Research Progress on the Effect of Grafting Technology on Disease Resistance and Stress Resistance of Watermelon
Abstract
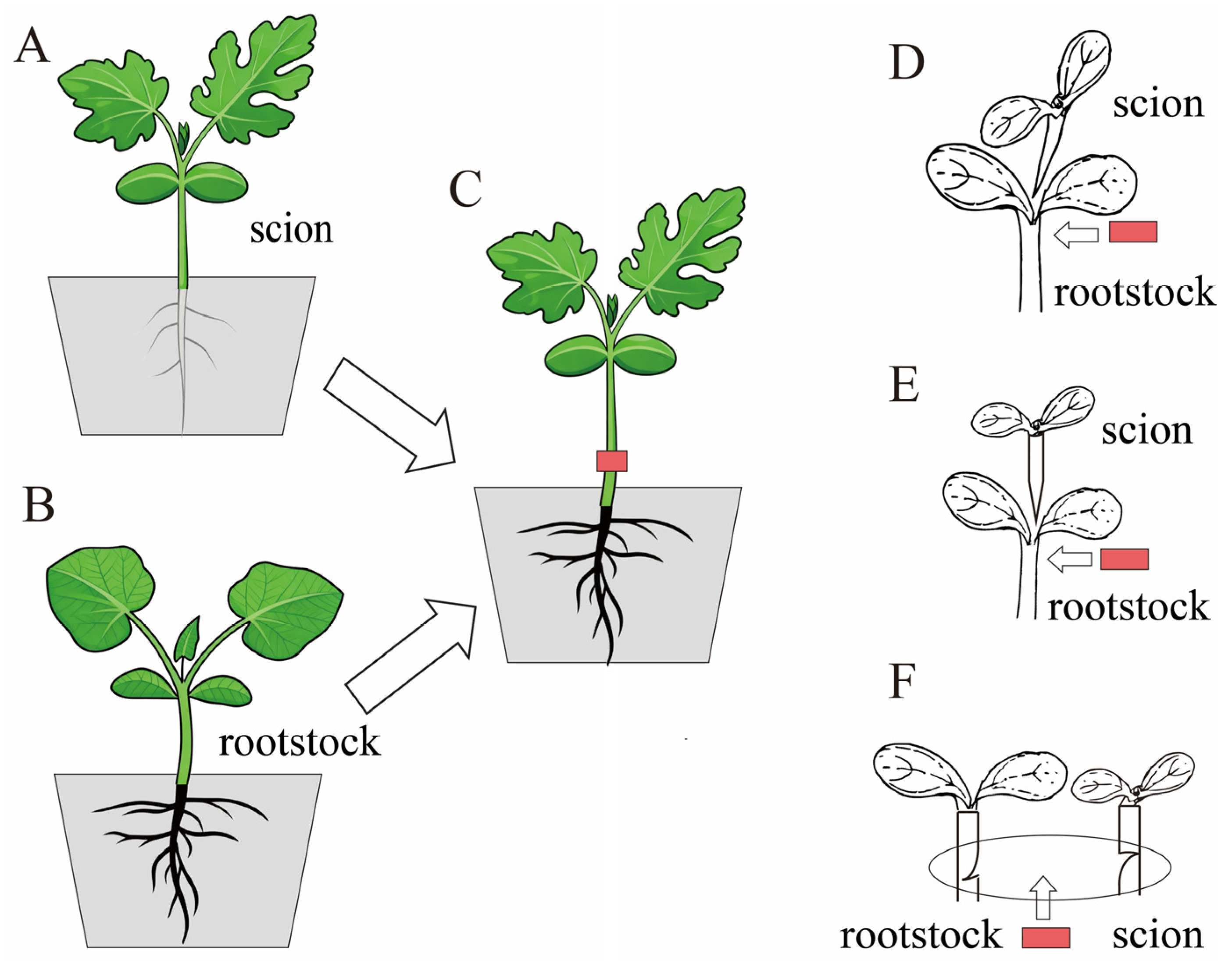
1. Introduction
2. Grafting Affects the Tolerance of Watermelon to Biotic Stress
2.1. Virus Disease
2.2. Fungal Diseases
2.3. Root-Knot Nematode
3. Grafting Affected the Tolerance of Watermelon to Abiotic Stress
3.1. Drought Stress
3.2. Temperature Stress
3.3. Salt Stress
4. Effects of Grafting on Fruit Quality of Watermelon
4.1. Soluble Solids Content
4.2. Organic Acid
4.3. Lycopene
4.4. Physical and Sensory Quality
5. Mechanism of Grafting Affecting Disease and Stress Resistance of Plants
5.1. Biotic Stress Resistance
5.2. Abiotic Stress Resistance
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bahadur, A.; Singh, P.M.; Rai, N.; Singh, A.K.; Singh, A.K.; Karkute, S.G.; Behera, T.K. Grafting in Vegetables to Improve Abiotic Stress Tolerance, Yield and Quality. J. Hortic. Sci. Biotechnol. 2024, 99, 385–403. [Google Scholar] [CrossRef]
- Augstein, F.; Melnyk, C.W. Modern and Historical Uses of Plant Grafting to Engineer Development, Stress Tolerance, Chimeras, and Hybrids. Plant J. 2025, 121, e70057. [Google Scholar] [CrossRef] [PubMed]
- Singathiya, P.; Mahala, P.; Yadav, L.P.; Varotariya, K.; Brahmani, G.; Sohi, A.; Choudhary, R.; Jangu, R.; Uikey, P.; Kumar, S. Advanced Grafting Techniques for Mitigating Biotic and Abiotic Stresses in Vegetable Crops: Breeding and Biotechnological Approaches. Biotechnol. Environ. 2025, 2, 9. [Google Scholar] [CrossRef]
- Liu, C.; Lin, W.; Feng, C.; Wu, X.; Fu, X.; Xiong, M.; Bie, Z.; Huang, Y. A New Grafting Method for Watermelon to Inhibit Rootstock Regrowth and Enhance Scion Growth. Agriculture 2021, 11, 812. [Google Scholar] [CrossRef]
- Kantor, M.; Levi, A. Utilizing Genetic Resources and Precision Agriculture to Enhance Resistance to Biotic and Abiotic Stress in Watermelon. Not. Sci. Biol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, J.; Ren, R.; Liu, G.; Yao, X.; Lou, L.; Xu, J.; Yang, X. Proteomic Analysis of Fusarium oxysporum-Induced Mechanism in Grafted Watermelon Seedlings. Front. Plant Sci. 2021, 12, 632758. [Google Scholar] [CrossRef]
- Kousik, C.S.; Mandal, M.; Hassell, R. Powdery Mildew Resistant Rootstocks that Impart Tolerance to Grafted Susceptible Watermelon Scion Seedlings. Plant Dis. 2018, 102, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Du, H.; Xiao, J.; Zhou, B.; Zheng, X.; Deng, Y.; Zheng, X.; Chen, M. Watermelon Wilt Disease: Causes, Harms, and Control Measures. Front. Microbiol. 2025, 16, 1601130. [Google Scholar] [CrossRef]
- Byun, H.-S.; Choi, H.-S.; Kim, H.R.; Kwak, H.-R.; Kil, E.-J.; Kim, M. First Report of Melon Aphid-Borne Yellows Virus Infecting Watermelon in Korea. Plant Dis. 2022, 106, 1766. [Google Scholar] [CrossRef]
- Morales, C.; Riveros-Burgos, C.; Espinoza Seguel, F.; Maldonado, C.; Mashilo, J.; Pinto, C.; Contreras-Soto, R.I. Rootstocks Comparison in Grafted Watermelon under Water Deficit: Effects on the Fruit Quality and Yield. Plants 2023, 12, 509. [Google Scholar] [CrossRef]
- Ma, G.; Kang, H. The Research Advance of Virus Diseases in the Cucurbitaceae. Heilongjiang Agric. Sci. 2001, 1, 44–47. [Google Scholar]
- Zhao, L.; Qiu, Y.; Zhang, X.; Liu, H.; Yang, J.; Zhang, J.; Zhang, H.; Xu, X.; Wen, C. The Detection of Citrullus lanatus Cryptic Virus Using TaqMan-qPCR Method. Sci. Agric. Sin. 2021, 54, 4337–4347. [Google Scholar]
- Huitrón-Ramírez, M.V.; Ricárdez-Salinas, M.; Camacho-Ferre, F. Influence of Grafted Watermelon Plant Density on Yield and Quality in Soil Infested with Melon Necrotic Spot Virus. HortScience 2009, 44, 1838–1841. [Google Scholar] [CrossRef]
- Felipe, A.; García López, F.; González-Eguiarte, D.; Macías, R.; Zarazúa, P.; María, V.; Huitrón, R. Watermelon Production with Rootstocks in Soils Infested with the Melon Necrotic Spot Virus. Rev. Mex. Cienc. Agríc. 2018, 9, 577. [Google Scholar]
- Hao, X.; Liu, F.; Liu, L.; Wu, H.; Liang, Z.; Zhao, W.; Wang, Y.; Gu, Q.; Kang, B. Zucchini yellow mosaic virus-induced hypersensitive response is associated with pathogenesis-related 1 protein expression and confers resistance in watermelon. Plant Cell Rep. 2024, 43, 277. [Google Scholar] [CrossRef]
- Yetışır, H.; Sari, N.; Yücel, S. Rootstock Resistance to Fusarium Wilt and Effect on Watermelon Fruit Yield and Quality. Phytoparasitica 2003, 31, 163–169. [Google Scholar] [CrossRef]
- Attavar, A.; Tymon, L.; Perkins-Veazie, P.; Miles, C.A. Cucurbitaceae Germplasm Resistance to Verticillium Wilt and Grafting Compatibility with Watermelon. HortScience 2020, 55, 141–148. [Google Scholar] [CrossRef]
- Mahapatra, S.; Rao, E.S.; Hebbar, S.S.; Rao, V.K.; Pitchaimuthu, M.; Sriram, S. Evaluation of rootstocks resistant to gummy stem blight and their effect on the fruit yield and quality traits of grafted watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). J. Hortic. Sci. Biotechnol. 2023, 98, 635–648. [Google Scholar] [CrossRef]
- Mohamed, F.; El-Hamed, K.; Elwan, M.; Hussien, M.-A. Impact of Grafting on Watermelon Growth, Fruit Yield and Quality. Veg. Crops Res. Bull. 2012, 76, 99. [Google Scholar] [CrossRef]
- Jang, Y.; Moon, J.-H.; Kim, S.-G.; Kim, T.; Lee, O.-J.; Lee, H.-J.; Wi, S.-H. Effect of Low-Temperature Tolerant Rootstocks on the Growth and Fruit Quality of Watermelon in Semi-Forcing and Retarding Culture. Agronomy 2023, 13, 67. [Google Scholar] [CrossRef]
- Thies, J.A.; Ariss, J.J.; Hassell, R.L.; Olson, S.; Kousik, C.S.; Levi, A. Grafting for Management of Southern Root-Knot Nematode, Meloidogyne incognita, in Watermelon. Plant Dis. 2010, 94, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Pofu, M.; Mashela, P.; Mphosi, M. Management of Meloidogyne incognita in nematodesusceptible watermelon cultivars using nematoderesistant Cucumis africanus and Cucumis myriocarpus rootstocks. Afr. J. Biotechnol. 2011, 10, 8790–8793. [Google Scholar] [CrossRef]
