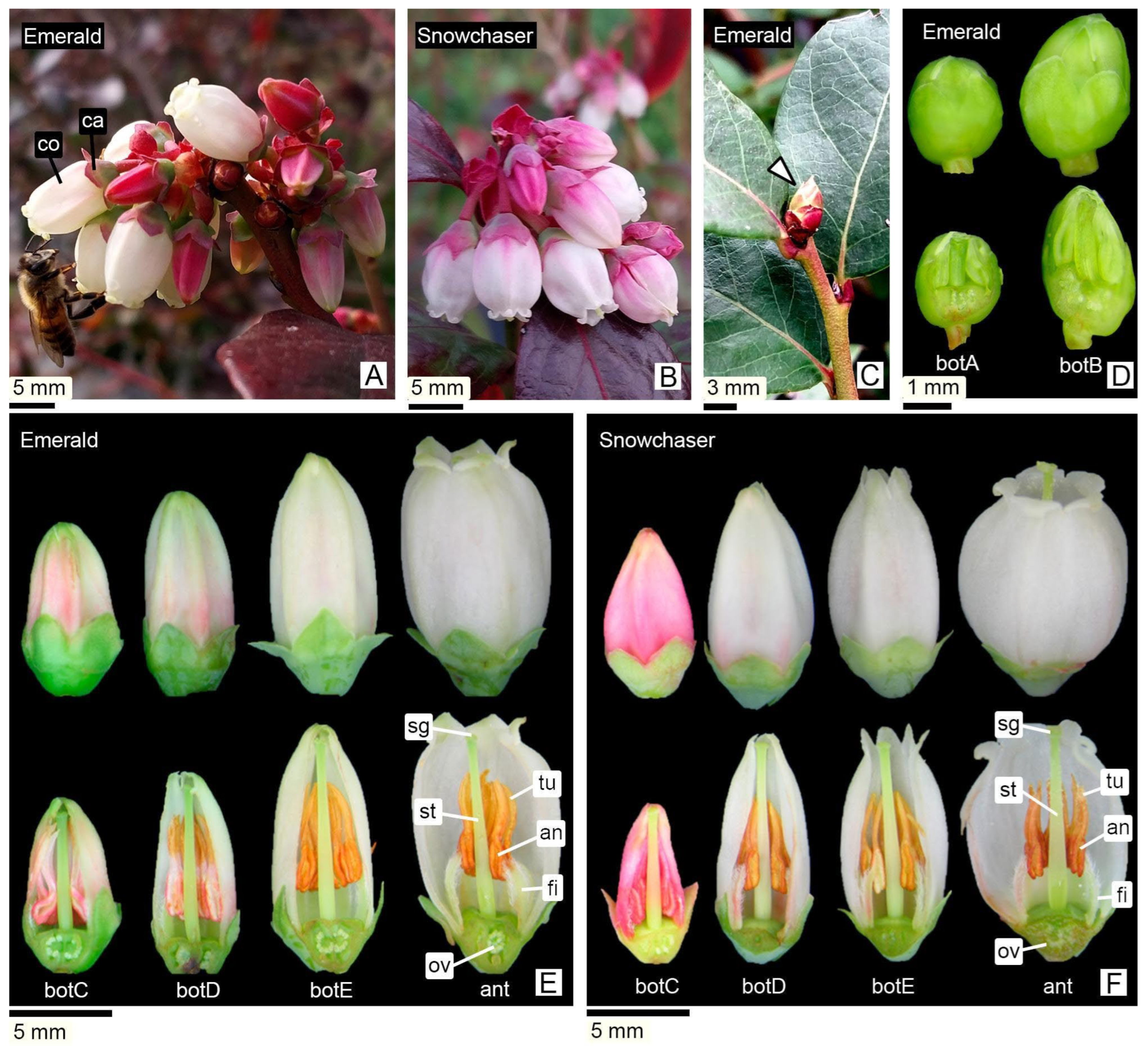

| Stage | Larger Diameter (mm) | Length (mm) | Perianth Color and Characteristics | Anther Color | Tubules Color | ||
|---|---|---|---|---|---|---|---|
| ‘Emerald’ | ‘Snowchaser’ | ‘Emerald’ | ‘Snowchaser’ | ||||
| db | <1 | <1.2 | Not visible. Flower bud covered by bracts of dormant reproductive bud. | Not externally visible | Not externally visible | ||
| botA | 1–1.5 | 1.2–1.6 | Green calyx, hidden corolla. | Green | Green | ||
| botB | 1.5–2 | 1.6–3 | Green calyx, hidden corolla. | Green | Green | ||
| botC | 2–2.5 | 3–8 | The pink corolla begins to emerge. | Soft pink | Deep pink | Whitish | Pink |
| botD | 2.5–4.5 | 8–10.5 | Pink corolla with white shades. | Whitish | Ochre | Orange–ochre | |
| botE | 4.5–5 | 10.5–11.5 | Corolla white, petals apex begin to separate. | Orange–ochre | Orange–ochre | ||
| ant | 5–7 | 10.5–11.5 | White corolla, flower at anthesis. | Orange–ochre | Orange–ochre | ||
| Floral Stages (This Paper) | MSU | BBCH | Pollen Development Stage | Main Morphological Event | Anther Tissues | Tubule |
|---|---|---|---|---|---|---|
| db | Dormant or tight bud. | 00 Dormancy. Flower buds are tightly closed. | Archesporial cells. | Anthers initially quadrangular, later tetralobed. | (1°) Epidermis, archesporial cells; (2°) Epidermis, primary parietal layer, sporogenous tissue; (3°) Epidermis, layer forming. | Developing. |
| botA | Bud swells. | 51 Inflorescence buds swelling. Bud scales elongated, with light colored margins. | Sporogenous tissue. | Compact MMCs observed in pollen sacs, with expanded nuclei and high chromatin content. | Epidermis, middle layers, tapetum, sporogenous tissue/MMCs, connective tissue, vascular bundle. | Solid. Epidermis encloses a mass of parenchymatous cells. |
| botB | Bud break or bud burst. | 53 Inflorescence bud burst. Bud scales separated, lightened bud areas are visible. | Meiosis. | Locules expand, and internal cells become loosely arranged. Microspore mother cells undergo meiosis. Binucleate tapetum. | Epidermis, middle layers, tapetum, MMCs, connective tissue, vascular bundle. | Onset of central parenchyma degradation. Formation of apical slit. |
