Induction and Transformation of Friable Callus in Chrysanthemum ‘Jimba’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Gene Expression Vector and Bacterial Strain
2.3. Basic Medium Formulation and Culture Conditions
2.4. Screening of Friable Callus Induction Medium
2.5. Observation of Callus Cells Under a Microscope
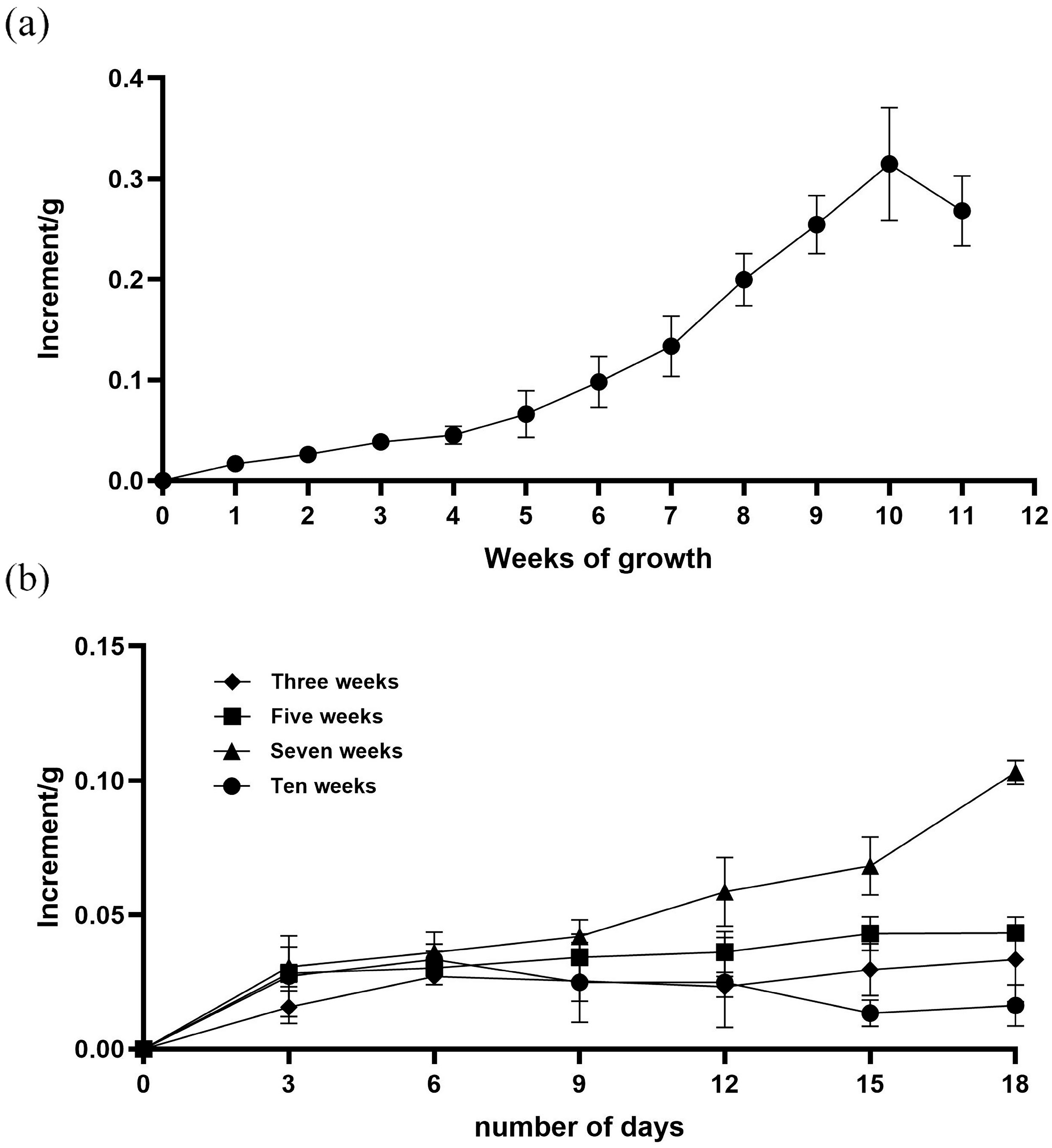
2.6. Growth Curve Plotting for Proliferation of Friable Callus
2.7. Screening of Subculture Time for Friable Callus
2.8. Culture of Agrobacterium Colonies and Preparation of Agrobacterium Infection Solution
2.9. Screening of Agrobacterium Infection Concentration and Duration
2.10. Total Anthocyanin Content Measurement
2.11. RNA Extraction and RT-qPCR
2.12. Data Statistics and Calculation Formulas
3. Results
3.1. Optimal PGR Combination for Friable Callus Induction in Chrysanthemum ‘Jimba’ Is 1.0 mg/L 6-BA + 0.4–0.6 mg/L NAA
3.2. Type I Calli Exhibit the Highest Nuclear-to-Cytoplasmic Ratio and Most Friable Morphology
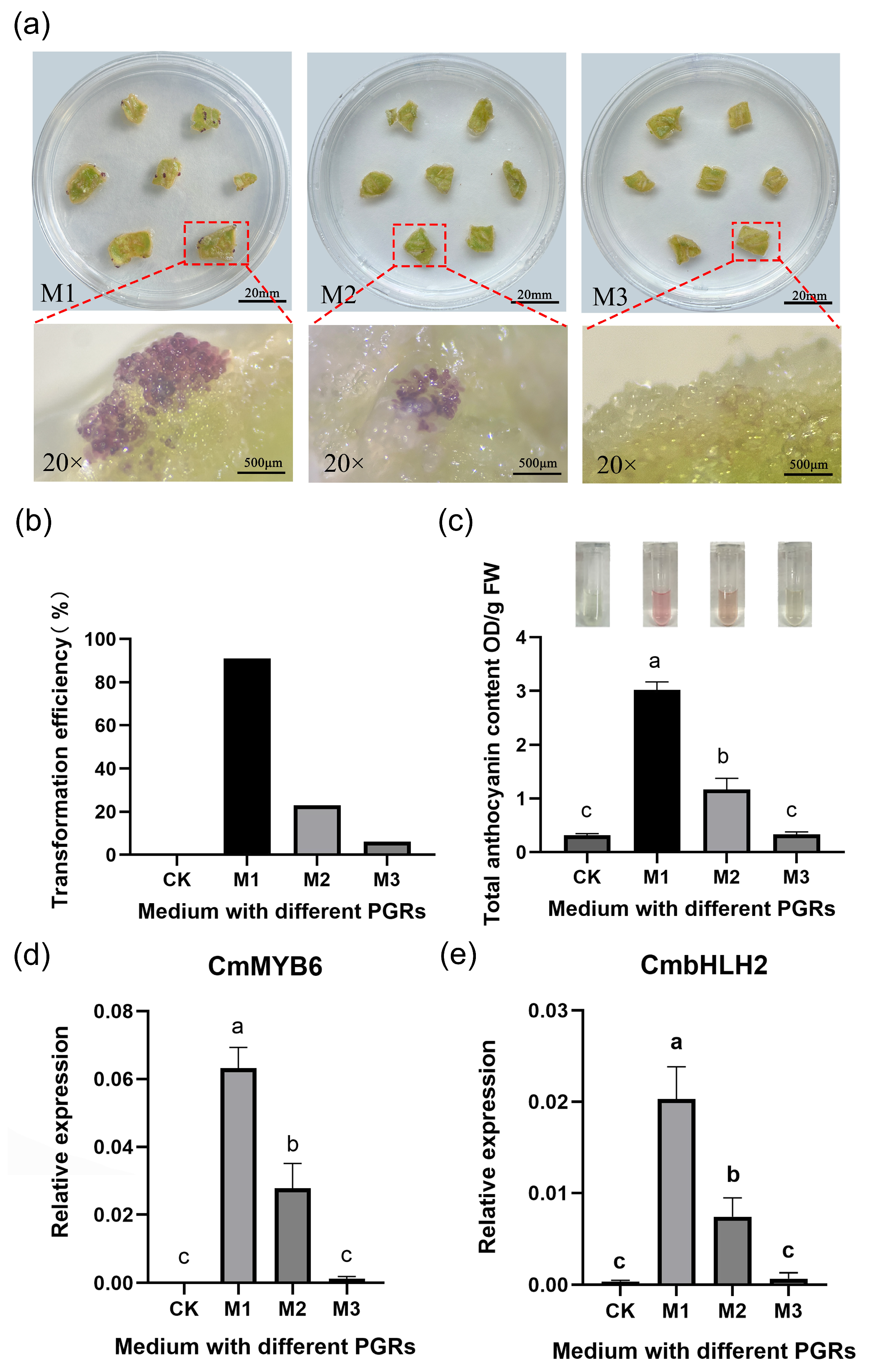
3.3. Comparative Analysis of Transformation Efficiency in Callus Induced by Different PGR Combinations
3.4. The 7th Week Is the Optimal Subculture Time for Friable Callus
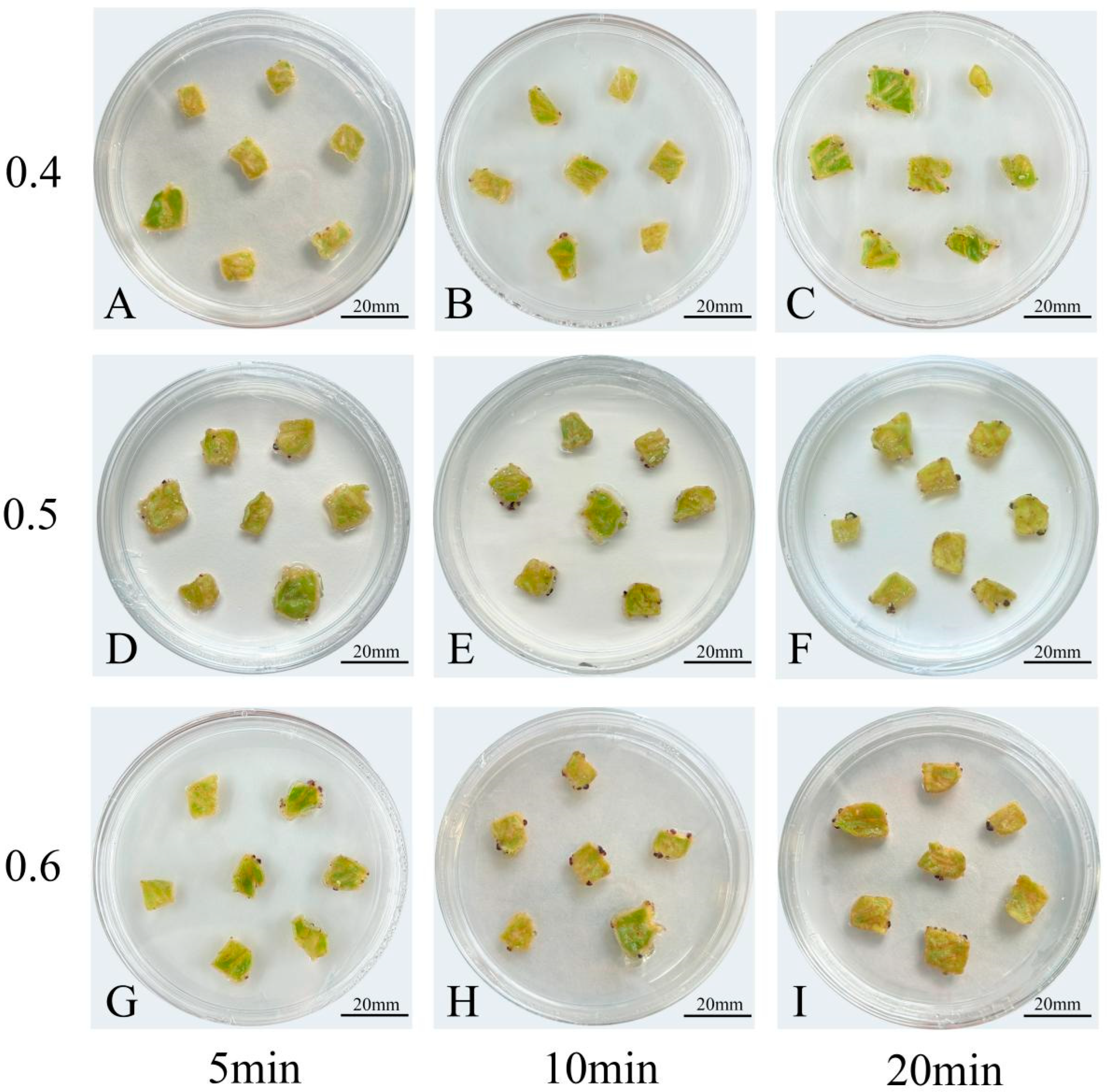
3.5. Optimal Agrobacterium Infection Conditions for Chrysanthemum Callus Were OD600 0.5 and 10-Min Infection
4. Discussion
4.1. The Optimal PRGs Combination for Inducing Friable Callus in Chrysanthemum ‘Jimba’ Is 6-BA + NAA
4.2. Effect of Subculture Time on the Proliferation Rate of Friable Callus in Chrysanthemum ‘Jimba’
4.3. Effects of Infection Concentration and Duration on the Growth and Proliferation of Friable Callus in Chrysanthemum ‘Jimba’
4.4. Comparison of Transformation Efficiency Among Callus Induced by Different PGRs
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Yan, S.; An, P.; Cao, Q.; Wang, C.; Wang, J.; Zhang, H.; Zhang, L. Embryogenic callus induction from immature zygotic embryos and genetic transformation of Larix kaempferi 3×Larix gmelinii 9. PLoS ONE 2021, 16, e258654. [Google Scholar] [CrossRef]