- Ellouze, W.; Mishra, V.; Howard, R.J.; Ling, K.-S.; Zhang, W. Preliminary Study on the Control of Cucumber Green Mottle Mosaic Virus in Commercial Greenhouses Using Agricultural Disinfectants and Resistant Cucumber Varieties. Agronomy 2020, 10, 1879. [Google Scholar] [CrossRef]
- Yu, T.-A.; Chiang, C.-H.; Wu, H.-W.; Li, C.-M.; Yang, C.-F.; Chen, J.-H.; Chen, Y.-W.; Yeh, S.-D. Generation of Transgenic Watermelon Resistant to Zucchini Yellow Mosaic Virus and Papaya Ringspot Virus type W. Plant Cell Rep. 2011, 30, 359–371. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ku, H.-M.; Chiang, Y.-H.; Ho, H.-Y.; Yu, T.-A.; Jan, F.-J. Development of Transgenic Watermelon Resistant to Cucumber Mosaic Virus and Watermelon Mosaic Virus by Using a Single Chimeric Transgene Construct. Transgenic Res. 2012, 21, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.A. Grafting for Managing Vegetable Crop Pests. Pest Manag. Sci. 2021, 77, 4825–4835. [Google Scholar] [CrossRef]
- Liu, G. Identification and Comprehensive Control of Main Infectious Diseases of Greenhouse Seedless Watermelon in North China. North. Hortic. 2013, 20, 118–120. [Google Scholar]
- Qiao, Y.; Wang, Z.; Sun, H.; Guo, H.; Song, Y.; Zhang, H.; Ruan, Y.; Xu, Q.; Huang, Q.; Shen, Q.; et al. Synthetic Community Derived from Grafted Watermelon Rhizosphere Provides Protection for Ungrafted Watermelon Against Fusarium oxysporum via Microbial Synergistic Effects. Microbiome 2024, 12, 101. [Google Scholar] [CrossRef]
- Kumar, V.; Mann, S.; Kumar, A. Screening of Vegetables Crop Genotype Against Root-knot Nematode (Meloidogyne incognita) under Polyhouse Conditions. Int. J. Agric. Sci. 2021, 17, 600–603. [Google Scholar] [CrossRef]
- Sumita, K.; Vivekananda, Y. A Southern Root-knot Nematode (Meloidogyne incognita) First Reported on Cucumber in Manipur. Indian J. Agric. Res. 2024, 58, 548–550. [Google Scholar] [CrossRef]
- Patel, T.; Quesada-Ocampo, L.M.; Wehner, T.C.; Bhatta, B.P.; Correa, E.; Malla, S. Recent Advances and Challenges in Management of Colletotrichum orbiculare, the Causal Agent of Watermelon Anthracnose. Horticulturae 2023, 9, 1132. [Google Scholar] [CrossRef]
- Saud, S.; Aryal, S.; Ojha, S.; Bhandari, P.; Ghimire, A.; Thapa, A. A Comprehensive Review: Root-knot Nematode; Biology and Management. Trop. Agroecosyst. 2024, 5, 10–15. [Google Scholar] [CrossRef]
- García-Mendívil, H.A.; Munera, M.; Giné, A.; Escudero, N.; Picó, M.B.; Gisbert, C.; Sorribas, F.J. Response of Two Citrullus amarus Accessions to Isolates of Three Species of Meloidogyne and Their Graft Compatibility with Watermelon. Crop Prot. 2019, 119, 208–213. [Google Scholar] [CrossRef]
- Phani, V.; Gowda, M.T.; Dutta, T.K. Grafting Vegetable Crops to Manage Plant-parasitic Nematodes: A Review. J. Pest Sci. 2024, 97, 539–560. [Google Scholar] [CrossRef]
- Liu, B.; Ren, J.; Zhang, Y.; An, J.; Chen, M.; Chen, H.; Xu, C.; Ren, H. A New Grafted Rootstock Against Root-knot Nematode for Cucumber, Melon, and Watermelon. Agron. Sustain. Dev. 2015, 35, 251–259. [Google Scholar] [CrossRef]
- Thies, J.A.; Ariss, J.J.; Kousik, C.S.; Hassell, R.L.; Levi, A. Resistance to Southern Root-knot Nematode (Meloidogyne incognita) in Wild Watermelon (Citrullus lanatus var. citroides). J. Nematol. 2016, 48, 14–19. [Google Scholar] [CrossRef]
- Thies, J.; Levi, A.; Ariss, J.; Hassell, R. RKVL-318, a Root-knot Nematode-resistant Watermelon Line as Rootstock for Grafted Watermelon. HortScience 2015, 50, 141–142. [Google Scholar] [CrossRef]
- Sehularo, M.N.; Kgwaakgwaa, P.; Madumane, K.; Sewelo, L.T.; Batlang, U.; Kobue-Lekalake, R.; Malambane, G. Grafting Susceptible Watermelon on Wild Watermelon Root Stocks Improves Response to Moisture Stress and Improves Growth and Yield. J. Agric. Sci. 2025, 17. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Colla, G.; Rea, E. Yield, Mineral Composition, Water Relations, and Water Use Efficiency of Grafted Mini-watermelon Plants Under Deficit Irrigation. HortScience 2008, 43, 730–736. [Google Scholar] [CrossRef]
- Yavuz, N.; Seymen, M.; Yavuz, D.; Kal, Ü.; Kurtar, E.S.; Kal, S.; Gür, A. Functional roles of plant growth-promoting rhizobacteria in ungrafted and grafted watermelons under various deficit irrigation strategies. Agric. Water Manag. 2025, 318, 109687. [Google Scholar] [CrossRef]
- Bikdeloo, M.; Colla, G.; Rouphael, Y.; Hassandokht, M.R.; Soltani, F.; Salehi, R.; Kumar, P.; Cardarelli, M. Morphological and Physio-Biochemical Responses of Watermelon Grafted onto Rootstocks of Wild Watermelon [Citrullus colocynthis (L.) Schrad] and Commercial Interspecific Cucurbita Hybrid to Drought Stress. Horticulturae 2021, 7, 359. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zheng, D.; Li, X.; Hu, X.; Khan, A.; Wang, X.; Li, M.; Du, Q.; Li, J.; et al. Transcription factor ClTCP4 maintains watermelon resilience to drought by stabilizing antioxidant and photosynthetic systems. Plant Cell Rep. 2025, 44, 168. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Kong, Q.; Cheng, F.; Niu, M.; Xie, J.; Muhammad Azher, N.; Bie, Z. Comprehensive Mineral Nutrition Analysis of Watermelon Grafted onto Two Different Rootstocks. Hortic. Plant J. 2016, 2, 105–113. [Google Scholar] [CrossRef]
- Kurtar, E.S.; Seymen, M.; Yavuz, D.; Acar, B.; Metin, D.; Atakul, Z.; Kal, Ü. Morphophysiological and biochemical investigation of the potential of citron watermelon (Citrullus lanatus var. citroides) rootstock under different irrigation regimes. Hortic. Environ. Biotechnol. 2024, 65, 1009–1023. [Google Scholar] [CrossRef]