| botC | Tight cluster. | 55 Green bud stage. First flower buds visible, tight flower clusters, flower buds are still closed. | Tetrads of microspores | Simultaneous cytokinesis in tetrads. Tetrads are surrounded by callose. | Epidermis, middle layers, tapetum, tetrads, connective tissue, vascular bundle. | Hollow in the central region. Epidermis with adjacent layers of parenchymatic cells. |
| botD | Early pink bud | 57 Early pink bud stage. Flower buds are separating, still closed and pink, pedicels are elongating. | Callose dissolves, releasing tetrads. | Fusion of pollen sacs within each theca occurs only at the apex, continuing into a tubule. | Epidermis, tetrads, connective tissue, vascular bundle. | Hollow. Tubule wall consisting of one or two cell layers. |
| botE | Late pink bud. | 59 Late pink bud. All flower buds are fully developed but still closed, the expanded petals are white now. | Tetrads of bicellular pollen grains. | Anthers contain tetrads of bicellular pollen grains. | Epidermis, tetrads, connective tissue, vascular bundle. | Hollow. Tubule wall consisting of one or two cell layers. |
| ant | Full bloom | 65 Full flowering. At least 50% of the flowers are open. | Anther dehiscence, tetrad release. | The permanent tetrads are released through the tubules. The anther remains composed of epidermis. | Epidermis, tetrads, connective tissue, vascular bundle. | Hollow. Tubule wall composed only of epidermis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recalde, J.M.; Garavello, M.F.; Alayón Luaces, P.; González, A.M. Anther Ontogeny and Pollen Development in Southern Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae 2025, 11, 1278. https://doi.org/10.3390/horticulturae11111278
Recalde JM, Garavello MF, Alayón Luaces P, González AM. Anther Ontogeny and Pollen Development in Southern Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae. 2025; 11(11):1278. https://doi.org/10.3390/horticulturae11111278
Chicago/Turabian StyleRecalde, José María, Miguel Fernando Garavello, Paula Alayón Luaces, and Ana María González. 2025. "Anther Ontogeny and Pollen Development in Southern Highbush Blueberry (Vaccinium corymbosum L.)" Horticulturae 11, no. 11: 1278. https://doi.org/10.3390/horticulturae11111278
APA StyleRecalde, J. M., Garavello, M. F., Alayón Luaces, P., & González, A. M. (2025). Anther Ontogeny and Pollen Development in Southern Highbush Blueberry (Vaccinium corymbosum L.). Horticulturae, 11(11), 1278. https://doi.org/10.3390/horticulturae11111278







