Determination of the Resistance of Tolerant Hybrids of Buxus to the Pathogen Cylindrocladium buxicola and the Effect of Nutrition and Climatic Conditions on Leaf Color
Abstract
1. Introduction
2. Materials and Methods
2.1. Screening
2.2. Molecular Analysis
2.3. Plant Material
2.4. Laboratory Testing
2.5. Open Field Testing
2.6. Pathogen Evaluation
2.7. Winter Coloring
2.8. Statistical Analysis
3. Results
3.1. Screening
3.2. Laboratory Tests
3.3. Open Field Tests
3.4. Winter Coloring
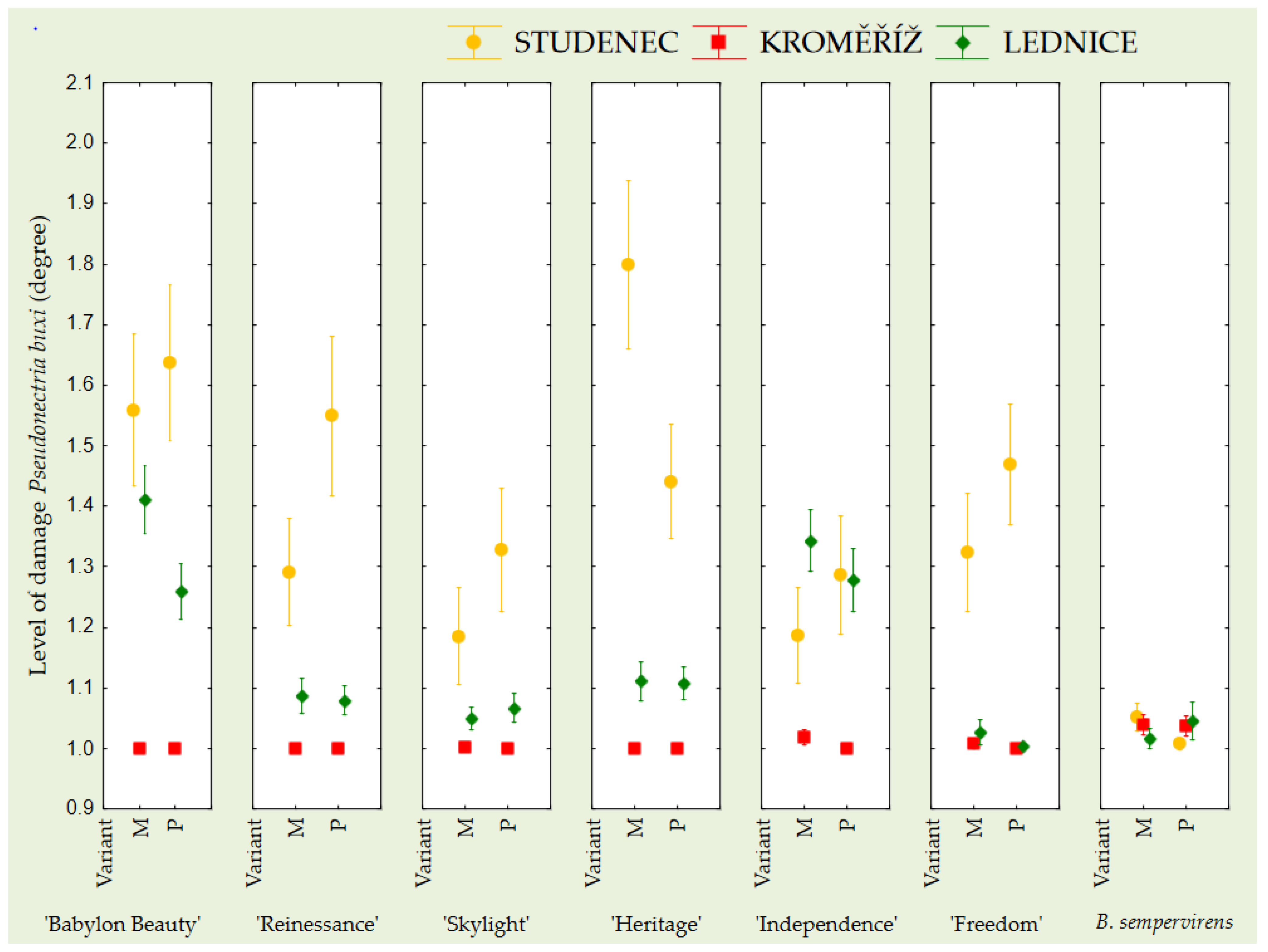
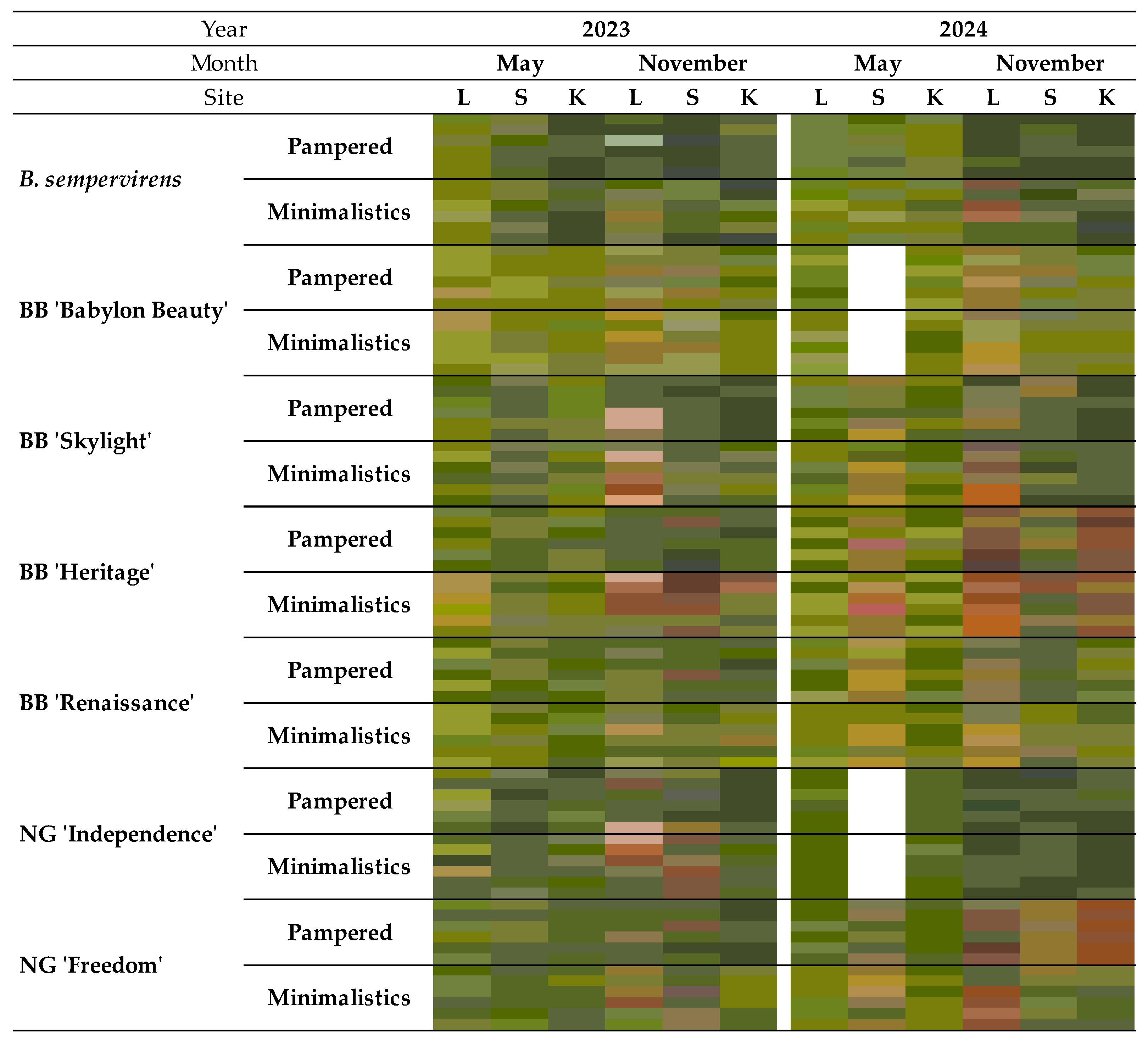

4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BB | BetterBuxus® |
| NG | NewGen® |
| NDVI | Normalized Difference Vegetation Index |
| IPM | Integrated Pest Management |
References
- Batdorf, L.R. Boxwood Handbook—A Practical Guide to Knowing and Growing Boxwood, 3rd ed.; The American Boxwood Society, Greater Valley Publications Inc.: Winchester, VA, USA, 2005; 123p, ISBN 978-1886833012. [Google Scholar]
- Kramer, M.; Guo, Y.; Pooler, M. Ranking resistance of Buxus cultivars to boxwood blight—An integrated analysis. J. Environ. Hortic. 2020, 38, 50–55. [Google Scholar] [CrossRef]
- Lehtijärvi, A.; Doğmuş-Lehtijärvi, H.T.; Oskay, F. Boxwood blight in Turkey: Impact on natural boxwood populations and management challenges. Balt. For. 2017, 23, 274–278. [Google Scholar]
- Shishkoff, N.; Miller, M.E.; Cubeta, M.A. Rooting response of boxwood cultivars to hot water treatment and thermal sensitivity of Calonectria henricotiae and C. pseudonaviculata in diseased boxwood (Buxus spp.). J. Environ. Hortic. 2021, 39, 1–10. [Google Scholar] [CrossRef]
- Lombard, L.; Crous, P.W.; Wingfield, B.D.; Wingfield, M.J. Systematics of Calonectria: A genus of root, shoot and foliar pathogens. Stud. Mycol. 2010, 66, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Gehesquière, B.; Crouch, J.A.; Marra, R.E.; Van Poucke, K.; Rys, F.; Maes, M.; Gobin, B.; Höfte, M.; Heungens, K. Characterization and taxonomic reassessment of the box blight pathogen Calonectria pseudonaviculata, introducing Calonectria henricotiae sp. nov. Plant Pathol. 2016, 65, 37–52. [Google Scholar] [CrossRef]