- Yavuz, D.; Gökmen Yılmaz, F.; Seymen, M.; Korkmaz, A.; Baştaş, K.K. Effects of Newly Isolated Rhizobacteria on the Physiological Characteristics and Nutrient Uptake of Watermelon Plants Grafted onto Different Rootstocks Under Water Stress. J. Crop Health 2024, 76, 865–881. [Google Scholar] [CrossRef]
- Seymen, M.; Yavuz, D.; Ercan, M.; Akbulut, M.; Çoklar, H.; Kurtar, E.S.; Yavuz, N.; Süheri, S.; Türkmen, Ö. Effect of wild watermelon rootstocks and water stress on chemical properties of watermelon fruit. Hortic. Environ. Biotechnol. 2021, 62, 411–422. [Google Scholar] [CrossRef]
- Lu, K.; Sun, J.; Li, Q.; Li, X.; Jin, S. Effect of Cold Stress on Growth, Physiological Characteristics, and Calvin-Cycle-Related Gene Expression of Grafted Watermelon Seedlings of Different Gourd Rootstocks. Horticulturae 2021, 7, 391. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, F.; Huang, Y.; Bie, Z. Grafting Watermelon Onto Pumpkin Increases Chilling Tolerance by Up Regulating Arginine Decarboxylase to Increase Putrescine Biosynthesis. Front. Plant Sci. 2022, 12, 812396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Gao, M.; Lu, J.; Huang, Y.; Bie, Z. Spatial–Temporal Response of Reactive Oxygen Species and Salicylic Acid Suggest Their Interaction in Pumpkin Rootstock-Induced Chilling Tolerance in Watermelon Plants. Antioxidants 2021, 10, 2024. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Jalal, A.G.; Ma, J.; Wei, C.; Zhang, X. Methyl Jasmonate Mediates Melatonin-induced Cold Tolerance of Grafted Watermelon Plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Wen, W.; Sun, J.; Shu, S.; Guo, S. Transcriptome and Proteome Analysis Identifies Salt Stress Response Genes in Bottle Gourd Rootstock-Grafted Watermelon Seedlings. Agronomy 2023, 13, 618. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.; Tian, J.; Duan, J.; Du, C. Effects of Grafting on the Isozymes and Activities of Antioxidant Enzymes of Watermelon Leaves under NaCl Stress. J. Chang. Veg. 2009, 4, 22–26. [Google Scholar]
- Yang, Y.; Lu, X.; Yan, B.; Li, B.; Sun, J.; Guo, S.; Tezuka, T. Bottle Gourd Rootstock-grafting Affects Nitrogen Metabolism in NaCl-stressed Watermelon Leaves and Enhances Short-term Salt Tolerance. J. Plant Physiol. 2013, 170, 653–661. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Tian, J.; Li, J.; Sun, J.; He, L.; Guo, S.; Tezuka, T. Proteomic Study Participating the Enhancement of Growth and Salt Tolerance of Bottle Gourd Rootstock-grafted Watermelon Seedlings. Plant Physiol. Biochem. 2012, 58, 54–65. [Google Scholar] [CrossRef]
- Yetisir, H.; Uygur, V. Responses of Grafted Watermelon onto Different Gourd Species to Salinity Stress. J. Plant Nutr. 2010, 33, 315–327. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, S.; Wei, M.; Gong, B.; Shi, Q. Effect of Different Rootstocks on the Salt Stress Tolerance in Watermelon Seedlings. Hortic. Plant J. 2018, 4, 239–249. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, S.; Lu, X.; He, N.; Gao, L.; Dou, J.; Bie, Z.; Liu, W. Genome Duplication Improves the Resistance of Watermelon Root to Salt Stress. Plant Physiol. Biochem. 2018, 133, 11–21. [Google Scholar] [CrossRef]
- Bőhm, V.; Fekete, D.; Balázs, G.; Gáspár, L.; Kappel, N. Salinity Tolerance of Grafted Watermelon Seedlings. Acta Biol. Hung. 2017, 68, 412–427. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, S.; Li, J.; Du, C.; Chen, L.; Wang, L. Effects of Grafting on the Soluble Protein Expression in Watermelon Seedling under Salt Stress. J. Nanjing Agric. Univ. 2011, 34, 54–60. [Google Scholar]
- Márkus, R.; Czigle, S.; Zana, B.; Somogyi, B.A.; Urbán, P.; Kutyáncsánin, D.; Helfrich, P.; Stranczinger, S. Drought and Salt Stressors Alter NAC and WRKY Gene Expression Profiles in Grafted Citrullus lanatus. Plant Mol. Biol. Report. 2025, 43, 1576–1587. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, K.; Li, Y.; Bai, C.; Xue, Z.; Wu, Y. Joint transcriptomic and metabolomic analysis provides new insights into drought resistance in watermelon (Citrullus lanatus). Front. Plant Sci. 2024, 15, 1364631. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Dufault, R. Short-term Cyclic Cold Temperature Stress on Watermelon Yield. HortScience 2002, 37, 487–489. [Google Scholar] [CrossRef]
- Yaseen, I.; Choi, S.; Mukhtar, T.; Park, J.-I.; Kim, H.-T. Quantification of Growth and Physiological Characteristics in Tolerant and Sensitive Watermelon Lines Under Cold Treatment. Hortic. Environ. Biotechnol. 2025, 66, 189–204. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Liu, G.; Yang, X.; Hou, X. Comparative Transcriptome Profiling of Chilling Stress Responsiveness in Grafted Watermelon Seedlings. Plant Physiol. Biochem. 2016, 109, 561–570. [Google Scholar] [CrossRef]
- Hou, L.; Yin, J.; Wu, L.; Yan, J.; Guo, Q.; XIan, W. Transcriptome Analysis Revealed that Grafting Improves the Resistance of Pepper to Phytophthora Capsici by Fine-tuning Growth-defense Tradeoff. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12705. [Google Scholar] [CrossRef]
- Lang, X.; Zhao, X.; Zhao, J.; Ren, T.; Nie, L.; Zhao, W. MicroRNA Profiling Revealed the Mechanism of Enhanced Cold Resistance by Grafting in Melon (Cucumis melo L.). Plants 2024, 13, 1016. [Google Scholar] [CrossRef]
- Smiti, K.; Mina, U.; Verma, M.; Arya, L. Impact of Climate Extremes and Other Key Abiotic Stresses on Cucurbits: A Systematic Review. Vegetos 2025. [Google Scholar] [CrossRef]
- Bayoumi, Y.; Abd-Alkarim, E.; El-Ramady, H.; El-Aidy, F.; Hamed, E.-S.; Taha, N.; Prohens, J.; Rakha, M. Grafting Improves Fruit Yield of Cucumber Plants Grown under Combined Heat and Soil Salinity Stresses. Horticulturae 2021, 7, 61. [Google Scholar] [CrossRef]
- El-Kafafi, S.; Abu El-Azm, N.; Hikal, M. Grafting Enables Cantaloupe to Tolerate More Saline Stress. J. Plant Prod. 2017, 8, 671–678. [Google Scholar] [CrossRef]