- Zhang, A.J.; Jiang, X.N.; Gai, Y. Optimization of genetic transformation method for embryogenic callus of Larix kaempferi. Mol. Plant Breed. 2024, 1–13. Available online: http://kns.cnki.net/kcms/detail/46.1068.S.20220606.1454.004.html (accessed on 30 September 2025).
- Armstrong, C.L.; Green, C.E. Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 1985, 164, 207–214. [Google Scholar] [CrossRef]
- Xu, S.R.; Zhao, W.Z.; Gong, X.Y.; Li, L.; Xiao, W. Resistance analysis of apple B-type cytokinin response factor MdARR11 to drought stress. J. Nucl. Agric. Sci. 2024, 38, 226–234. [Google Scholar]
- Zhang, K.C.; Li, X.Y.; Zhang, Y.G.; Zhang, D.Y.; Wang, Y.C. Cloning and Expression Analysis of MsCBF1 Gene from Malus sieversii (Ldb.) Roem. Plant Physiol. J. 2018, 54, 803–811. [Google Scholar]
- Wang, Y.; Yuan, J.; Wei, X.; Chen, Y.; Chen, Q.; Ge, X. GhLBDs Promote Callus Initiation and Act as Selectable Markers to Increase Transformation Efficiency. Front. Plant Sci. 2022, 13, 861706. [Google Scholar] [CrossRef]
- Xue, Y.; Shan, Y.; Yao, J.L.; Wang, R.; Xu, S.; Liu, D.; Ye, Z.; Lin, J.; Li, X.; Xue, C.; et al. The transcription factor PbrMYB24 regulates lignin and cellulose biosynthesis in stone cells of pear fruits. Plant Physiol. 2023, 192, 1997–2014. [Google Scholar] [CrossRef]
- Cordeiro, D.; Alves, A.; Ferraz, R.; Casimiro, B.; Canhoto, J.; Correia, S. An efficient Agrobacterium-mediated genetic transformation method for Solanum betaceum Cav. embryogenic callus. Plants 2023, 12, 1202. [Google Scholar] [CrossRef]
- Li, X.L.; Ding, N.; Jia, M.R.; Wei, L.Z.; Jiang, J.Z.; Li, B.B.; Jia, W.S. Establishment of gene transformation system in fruit callus and its application in gene functional analysis for apple plant. J. China Agric. Univ. 2015, 20, 108–113. [Google Scholar]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef]
- Yi, Y.H.; Xu, D.F.; Dong, H.Y.; Liu, Y.J. Induction of loose embryogenic callus in melon (Cucumis melo L.). Seed 2023, 42, 144–150. [Google Scholar]
- Ramírez-Mosqueda, M.A. Overview of Somatic Embryogenesis. Somat. Embryog. Methods Protoc. 2022, 2527, 1–8. [Google Scholar]
- Tokuji, Y.; Kuriyama, K. Involvement of gibberellin and cytokinin in the formation of embryogenic cell clumps in carrot (Daucus carota). J. Plant Physiol. 2003, 160, 133–141. [Google Scholar] [CrossRef]