- Daughtrey, M.L. Boxwood blight: Threat to ornamentals. Annu. Rev. Phytopathol. 2019, 57, 189–209. [Google Scholar] [CrossRef]
- Gehesquière, B. Cylindrocladium buxicola Nom. Cons. Prop. (syn. Calonectria pseudonaviculata) on Buxus: Molecular Characterization, Epidemiology, Host Resistance and Fungicide Control. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2014. [Google Scholar]
- Brand, T.; Beltz, H.; Adhikari, U.; Daughtrey, M.L.; Luster, D.G.; Kong, P.; Hong, C. Evaluation of fungicides for management of boxwood blight caused by Calonectria spp. under field conditions in Northern Germany. J. Plant Dis. Prot. 2023, 130, 325–335. [Google Scholar] [CrossRef]
- LeBlanc, N.; Salgado-Salazar, C.; Crouch, J.A. Boxwood blight: An ongoing threat to ornamental and native boxwood. Appl. Microbiol. Biotechnol. 2018, 102, 4371–4380. [Google Scholar] [CrossRef]
- Khaliq, I.; Brand, T.; Weiland, J.E.; Hong, C.; Daughtrey, M.L.; Shishkoff, N. Investigating Weather Variables Driving Boxwood Blight Epidemics. Plant Pathol. 2024, 73, e13969. [Google Scholar] [CrossRef]
- Šafránková, I.; Holková, L.; Kmoch, M. First report of Cylindrocladium buxicola on box in the Czech Republic. New Dis. Rep. 2012, 25, 5. [Google Scholar] [CrossRef]
- Garibaldi, A.; Bertetti, D.; Ortu, G.; Gullino, M.L. First report of Volutella blight caused by Pseudonectria buxi on Japanese boxwood (Buxus microphylla) in Italy. Plant Dis. 2016, 100, 1499. [Google Scholar] [CrossRef]
- Douglas, S.M. Boxwood Blight—A New Disease for Connecticut and the U.S; The Connecticut Agricultural Experiment Station: New Haven, CT, USA, 2011. [Google Scholar]
- Elmhirst, J.F.; Auxier, B.E.; Wegener, L.A. First report of box blight caused by Cylindrocladium pseudonaviculatum (C. buxicola) in British Columbia, Canada. Plant Dis. 2013, 97, 559. [Google Scholar] [CrossRef] [PubMed]