- Song, Q.; Joshi, M.; Joshi, V. Transcriptomic Analysis of Short-Term Salt Stress Response in Watermelon Seedlings. Int. J. Mol. Sci. 2020, 21, 6036. [Google Scholar] [CrossRef]
- Sun, X.; Chen, S.; Yang, L.; Li, J.; Zhou, B.; Chen, N. Effects of Salt Stress on Plant Growth and Stoichiometric Characteristics of C, N, P and K of Grafted Watermelon Seedlings. Agric. Res. Arid. Areas 2020, 38, 170–176. [Google Scholar]
- Zhu, Y.; Yuan, G.; Gao, B.; An, G.; Li, W.; Si, W.; Sun, D.; Liu, J. Comparative Transcriptome Profiling Provides Insights into Plant Salt Tolerance in Watermelon (Citrullus lanatus). Life 2022, 12, 1033. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Elango, D.; Liu, H.; Jin, D.; Wang, X.; Wu, Y. Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Watermelon under Salt Stress. Environ. Exp. Bot. 2023, 206, 105200. [Google Scholar] [CrossRef]
- Llanderal, A.; Vasquez Muñoz, G.; Pincay-Solorzano, M.S.; Ceasar, S.A.; García-Caparros, P. Growth, Spectral Vegetation Indices, and Nutritional Performance of Watermelon Seedlings Subjected to Increasing Salinity Levels. Agronomy 2025, 15, 1620. [Google Scholar] [CrossRef]
- Niu, M.; Luo, W.; Luo, L.; Chen, S.; Zhao, H.; Zhang, H.; Qian, Y. Non-Invasive Micro-Test Technology and Reciprocal Grafting Provide Direct Evidence of Contrasting Na+ Transport Strategies between Cucurbita moschata and Cucurbita maxima. Agronomy 2023, 13, 1843. [Google Scholar] [CrossRef]
- Başak, H.; Aydin, A.; Yetişir, H.; Turan, M. Salt Stress Effects On Hybrid Bottle Gourd (Lagenaria siceraria) Rootstock Candidates Plant Growth, Hormones and Nutrient Content. J. Crop Health 2025, 77, 28. [Google Scholar] [CrossRef]
- Yuan, G.; He, Y.; Sun, D.; Shi, M.; Li, W.; Zhang, J.; Zhu, Y. ClaDREB14 Enhances the Salt Tolerance of Watermelon by Positively Regulating the Expression of ClaPOD6. Hortic. Plant J. 2025. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and Melon Fruit Quality: The Genotypic and Agro-environmental Factors Implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Dia, M.; Wehner, T.C.; Perkins-Veazie, P.; Hassell, R.; Price, D.S.; Boyhan, G.E.; Olson, S.M.; King, S.R.; Davis, A.R.; Tolla, G.E. Stability of Fruit Quality Traits in Diverse Watermelon Cultivars Tested in Multiple Environments. Hortic. Res. 2016, 3, 16066. [Google Scholar] [CrossRef] [PubMed]
- Ying, Q.; He, Y.; Wang, Y.; Cao, T.; Gu, B.; Wang, Y. Effects of Grafting with Different Rootstocks on Watermelon Fruit Quality. Acta Agric. Zhejiangensis 2017, 29, 590–596. [Google Scholar]
- Sun, N.; Ma, Y.; Wang, X.; Ying, Q.; Huang, Y.; Zhang, L.; Zhu, Z.; Wang, Y.; He, Y. Grafting onto Pumpkin Alters the Evolution of Fruit Sugar Profile and Increases Fruit Weight through Invertase and Sugar Transporters in Watermelon. Sci. Hortic. 2023, 314, 111936. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Kyriacou, M.C.; Siomos, A.S.; Gerasopoulos, D. Evolution of Watermelon Fruit Physicochemical and Phytochemical Composition during Ripening as Affected by Grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Brecht, J.K.; Sims, C.A.; Sanchez, T.; Dufault, N.S. Fruit Quality of Seedless Watermelon Grafted onto Squash Rootstocks under Different Production Systems. J. Sci. Food Agric. 2017, 97, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lozano, M.; Dutta, S.K.; Natarajan, P.; Tomason, Y.R.; Lopez, C.; Katam, R.; Levi, A.; Nimmakayala, P.; Reddy, U.K. Transcriptome Changes in Reciprocal Grafts Involving Watermelon and Bottle Gourd Reveal Molecular Mechanisms Involved in Increase of the Fruit SIze, RInd Toughness and Soluble Solids. Plant Mol. Biol. 2020, 102, 213–223. [Google Scholar] [CrossRef]
- Ning, K.; Cai, X.; Yan, L.; Zhou, W.; Xie, A.; Wang, Y.; Xu, P. Transcriptomic and Metabolomic Analysis Reveals Improved Fruit Quality in Grafted Watermelon. Horticulturae 2024, 10, 1269. [Google Scholar] [CrossRef]
- Aslam, A.; Zhao, S.; Azam, M.; Lu, X.; He, N.; Li, B.; Dou, J.; Zhu, H.; Liu, W. Comparative Analysis of Primary Metabolites and Transcriptome Changes Between Ungrafted and Pumpkin-grafted Watermelon During Fruit Development. PeerJ 2020, 8, e8259. [Google Scholar] [CrossRef]
- Fekete, D.; Stéger-Máté, M.; Bőhm, V.; Balázs, G.; Kappel, N. Lycopene and Flesh Colour Differences in Grafted and Non-grafted Watermelon. Acta Univ. Sapientiae Aliment. 2015, 8, 111–117. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Liu, P.; Cao, L.; Huang, Y.; Zhao, L.; Lv, H.; Bie, Z. Transcriptional Regulation of Lycopene Metabolism Mediated by Rootstock During the Ripening of Grafted Watermelons. Food Chem. 2017, 214, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Soteriou, G.A.; Rouphael, Y.; Siomos, A.S.; Gerasopoulos, D. Configuration of Watermelon Fruit Quality in Response to Rootstock-Mediated Harvest Maturity and Postharvest Storage. J. Sci. Food Agric. 2016, 96, 2400–2409. [Google Scholar] [CrossRef]
- Guo, S.; Sun, H.; Tian, J.; Zhang, G.; Gong, G.; Ren, Y.; Zhang, J.; Li, M.; Zhang, H.; Li, H.; et al. Grafting Delays Watermel on Fruit Ripening by Altering Gene Expression of ABA Centric Phytohormone Signaling. Front. Plant Sci. 2021, 12, 624319. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-Veazie, P. Rootstock Effects on Plant Vigor and Watermelon Fruit Quality. Rep.-Cucurbit Genet. Coop. 2005, 28, 39. [Google Scholar]
- Soteriou, G.A.; Siomos, A.S.; Gerasopoulos, D.; Rouphael, Y.; Georgiadou, S.; Kyriacou, M.C. Biochemical and Histological Contributions to Textural Changes in Watermelon Fruit Modulated by Grafting. Food Chem. 2017, 237, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, M.C.; Soteriou, G. Quality and Postharvest Performance of Watermelon Fruit in Response to Grafting on Interspecific Cucurbit Rootstocks. J. Food Qual. 2015, 38, 21–29. [Google Scholar] [CrossRef]