- Yu, Q.Y.; Guo, M.M.; Xu, K.X.; Liu, J.B.; Wang, C.; Zheng, J.; Zhang, K.Z.; Zhang, Y. Callus induction and suspension culture of inflorescence and rachis from Syringa vulgaris ‘Downfield’. J. Northeast. For. Univ. 2023, 51, 47–53. [Google Scholar]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Thidiazuron-induced somatic embryogenesis and changes of antioxidant properties in tissue cultures of half-high blueberry plants. Sci. Rep. 2018, 8, 16978. [Google Scholar]
- Yi, X. Basic Research on Embryogenic Callus Induction and Genetic Transformation of Rosa chinensis ‘Yueyuehong’. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2014. [Google Scholar]
- Wang, L.; Li, Y.; Dai, W.N.; Yan, J.; Zhang, C.H. Establishment and optimization of grape cell suspension culture system. Biotechnol. Bull. 2018, 34, 80–86. [Google Scholar]
- Cheng, J.H.; Ren, Q.; Li, B.H.; Xun, H.F.; Ding, Z.E. Optimization of loose callus induction conditions for Punica granatum L. Nonwood For. Res. 2009, 27, 75–78. [Google Scholar]
- Wang, J.; Zhang, J.; Ji, Q.L.; Yao, Y.C. Induction and proliferation of callus in ornamental (Malus spp.). J. Beijing Univ. Agric. 2016, 31, 95–101. [Google Scholar]
- Wu, L.F.; Wei, X.M.; Ying, Q.Q.; Yang, C.R.; Ye, M. Analysis of callus induction quality from Sophora davidii leaves with different growth regulators. North. Hortic. 2018, 10, 63–69. [Google Scholar]
- Basnayake, S.W.; Moyle, R.; Birch, R.G. Embryogenic callus proliferation and regeneration conditions for genetic transformation of diverse sugarcane cultivars. Plant Cell Rep. 2011, 30, 439–448. [Google Scholar] [PubMed]
- Yang, S.S.; Li, S.Z.; Zhang, J.B.; Li, J.C. In vitro induction culture and browning inhibition of Taxus media Rehd., an anticancer plant. Chin. J. Biotechnol. 2024, 40, 3823–3832. [Google Scholar] [CrossRef]