- Henricot, B.; Culham, A. Cylindrocladium buxicola, a new species affecting Buxus spp., and its phylogenetic status. Mycologia 2002, 94, 980–997. [Google Scholar] [CrossRef] [PubMed]
- Gräfenhan, T.; Schroers, H.J.; Nirenberg, H.I.; Seifert, K.A. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Stud. Mycol. 2011, 68, 79–113. [Google Scholar] [CrossRef]
- Lombard, L.; van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Generic concepts in Nectriaceae. Stud. Mycol. 2015, 80, 189–245. [Google Scholar] [CrossRef]
- Castroagudín, V.L.J.; Weiland, E.; Baysal-Gurel, F.; Cubeta, M.A.; Daughtrey, M.L.; Gauthier, N.W.; LaMondia, J.; Luster, D.G.; Hand, F.P.; Shishkoff, N. One clonal lineage of Calonectria pseudonaviculata is primarily responsible for the boxwood blight epidemic in the United States. Phytopathology 2020, 110, 1845–1853. [Google Scholar] [CrossRef]
- Salgado-Salazar, C.; Shiskoff, N.; LeBlanc, N.; Ismaiel, A.A.; Collins, M.; Cubeta, M.A.; Crouch, J.A. Coccinonectria pachysandricola, causal agent of a new foliar blight disease of Sarcococca hookeriana. Plant Dis. 2019, 103, 1337–1346. [Google Scholar] [CrossRef]
- Špetík, M.; Berraf-Tebbal, A.; Cechova, J.; Eichmeier, A. Occurrence of Pseudonectria foliicola causing Volutella blight on boxwood in Czech Republic. Plant Dis. 2020, 104, 1547. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Bika, R.; Avin, F.A.; Jennings, C.; Simmons, T. Occurrence of Volutella blight caused by Pseudonectria foliicola on boxwood in Tennessee. Plant Dis. 2021, 105, 2014. [Google Scholar] [CrossRef]
- Rivera, Y.; Salgado-Salazar, C.; Veltri, D.; Malapi-Wight, M.; Crouch, J.A. Genome analysis of the ubiquitous boxwood pathogen Pseudonectria foliicola. PeerJ 2018, 6, e5401. [Google Scholar] [CrossRef]
- Shi, F.; Hsiang, T. First report of Pseudonectria buxi causing Volutella blight on boxwood (Buxus sp.) in Beijing, China. Plant Dis. 2014, 98, 1282. [Google Scholar] [CrossRef]