- Kurum, R.; Çelik, İ.; Eren, A. Effects of Rootstocks on Fruit Yield and Some Quality Traits of Watermelon (Citrullus lanatus). Derim 2017, 34, 91–98. [Google Scholar] [CrossRef]
- Fredes, A.; Roselló, S.; Beltran Arandes, J.; Cebolla-Cornejo, J.; Pérez de Castro, A.; Gisbert, P.; Picó, M.B. Fruit Quality Assessment of Watermelons Grafted onto Citron Melon Rootstock. J. Sci. Food Agric. 2017, 97, 1646–1655. [Google Scholar] [CrossRef]
- Zaaroor-Presman, M.; Alkalai-Tuvia, S.; Chalupowicz, D.; Beniches, M.; Gamliel, A.; Fallik, E. Watermelon Rootstock/Scion Relationships and the Effects of Fruit-Thinning and Stem-Pruning on Yield and Postharvest Fruit Quality. Agriculture 2020, 10, 366. [Google Scholar] [CrossRef]
- Aras, V.; Sari, N.; Solmaz, I. Effects of Cucurbita, Lagenaria and Citrullus rootstocks on pollen and fruit characters, seed yield and quality of F 1 hybrid watermelon. Int. J. Agric. Environ. Food Sci. 2022, 6, 683–693. [Google Scholar] [CrossRef]
- Chen, W.; Zhong, C.; Liao, J.; Bao, W.; Yang, Y.; Yu, W. Effects of Double-rootstock Grafting on Growth and Fruit Quality of Watermelon. China Cucurbits Veg. 2017, 30, 13–16. [Google Scholar]
- Ning, K.; Zhou, W.; Cai, X.; Yan, L.; Ma, Y.; Xie, A.; Wang, Y.; Xu, P. Rootstock–Scion Exchanging mRNAs Participate in Watermelon Fruit Quality Improvement. Int. J. Mol. Sci. 2025, 26, 5121. [Google Scholar] [CrossRef] [PubMed]
- Gölükcü, M.; Tokgöz, H. Variation in Sugar, Organic Acid and Volatile Flavor Compounds of Watermelon (Citrullus lanatus) Grafted on Different Rootstocks at Different Harvest Time. Akad. Gıda 2018, 16, 381–386. [Google Scholar] [CrossRef]
- Liu, J.; Li, J. Grafting Experiment with Different Stocks on Watermelon and Muskmelon. North. Hortic. 2008, 1, 33–35. [Google Scholar]
- Gao, L.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Dou, J.; Liu, W. Comparative Transcriptome Analysis Reveals Key Genes Potentially Related to Soluble Sugar and Organic Acid Accumulation in Watermelon. PLoS ONE 2018, 13, e0190096. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.J.; Bin Safdar, L.; Gebremeskel, H.; Zhao, S.; Yuan, P.; Zhu, H.; Kaseb, M.O.; Anees, M.; Lu, X.; He, N.; et al. Identification of Key Gene Networks Controlling Organic Acid and Sugar Metabolism during Watermelon Fruit Development by Integrating Metabolic Phenotypes and Gene Expression Profiles. Hortic. Res. 2020, 7, 193. [Google Scholar] [CrossRef]
- Li, Y.; Cui, Z.; Hu, L. Recent Technological Strategies for Enhancing the Stability of Lycopene in Processing and Production. Food Chem. 2023, 405, 134799. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.J.; Wiley, E.R.; Brown, E.D.; Clevidence, B.A.; Vinyard, B.T.; Collins, J.K.; Perkins-Veazie, P.; Baker, R.A. Consumption of Watermelon Juice Increases Plasma Concentrations of Lycopene and β-Carotene in Humans. J. Nutr. 2003, 133, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, N.; Zhao, S.; Lu, X. Advances in Watermelon Breeding in China. China Cucurbits Veg. 2016, 29, 1–7. [Google Scholar] [CrossRef]
- Shafe, M.O.; Gumede, N.M.; Nyakudya, T.T.; Chivandi, E. Lycopene: A Potent Antioxidant with Multiple Health Benefits. J. Nutr. Metab. 2024, 2024, 6252426. [Google Scholar] [CrossRef]
- Noreen, S.; Shehzadi, S.; Egbuna, C.; Aja, P.M. Lycopene Alleviates Lipid Dysregulation, Oxidative Stress, and Hypercholesterolemia in Obese Rats Subjected to a High-Fat Diet. Food Sci. Nutr. 2025, 13, e70549. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Das, R.; Ray, A.K.; Mishra, S.K.; Anand, S. Recent Insights on Pharmacological Potential of Lycopene and its Nanoformulations: An Emerging Paradigm towards Improvement of Human Health. Phytochem. Rev. 2025, 24, 1091–1118. [Google Scholar] [CrossRef]
- Perkins Veazie, P.; Olson, S.; Hassell, R.; Schultheis, J.; Miller, G.; Kelly, W. Rootstock of Interspecific Squash Hybrids (Cucarbita maxima x Cucurbita moschata) Increases Lycopene Content of Watermelon. In Proceedings of the HortScience, Orlando, FL, USA, 21–24 July 2008; pp. 1199–1200. [Google Scholar]
- Xu, X.; Bie, Z.; Sun, D.; Deng, Y.; Li, W.; An, G.; Liu, J. Effects of Grafting Combinations on the Plant Growth and Fruit Quality in Watermelon (Citrullus lanatus). China Cucurbits Veg. 2011, 24, 10–14. [Google Scholar] [CrossRef]
- Roberts, W.; Bruton, B.; Fish, W.; Taylor, M. Year Two: Effects of Grafting on Watermelon Yield and Quality. HortScience 2006, 41, 519. [Google Scholar] [CrossRef]
- Alan, O.; Ozdemir, N.; Gunen, Y. Effect of Grafting on Watermelon Plant Growth, Yield and Quality. J. Agron. 2007, 6, 362–365. [Google Scholar] [CrossRef]
- Fallik, E.; Alkalai-Tuvia, S.; Chalupowicz, D.; Popovsky-Sarid, S.; Zaaroor-Presman, M. Relationships between Rootstock-Scion Combinations and Growing Regions on Watermelon Fruit Quality. Agronomy 2019, 9, 536. [Google Scholar] [CrossRef]
- Devi, P.; Perkins-Veazie, P.; Miles, C. Impact of Grafting on Watermelon Fruit Maturity and Quality. Horticulturae 2020, 6, 97. [Google Scholar] [CrossRef]
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting Fruiting Vegetables to Manage Soilborne Pathogens, Foliar Pathogens, Arthropods and Weeds. Sci. Hortic. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Mozafarian Meimandi, M.; Kappel, N. Grafting Plants to Improve Abiotic Stress Tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II: Mechanisms of Adaptation and Stress Amelioration; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 477–490. [Google Scholar]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication Between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Notaguchi, M. The Use of Grafting to Study Systemic Signaling in Plants. Plant Cell Physiol. 2017, 58, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Chachar, M.; Ahmed, N.; Zhang, P.; Chachar, Z.; Geng, Y.; Guo, D.; Chachar, S. Harnessing Epigenetics through Grafting: Revolutionizing Horticultural Crop Production. Horticulturae 2023, 9, 672. [Google Scholar] [CrossRef]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, biochemical, and molecular aspects of grafting in fruit trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef]