- Mahood, H.E.; Sarropoulou, V.; Tzatzani, T.T. Effect of explant type (leaf, stem) and 2,4-D concentration on callus induction: Influence of elicitor type (biotic, abiotic), elicitor concentration and elicitation time on biomass growth rate and costunolide biosynthesis in gazania (Gazania rigens) cell suspension cultures. Bioresour. Bioprocess. 2022, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, D.; Long, X.; Hao, Z.; Lu, Y.; Zhou, Y.; Peng, Y.; Cheng, T.; Shi, J.; Chen, J. Agrobacterium-mediated genetic transformation of embryogenic callus in a Liriodendron hybrid (L. chinense × L. tulipifera). Front. Plant Sci. 2022, 13, 802128. [Google Scholar] [CrossRef]
- Teng, R.P.; Zhang, J.Q.; Liu, X.F.; Yu, L.; Zhang, C.; Li, F. Optimization of tissue culture regeneration system for chrysanthemum ‘Jimba’. Mol. Plant Breed. 2025, 23, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, C. Optimization of Genetic Transformation System and Preliminary Establishment of ‘Jimba’ Mutant Library Using Gene Editing Technology in Chrysanthemum. Master’s Thesis, Shandong Agricultural University, Taian, China, 2021. [Google Scholar]
- Xiang, L.; Liu, X.; Li, H.; Yin, X.; Grierson, D.; Li, F.; Chen, K. CmMYB#7, an R3 MYB transcription factor, acts as a negative regulator of anthocyanin biosynthesis in chrysanthemum. J. Exp. Bot. 2019, 70, 3111–3123. [Google Scholar] [CrossRef]
- Madke, S.S.; Cherian, J.K.; Badere, S.R. A modified Murashige and Skoog media for efficient multiple shoot induction in G. arborea Roxb. J. For. Res. 2014, 25, 557–564. [Google Scholar] [CrossRef]
- Xue, Y. Study on Induction of Loose Embryogenic Callus in Lycium barbarum L. from Ningxia. Master’s Thesis, Tianjin Agricultural University, Tianjin, China, 2023. [Google Scholar]
- Nakatsuka, A.; Mizuta, D.; Kii, Y.; Miyajima, I.; Kobayashi, N. Isolation and expression analysis of flavonoid biosynthesis genes in evergreen azalea. Sci. Hortic. 2008, 118, 314–320. [Google Scholar] [CrossRef]
- Xiang, L.L.; Liu, X.F.; Li, X.; Yin, X.R.; Grierson, D.; Li, F.; Chen, K.S. A Novel bHLH Transcription Factor Involved in Regulating Anthocyanin Biosynthesis in Chrysanthemums (Chrysanthemum morifolium Ramat). PLoS ONE 2015, 10, e0143892. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.S. Key Factors and Mechanism of Loose Callus Formation in Pinellia ternata. Master’s Thesis, Guizhou University, Guiyang, China, 2018. [Google Scholar]