- Brand, T.; Grüner, J. Gehölzkrankheiten; Eugen Ulmer KG: Stuttgart, Germany, 2024; 195p, ISBN 978-3-8186-2052-3. [Google Scholar]
- Hormaetxe, K.; Hernández, A.; Becerril, J.M.; García-Plazaola, J.I. Role of red carotenoids in photoprotection during winter acclimation in Buxus sempervirens leaves. Plant Biol. 2004, 6, 325–332. [Google Scholar] [CrossRef]
- Hughes, N.M. Winter leaf reddening in ‘evergreen’ species. New Phytol. 2011, 190, 573–581. [Google Scholar] [CrossRef]
- Bartíková, M.; Holková, L.; Šafránková, I. Occurrence of boxwood blight (Calonectria pseudonaviculata and C. henricotiae) in historical gardens in the Czech Republic. Eur. J. Plant Pathol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef]
- Van Laere, K.; Heungens, K.; Gehesquière, B.; Leus, L.; Hermans, D.; Van Huylenbroeck, J. Breeding and selection of Buxus for resistance to Calonectria pseudonaviculata. J. Phytopathol. 2019, 167, 363–370. [Google Scholar] [CrossRef]
- Omolehin, O.; Keller, J.; Gouker, F.; Daughtrey, M.; Luster, D.; Pscheidt, J.; Hong, C. Combating an invasive boxwood pathogen—Calonectria pseudonaviculata—In the United States by shifting production to less-susceptible cultivars. Plant Dis. 2023, 107, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Leus, L. Breeding for disease resistance in ornamentals. In Ornamental Crops; Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; Volume 11, pp. 225–246. [Google Scholar] [CrossRef]
- Mekapogu, M.; Jung, J.-A.; Kwon, O.-K.; Ahn, M.-S.; Song, H.-Y.; Jang, S. Recent progress in enhancing fungal disease resistance in ornamental plants. Int. J. Mol. Sci. 2021, 22, 7956. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J.; Bhattarai, K. Ornamental plant breeding: Entering a new era? Ornam. Hortic. 2022, 28, 297–305. [Google Scholar] [CrossRef]
- Van Laere, K.; Hokanson, S.C.; Contreras, R.; Van Huylenbroeck, J. Woody ornamentals of the temperate zone. In Ornamental Crops; Handbook of Plant Breeding; Van Huylenbroeck, J., Ed.; Springer: Cham, Switzerland, 2018; Volume 11. [Google Scholar] [CrossRef]