- Lal, N.; Ramteke, V.; Diwan, G.; Singh, P.; Sahu, N.; Hota, D.; S.R., M.; Meena, N.K.; Sharma, K.M.; Shiurkar, G.B.; et al. Physiological, Biochemical, and Molecular Responses During Grafting in Horticultural Crops. Plant Mol. Biol. Report. 2025. [Google Scholar] [CrossRef]
- Dong, D.; Shi, Y.-N.; Mou, Z.-M.; Chen, S.-Y.; Zhao, D.-K. Grafting: A potential method to reveal the differential accumulation mechanism of secondary metabolites. Hortic. Res. 2022, 9, uhac050. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, T.A.; Dinh, S.T.; Baldwin, I.T. JA but not JA-Ile is the cell-nonautonomous signal activating JA mediated systemic defenses to herbivory in Nicotiana attenuata. J. Integr. Plant Biol. 2017, 59, 552–571. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.; Dong, X. Inducers of Plant Systemic Acquired Resistance Regulate NPR1 Function through Redox Changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Tonelli, M.L.; Figueredo, M.S.; Rodríguez, J.; Fabra, A.; Ibañez, F. Induced systemic resistance -like responses elicited by rhizobia. Plant Soil 2020, 448, 1–14. [Google Scholar] [CrossRef]
- Kushalappa, A.C.; Yogendra, K.N.; Karre, S. Plant Innate Immune Response: Qualitative and Quantitative Resistance. Crit. Rev. Plant Sci. 2016, 35, 38–55. [Google Scholar] [CrossRef]
- Rabari, A.; Ruparelia, J.; Jha, C.K.; Sayyed, R.Z.; Mitra, D.; Priyadarshini, A.; Senapati, A.; Panneerselvam, P.; Das Mohapatra, P.K. Articulating beneficial rhizobacteria-mediated plant defenses through induced systemic resistance: A review. Pedosphere 2023, 33, 556–566. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Chen, J.; Wang, R.; Zhang, J.; Hou, J.; Liu, T. Insights into the molecular mechanism of Trichoderma stimulating plant growth and immunity against phytopathogens. Physiol. Plant. 2023, 175, e14133. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Xu, Y.; Zhang, G.; Shen, Q.; Zhang, R. Exploring Elicitors of the Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 to Induce Plant Systemic Resistance and Their Interactions With Plant Signaling Pathways. Mol. Plant-Microbe Interact. 2018, 31, 560–567. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Avramidou, E.V.; Abraham, E.M.; Gerakari, M.; Megariti, S.; Doupis, G.; Doulis, A.G. Epigenetic Changes and Transcriptional Reprogramming Upon Woody Plant Grafting for Crop Sustainability in a Changing Environment. Front. Plant Sci. 2021, 11, 613004. [Google Scholar] [CrossRef]
- Cerruti, E.; Gisbert, C.; Drost, H.-G.; Valentino, D.; Portis, E.; Barchi, L.; Prohens, J.; Lanteri, S.; Comino, C.; Catoni, M. Grafting vigour is associated with DNA de-methylation in eggplant. Hortic. Res. 2021, 8, 241. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Su, X.; Xia, Z. Grafting improves drought tolerance by regulating antioxidant enzyme activities and stress-responsive gene expression in tobacco. Environ. Exp. Bot. 2014, 107, 173–179. [Google Scholar] [CrossRef]
- Monteiro, G.C.; Goto, R.; Minatel, I.O.; de Sousa da Silva, E.; da Silva, E.G.; Vianello, F.; Lima, G.P.P. Grafting, Agrochemicals, and Oxidative Enzymes as Factor for Plant Biotic Resistance. In Plant Health Under Biotic Stress: Volume 1: Organic Strategies; Ansari, R.A., Mahmood, I., Eds.; Springer: Singapore, 2019; pp. 37–57. [Google Scholar]
- Liu, Y.; Liu, H.; Zhang, T.; Liu, J.; Sun, X.; Sun, X.; Wang, W.; Zheng, C. Interactions Between Rootstock and Scion during Grafting and Their Molecular Rgulation Mechanism. Sci. Hortic. 2023, 308, 111554. [Google Scholar] [CrossRef]
- Williams, B.; Ahsan, M.U.; Frank, M.H. Getting to the root of grafting-induced traits. Curr. Opin. Plant Biol. 2021, 59, 101988. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to Modify Ion Accumulation in Horticultural Crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Pérez-Alfocea, F.; Galmés, J. The influence of grafting on crops’ photosynthetic performance. Plant Sci. 2020, 295, 110250. [Google Scholar] [CrossRef]
- Coşkun, Ö.F. The Effect of Grafting on Morphological, Physiological and Molecular Changes Induced by Drought Stress in Cucumber. Sustainability 2023, 15, 875. [Google Scholar] [CrossRef]
- Jiao, S.; Zeng, F.; Huang, Y.; Zhang, L.; Mao, J.; Chen, B. Physiological, biochemical and molecular responses associated with drought tolerance in grafted grapevine. BMC Plant Biol. 2023, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Nadoda, N.A.; Barot, D.C.; Baria, V.K.; Chaudhari, V.M. Exploitation of Grafting for Abiotic and Biotic Stress Management in Vegetable Crops: A Review. Adv. Res. Teach. 2024, 25, 125–131. [Google Scholar] [CrossRef]
- Wang, X.; Cao, M.; Li, H.; Liu, Y.; Fan, S.; Zhang, N.; Guo, Y. Strategies and prospects for melatonin to alleviate abiotic stress in horticultural plants. Hortic. Plant J. 2024, 10, 601–614. [Google Scholar] [CrossRef]
- Siddique, A.B.; Parveen, S.; Rahman, M.Z.; Rahman, J. Revisiting plant stress memory: Mechanisms and contribution to stress adaptation. Physiol. Mol. Biol. Plants 2024, 30, 349–367. [Google Scholar] [CrossRef]
- Davoudi, M.; Song, M.; Zhang, M.; Chen, J.; Lou, Q. Long-distance control of the scion by the rootstock under drought stress as revealed by transcriptome sequencing and mobile mRNA identification. Hortic. Res. 2022, 9, uhab033. [Google Scholar] [CrossRef]