- Tao, X.K.; Gao, G.Z.; Zhang, X.T.; Wang, H.C. Studies on Induction and Proliferation of Loose Callus in Pinellia ternate. J. Shanxi Datong Univ. 2020, 36, 77–80. [Google Scholar]
- Liu, J. Establishment of Chrysanthemum Regeneration System and Agrobacterium-Mediated Transformation of Green Fluorescent Protein (GFP) Gene. Master’s Thesis, Shandong Agricultural University, Taian, China, 2004. [Google Scholar]
- Wang, S. Improvement of Regeneration Efficiency During Chrysanthemum Transformation and Study on SVP Transformation of ‘Jinba’. Master’s Thesis, Shandong Agricultural University, Taian, China, 2020. [Google Scholar]
- An, Y. Establishment of a Genetic Transformation System for Embryogenic Callus of Fraxinus mandshurica. Master’s Thesis, Northeast Forestry University, Harbin, China, 2023. [Google Scholar]
- Gill, R.; Saxena, P.K. Somatic embryogenesis in Nicotiana tabacum L.: Induction by thidiazuron of direct embryo differentiation from cultured leaf discs. Plant Cell Rep. 1993, 12, 154–159. [Google Scholar] [CrossRef]
- Murthy, B.N.S.; Saxena, P.K. Somatic embryogenesis and plant regeneration of neem (Azadirachta indica A. Juss.). Plant Cell Rep. 1998, 17, 469–475. [Google Scholar] [CrossRef]
- Cai, Y.F.; Tang, L.T.; Chen, H.X.; Li, Y.F.; Liu, R.; Chen, J.R. Somatic embryogenesis in Rosa chinensis cv. ‘Old Blush’. Plant Cell Tissue Organ Cult. 2022, 149, 645–656. [Google Scholar] [CrossRef]
- Jin, H.J.; Zhang, J.; Shen, X.F.; Xiao, C.H.; Zhang, Y.P. Induction of callus and analysis of physiological and biochemical characteristics in Gentiana rhodantha Franch. Mol. Plant Breed. 2021, 19, 5482–5488. [Google Scholar]
- Chen, Y. Response of ‘Qiuhong’ Hemerocallis to Low Temperature Stress and Isolation of Pathogenic Bacteria Causing Leaf Blight and Screening of Fungicides. Master’s Thesis, Shanghai Institute of Technology, Shanghai, China, 2023. [Google Scholar]
- Wei, R. Construction of Callus Genetic Transformation System for Juglans sigillata and Genetic Transformation of JsFLS5. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar]
- Wei, Q.; Li, C.; Yang, Y.J.; Xu, Y.J.; Gao, J.P.; Hong, B. Isolation and homologous genetic transformation of DREB1A gene from chrysanthemum ‘Jinba’. Plant Physiol. J. 2011, 47, 153–159. [Google Scholar]
- He, S. Agrobacterium-Mediated Genetic Transformation of Chrysanthemum with CmWRKY15-1 Gene. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Bi, M.M. Functional Study of Chrysanthemum White Rust Resistance-RELATED Gene CmWRKY15-1. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar]
- Fan, X.X. Construction of RNAi Vector for Chrysanthemum CmMET1 Gene and Its Genetic Transformation in Chrysanthemum and Artemisia annua. Master’s Thesis, Henan University, Kaifeng, China, 2016. [Google Scholar]

| Serial Number | Plant Growth Regulators (mg/L) | |||
|---|---|---|---|---|
| 6-BA | NAA | 2,4-D | TDZ | |
| Ck | 0 | 0 | 0 | 0 |
| Y1 | 1 | 0.2 | 0 | 0 |
| Y2 | 1 | 0.4 | 0 | 0 |
| Y3 | 1 | 0.6 | 0 | 0 |
| Y4 | 1 | 0.8 | 0 | 0 |
| Y5 | 1 | 0 | 0.2 | 0 |
| Y6 | 1 | 0 | 0.4 | 0 |
| Y7 | 1 | 0 | 0.6 | 0 |
| Y8 | 1 | 0 | 0.8 | 0 |
| Y9 | 0 | 0 | 0.2 | 0.2 |
| Y10 | 0 | 0 | 0.2 | 0.5 |
| Y11 | 0 | 0 | 0.2 | 0.8 |
| Y12 | 0 | 0 | 0.2 | 1.0 |
| Y13 | 0 | 0 | 0.5 | 0.2 |
| Y14 | 0 | 0 | 0.5 | 0.5 |
| Y15 | 0 | 0 | 0.5 | 0.8 |
| Y16 | 0 | 0 | 0.5 | 1.0 |
| Y17 | 0 | 0 | 1.0 | 0.2 |
| Y18 | 0 | 0 | 1.0 | 0.5 |
| Y19 | 0 | 0 | 1.0 | 0.8 |
| Y20 | 0 | 0 | 1.0 | 1.0 |
| Y21 | 0 | 0 | 1.5 | 0.2 |
| Y22 | 0 | 0 | 1.5 | 0.5 |
| Y23 | 0 | 0 | 1.5 | 0.8 |
| Y24 | 0 | 0 | 1.5 | 1.0 |
| Purpose | Primer Name | Sequence (5′–3′) |
|---|---|---|
| RT-qPCR | CmActin | F: CACCCCCAGAGAGAAAATAC R: ATCTGTTGGAAGGTGCTGAG |
| CmMYB6 | F: ATGGGGGAGTACAGAAAAATG R: TCATAGTTGGTCCGAATTTA | |
| CmbHLH7 | F: GGCTGCCAGCGGACCACCTCG R: GTAGTATCCATCTCCCCATACC |
| Serial Number | Browning Rate (%) | Proliferation Ratio | Callus Induction Time/d | Callus Growth Status | Callus Type |
|---|---|---|---|---|---|
| CK | 42 | 1.91 | 15 | Compact structure, soft texture, and white translucent water-stained appearance | IV |
| Y1 | 0 | 4.28 | 8 | Compact structure, soft texture, yellow-green coloration, with occasional adventitious bud formation | II |
| Y2 | 0 | 5.45 | 7 | Loose structure, relatively soft texture, green coloration | I |