- Lin, J. Molecular Mechanisms of Plant Response to Abiotic Stresses and Breeding for Stress Tolerance. Theor. Nat. Sci. 2024, 63, 123–127. [Google Scholar] [CrossRef]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable Grafting From a Molecular Point of View: The Involvement of Epigenetics in Rootstock-Scion Interactions. Front. Plant Sci. 2021, 11, 621999. [Google Scholar] [CrossRef]
- Apostolova, E.L. Molecular Mechanisms Associated with Plant Tolerance upon Abiotic Stress. Plants 2024, 13, 3532. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, C.; Wang, W.; Cao, Z.; Li, S.; Li, H.; Zhu, W.; Huang, Y.; Bao, M.; He, Y.; et al. Comparative transcriptome analysis of different heat stress responses between self-root grafting line and heterogeneous grafting line in rose. Hortic. Plant J. 2021, 7, 243–255. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Xu, J.; Liu, X.; Chi, Y.; Li, R.; Mo, L.; Shi, L.; Liang, S.; Yu, W.; et al. Recent progress of molecular mechanisms of DNA methylation in plant response to abiotic stress. Environ. Exp. Bot. 2024, 218, 105599. [Google Scholar] [CrossRef]
- Fuentes-Merlos, M.I.; Bamba, M.; Sato, S.; Higashitani, A. Self-grafting-induced Epigenetic Changes Leading to Drought Stress Tolerance in Tomato Plants. DNA Res. 2023, 30, dsad016. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Rasheed, Y.; Asif, K.; Ashraf, H.; Maqsood, M.F.; Shahbaz, M.; Zulfiqar, U.; Sardar, R.; Haider, F.U. Plant Biostimulants: Mechanisms and Applications for Enhancing Plant Resilience to Abiotic Stresses. J. Soil Sci. Plant Nutr. 2024, 24, 6641–6690. [Google Scholar] [CrossRef]
- Morcillo, R.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.C.; Torres, L.F.; Kuramae, E.E.; Andrade, S.A.L.d.; Mazzafera, P. Plant Gafting: Maximizing Beneficial Microbe-plant Interactions. Rhizosphere 2024, 29, 100825. [Google Scholar] [CrossRef]
| Type of Stress | Rootstock | Scion | Resistance | References | |
|---|---|---|---|---|---|
| Virus | Cucurbita maxima × C. moschata | RS841, Shintosa Camelforce | Tri-X 313 | Increased yield; fruit firmness increased, but did not affect the soluble solids content. | [13] |
| Cucurbita maxima × C. moschata | RS-841, Ercole | Mielheart | Significantly increased the resistance of scions to MNSV. | [14] | |
| Citrullus lanatus var citroides | Robust | ||||
| Citrullus mucosospermus | 938-16-B | 938-16-B, H1, Hongzi | Resistant to virus disease. | [15] | |
| Citrullus lanatus | H1, Hongzi | Susceptible to viral diseases. | |||
| Fungus | Cucurbita moschata | Landrace | Crimson Tide | The incidence rate was reduced by 88–100%, and even completely controlled. | [16] |
| Cucurbita maxima | Landrace | ||||
| Lagenaria siceraria | Landrace | ||||
| Luffa cylindrica | Landrace | ||||
| Benincasa hispida | Landrace | ||||
| Lagenaria hybrid | 216, Emphasis, Skopje, FR Gold | ||||
| Cucurbita hybrid | P360, Strong Tosa | ||||
| Lagenaria siceraria | USVL482-PMR, USVL351-PMR | Mickey Lee | Significantly enhance the resistance of the scion to powdery mildew. | [7] | |
| Lagenaria siceraria | Chaofengkangshengwang | Sumi 1 | High resistance to Fusarium wilt, and the incidence was only 3.4%. | [6] | |
| Cucurbita maxima × C. moschata | Tetsukabuto | Secretariat | Resistance to verticillium wilt. | [17] | |
| Citrullus lanatus var. citroides | IIHR-82, IIHR-617, BIL-53 | NS-295 | Resistance to gummy stem blight. | [18] | |
| Lagenaria siceraria | BG-95, BG-77-6-1 | ||||
| Citrullus lanatus var. citroides | IIHR-617 × Arka Manik, IIHR-82 × Arka Manik, IIHR-82 × IIHR-617 | ||||
| Cucurbita maxima × C. moschata | Nun 6001, Strongtosa, Tetsukabuto, Ferro, Shintoza | Aswan F1 | Enhance disease resistance. | [19] | |
| Lagenaria siceraria | IT207112 | Sambokkul | Resistance to Fusarium wilt, single spore root rot, and vine recession. | [20] | |
| Lagenaria siceraria | FRD22 | Moderate resistance to single spore root rot and vine decline disease. | |||
| Nematodes | Lagenaria siceraria | Emphasis, WMXP 3938, WMXP 3944, WMXP3445 | Fiesta, Tri-X 313 | Significantly reduce the root knot rate and infection level; increase production. | [21] |
| Cucurbita moschata × C. maxima | Strong Tosa | Significant nematode resistance in field trials. | |||
| Citrullus lanatus var. citroides | RKVL 301, RKVL 302, RKVL 303, RKVL 315, RKVL 318, Ojakkyo | Significant resistance; root knot index and nematode reproduction were low. | |||
| Cucumis africanus, Cucumis myriocarpus | Congo, Charleston Gray | Resistance to root-knot nematodes. | [22] | ||
| Type of Stress | Rootstock | Scion | Resistance | References | |
|---|---|---|---|---|---|
| Drought stress | Lagenaria siceraria | Illapel, Osorno, GC | Santa Amelia | Drought tolerance, significantly increased yield; improve root structure. | [10] |
| BG-48, Philippines | Not drought-tolerant. | ||||
| Cucurbita maxima × C. moschata | Shintoza | Crimson Sweet | Better growth performance and water status. | [41] | |
| Citrullus colocynthis (L.) Schrad | Esfahan | Better drought resistance, growth and biomass decreased less, and showed higher antioxidant activity and lower oxidative stress. | |||
| Cucurbita moschata | Naihan 1 | Up-regulation of ClTCP4 gene expression in scions helped to maintain higher photosynthetic efficiency and cell membrane stability. | [42] | ||
| Lagenaria siceraria | Jingxinzhen 1 | Zaojia 8424 | Better growth performance. | [43] | |
| Cucurbita maxima × C. moschata | Qingyanzhen 1 | ||||
| Citrullus lanatus subsp. mucosospermus | Crimson Sweet | Improve the ability to resist water stress, improve growth and yield. | [38] | ||
| Cucurbita maxima × C. moschata | Strong Tosa | Crimson Tide F1 | Enhance drought tolerance. | [44] | |
| Citrullus lanatus var. citroides | Crimson Tide | Enhance drought tolerance and affect physiological characteristics and nutrient uptake. | [45] | ||
| Cucurbita maxima × C. moschata | TZ-148 | ||||
| Citrullus lanatus var. citroides | A1, A2 | Crimson Tide | Enhance drought tolerance. | [46] | |