| Y3 | 0 | 5.40 | 7 | Loose structure, soft texture, green coloration | I |
| Y4 | 4 | 5.16 | 7 | Loose structure, soft texture, yellow-green coloration, with occasional adventitious bud formation | I |
| Y5 | 16 | 3.24 | 10 | Compact structure, firm texture, dark green coloration, with adventitious bud formation | III |
| Y6 | 10 | 4.00 | 9 | Compact structure, firm texture, dark green coloration, with adventitious bud formation | III |
| Y7 | 12 | 4.32 | 9 | Compact structure, soft texture, green coloration, with occasional adventitious bud formation | II |
| Y8 | 7 | 4.28 | 9 | Compact structure, soft texture, green coloration, with occasional adventitious bud formation | II |
| Y9 | 0 | 3.01 | 9 | Compact structure, firm texture, yellow-green coloration | III |
| Y10 | 0 | 4.89 | 7 | Loose structure, soft texture, yellowish-white coloration | II |
| Y11 | 0 | 3.63 | 10 | Compact structure, firm texture, yellowish-white coloration | III |
| Y12 | 0 | 2.88 | 10 | Compact structure, soft texture, yellowish-white coloration | IV |
| Y13 | 0 | 3.95 | 7 | Loose structure, firm texture, yellow-green coloration | III |
| Y14 | 0 | 4.30 | 7 | Loose structure, soft texture, yellow-green coloration | II |
| Y15 | 0 | 3.24 | 9 | Compact structure, soft texture, yellowish-white coloration | IV |
| Y16 | 0 | 2.33 | 10 | Compact structure, soft texture, yellowish-white coloration | IV |
| Y17 | 0 | 4.00 | 9 | Compact structure, firm texture, dark green coloration | III |
| Y18 | 0 | 3.46 | 9 | Compact structure, firm texture, yellow-green coloration | III |
| Y19 | 0 | 2.11 | 10 | Compact structure, soft texture, yellowish-white translucent water-stained appearance | IV |
| Y20 | 0 | 2.15 | 10 | Loose structure, soft texture, white translucent water-stained appearance | IV |
| Y21 | 0 | 2.39 | 10 | Loose structure, soft texture, yellowish-white coloration | IV |
| Y22 | 0 | 2.23 | 9 | Compact structure, soft texture, yellow translucent water-stained appearance | IV |
| Y23 | 0 | 2.55 | 9 | Compact structure, soft texture, yellowish-white translucent water-stained appearance | IV |