| Cucurbita maxima × C. moschata | TZ-148 | Enhance drought resistance and improve fruit quality. | |||
| Temperature stress | Lagenaria siceraria | FR79 | Sambokkul | Tolerance to low temperature, little effect on fruit quality. | [20] |
| Lagenaria siceraria | 0526, 2505 | Zaojia 8424 | Enhance cold resistance. | [47] | |
| Cucurbita maxima × C. moschata | Qingyan No. 1 | 97103 | Enhance cold resistance. | [48,49] | |
| Cucurbita moschata | Weizhen No. 1 | Nongkeda No. 5 | Enhance cold resistance. | [50] | |
| Cucurbita ficifolia Bouché | Cf | ||||
| Salt stress | Lagenaria siceraria | Chaofeng Kangshengwang | Xiuli | Enhance salt tolerance. | [51,52,53,54] |
| Cucurbita maxima | Cma | Crimson Tide | Enhance salt tolerance. | [55] | |
| Lagenaria siceraria | Skp, Birecik | ||||
| Citrullus lanatus | Jingxin No. 2 | Jingxin No. 2 | General salt tolerance. | [56] | |
| Cucurbita moschata | Quanneng Tiejia | General salt tolerance. | |||
| Kaijia No. 1 | High salt tolerance. | ||||
| Lagenaria siceraria | Hanzhen No. 3 | High salt tolerance. | |||
| Citrullus lanatus | Zhongyu No. 9 tetraploid | Zhongyu No. 9 | Enhance salt tolerance. | [57] | |
| Lagenaria siceraria | C. lanatus | Enhance salt tolerance. | [58,59,60] | ||
| Cucurbita maxima × C. moschata | Shintosa F-90 | C. lanatus | Enhance salt tolerance. | [58] | |
| Rootstock | Scion | Changes in Quality After Grafting | References | |
|---|---|---|---|---|
| Lagenaria siceraria | Yongzhen No. 1, Yongzhen No. 3, Yongzhen No. 8 | Zaojia 8424 | Increase SSC content. | [80] |
| Cucurbita maxima × C. moschata | Yongzhen No. 7 | Zaojia 8424 | Increase fruit weight (SSC content did not change). | [81] |
| Cucurbita maxima × C. moschata | TZ148 | Pegasus | The flesh firmness, color and other physical qualities were improved, and the contents of bioactive compounds such as lycopene and citrulline were increased, but the acidity was slightly increased. | [82] |
| Cucurbita maxima × C. moschata | Super Shintosa | Melody | Increase the lycopene content. | [83] |
| Cucurbita moschata | Marvel | Increase fruit firmness. | ||
| Cucurbita maxima × C. moschata | Root Power | |||
| Lagenaria siceraria | Macis | Crimson Sweet | Increased the size and rind thickness of fruits. | [84] |
| Cucurbita moschata | SiZhuang | 8424 | Improve quality, increase beneficial metabolites, and reduce bitter compounds. | [85] |
| Cucurbita moschata | Xi Jia Qiang Sheng | Zhongyu No. 1 | Increase the total sugar, total amino acid and total acid content. | [86] |
| Lagenaria siceraria | FR STRONG | RX 467 | Reduce the lycopene content. | [87] |
| Cucurbita maxima × C. moschata | RS 841 | Increase the lycopene content. | ||
| Lagenaria siceraria | Jingxinzhen No. 1 | Zaojia | Increase the lycopene content. | [88] |
| Citrullus lanatus var. citroides | Yongshi | |||
| Cucurbita maxima × C. moschata | Qingyanzhen No. 1 | No effect on lycopene content. | ||
| Cucurbita maxima × C. moschata | Ferro, Nun 6001, Shintoza | Aswan | Increase the lycopene content. | [19] |
| Cucurbita maxima × C. moschata | TZ148 | Pegasus | Increase fruit firmness and citrulline content. | [89] |
| Cucurbita maxima × C. moschata | Jingxinzhen No. 2 | 97103 | Maturity extended. | [90] |
| Cucurbita argyrosperma | 451 | Summer Flavor 800, Summer Sweet 5244 | Reduce fruit weight, lycopene content (diploid). | [91] |
| Cucurbita maxima × C. moschata | N101 | Pegasus | Increase fruit firmness | [92] |
| Cucurbita maxima × C. moschata | TZ148, Bombo, N101 | Celebration, Gallery, Pegasus, Torpilla | Increase fruit firmness, lycopene content; SSC content decreased slightly. | [93] |
| Cucurbita maxima × C. moschata | Ferro RZ, Nun 9075 | Crimson Tide | Increase SSC content, peel thickness, fruit firmness. | [94] |
| RS 841, Strong Tosa | Increase lycopene content, peel thickness, fruit firmness. | |||
| Citrullus lanatus var. citroides | BGV0005167 | Oneida | Increase fruit thickness, flesh firmness, SSC content. | [95] |
| Cucurbita maxima VAV 1860 × C. moschata PI 550689 | GMM1 | Increase fruit thickness and flesh firmness; change the fruit aroma. | ||
| Cobalt | ||||
| Cucurbita maxima × C. moschata | TZ-148 | 1262 | Improve fruit taste. | [96] |
| Nurit | Improve fruit taste, increase lycopene and SSC content. | |||
| Lagenaria siceraria | A3 | Crimson Tide | Increase the sugar content of fruit. | [46] |
| Lagenaria spp. | Argentario, 3335 | 187 × 125, 11 × 162 | Increase the SSC content, fruit diameter, peel thickness and fruit weight. | [97] |
| Cucurbita maxima × C. moschata | TZ148, Nun9075 | Increase fruit weight. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; La, S.; Chen, C.; Shi, A.; Wang, M.; Zhang, Y.; Guo, J.; Dong, L. Research Progress on the Effect of Grafting Technology on Disease Resistance and Stress Resistance of Watermelon. Horticulturae 2025, 11, 1271. https://doi.org/10.3390/horticulturae11101271
Liu X, La S, Chen C, Shi A, Wang M, Zhang Y, Guo J, Dong L. Research Progress on the Effect of Grafting Technology on Disease Resistance and Stress Resistance of Watermelon. Horticulturae. 2025; 11(10):1271. https://doi.org/10.3390/horticulturae11101271
Chicago/Turabian StyleLiu, Xuena, Shikai La, Chang Chen, Ainong Shi, Mingjiao Wang, Yingying Zhang, Jinghua Guo, and Lingdi Dong. 2025. "Research Progress on the Effect of Grafting Technology on Disease Resistance and Stress Resistance of Watermelon" Horticulturae 11, no. 10: 1271. https://doi.org/10.3390/horticulturae11101271
APA StyleLiu, X., La, S., Chen, C., Shi, A., Wang, M., Zhang, Y., Guo, J., & Dong, L. (2025). Research Progress on the Effect of Grafting Technology on Disease Resistance and Stress Resistance of Watermelon. Horticulturae, 11(10), 1271. https://doi.org/10.3390/horticulturae11101271