| Y24 | 0 | 2.09 | 10 | Compact structure, soft texture, and white translucent water-stained appearance | IV |
| Callus Type | Nuclear-to-Cytoplasmic Ratio (%) | Microscopic Cell Morphology |
|---|---|---|
| Type I | 21.07 ± 0.07 a | Small, regularly shaped cells with larger nuclei and dense cytoplasm |
| Type II | 8.79 ± 0.23 b | Irregularly shaped cells with thinner cytoplasm and smaller nuclei |
| Type III | 7.74 ± 0.04 c | Irregularly shaped cells with smaller nuclei |
| Type IV | 5.44 ± 0.15 d | Irregularly shaped cells; some lacked nuclei, with thinner cytoplasm and smaller nuclei |
| Agrobacterium Concentration /OD600 | Infection Duration /min | Agrobacterium Contamination Rate (%) | Transformation Efficiency (%) | Total Anthocyanin Content (OD/g FW) |
|---|---|---|---|---|
| 0.4 | 5 | 0 | 72 | 0.31 ± 0.14 d |
| 0.4 | 10 | 0 | 82 | 2.16 ± 0.10 c |
| 0.4 | 20 | 7 | 89 | 2.46 ± 0.46 abc |
| 0.5 | 5 | 0 | 75 | 2.23 ± 0.22 bc |
| 0.5 | 10 | 0 | 91 | 3.25 ± 0.65 ab |
| 0.5 | 20 | 11 | 85 | 3.43 ± 0.27 a |
| 0.6 | 5 | 9 | 75 | 2.82 ± 0.19 abc |
| 0.6 | 10 | 20 | 72 | 2.86 ± 0.24 abc |
| 0.6 | 20 | 33 | 54 | 1.96 ± 0.18 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, R.; Deng, Q.; Wang, J.; Li, Y.; Xu, L.; Tang, G.; Li, W.; Yu, X.; Xiang, L. Induction and Transformation of Friable Callus in Chrysanthemum ‘Jimba’. Horticulturae 2025, 11, 1267. https://doi.org/10.3390/horticulturae11101267
Fu R, Deng Q, Wang J, Li Y, Xu L, Tang G, Li W, Yu X, Xiang L. Induction and Transformation of Friable Callus in Chrysanthemum ‘Jimba’. Horticulturae. 2025; 11(10):1267. https://doi.org/10.3390/horticulturae11101267
Chicago/Turabian StyleFu, Ruoni, Qiwei Deng, Jishu Wang, Yanlin Li, Lu Xu, Guimei Tang, Weidong Li, Xiaoying Yu, and Lili Xiang. 2025. "Induction and Transformation of Friable Callus in Chrysanthemum ‘Jimba’" Horticulturae 11, no. 10: 1267. https://doi.org/10.3390/horticulturae11101267
APA StyleFu, R., Deng, Q., Wang, J., Li, Y., Xu, L., Tang, G., Li, W., Yu, X., & Xiang, L. (2025). Induction and Transformation of Friable Callus in Chrysanthemum ‘Jimba’. Horticulturae, 11(10), 1267. https://doi.org/10.3390/horticulturae11101267