- Dhakal, K.; Bika, R.; Ghimire, B.; Parajuli, M.; Neupane, S.; Neupane, K.; Addesso, K.; Baysal-Gurel, F. Arthropod and disease management in boxwood production. J. Integr. Pest Manag. 2022, 13, 18. [Google Scholar] [CrossRef]
- Yang, X.; Castroagudín, V.L.; Daughtrey, M.L.; Loyd, A.L.; Weiland, J.E.; Shishkoff, N.; Baysal-Gurel, F.; Santamaria, L.; Salgado-Salazar, C.; LaMondia, J.A.; et al. A diagnostic guide for Volutella blight affecting Buxaceae. Plant Health Prog. 2021, 22, 578–590. [Google Scholar] [CrossRef]
- Shi, F.; Hsiang, T. Volutella blight of boxwood. Landsc. Trades 2012, 34, 16–17. [Google Scholar]
- Budziszewska, M.; Bereś, P.K. The box tree moth Cydalima perspectalis: A review of biology, invasiveness, management practices, and future perspectives of control strategy in Europe. Plant Prot. 2024, 64, 275–286. [Google Scholar] [CrossRef]
- Barker, B.S.; Coop, L.; Hong, C. Potential distribution of invasive boxwood blight pathogen Calonectria pseudonaviculata as Predicted by Process-Based and Correlative Models. Biology 2022, 11, 849. [Google Scholar] [CrossRef]
- Likins, M.; Davis, B.; Anderson, P.; Gillis, B.; Hong, C.X. Flutriafol drench provides season-long protection of boxwood plantings pre-infected by Calonectria pseudonaviculata in the Mid-Atlantic. Plant Dis. 2025, 109, 2185–2196. [Google Scholar] [CrossRef]
- Weiland, J.E.; Beck, B.R.; Brand, T.; Hong, C.; Daughtrey, M.L. Cool Temperatures Favor Growth of Oregon Isolates of Boxwood Blight Pathogen. Plant Dis. 2022, 106, 3421–3430. [Google Scholar] [CrossRef]
- Loyd, L.; Zwart, D. Why is my boxwood orange? Factors that influence winter color change in boxwood foliage in Charlotte, North Carolina, USA. Arboric. Urban For. 2023, 49, 213–229. [Google Scholar] [CrossRef]
- Kureel, N.; Sarup, J.; Matin, S.; Goswami, S.; Kureel, K. Modelling vegetation health and stress using hyperspectral remote sensing data. Model. Earth Syst. Environ. 2021, 8, 733–748. [Google Scholar] [CrossRef]
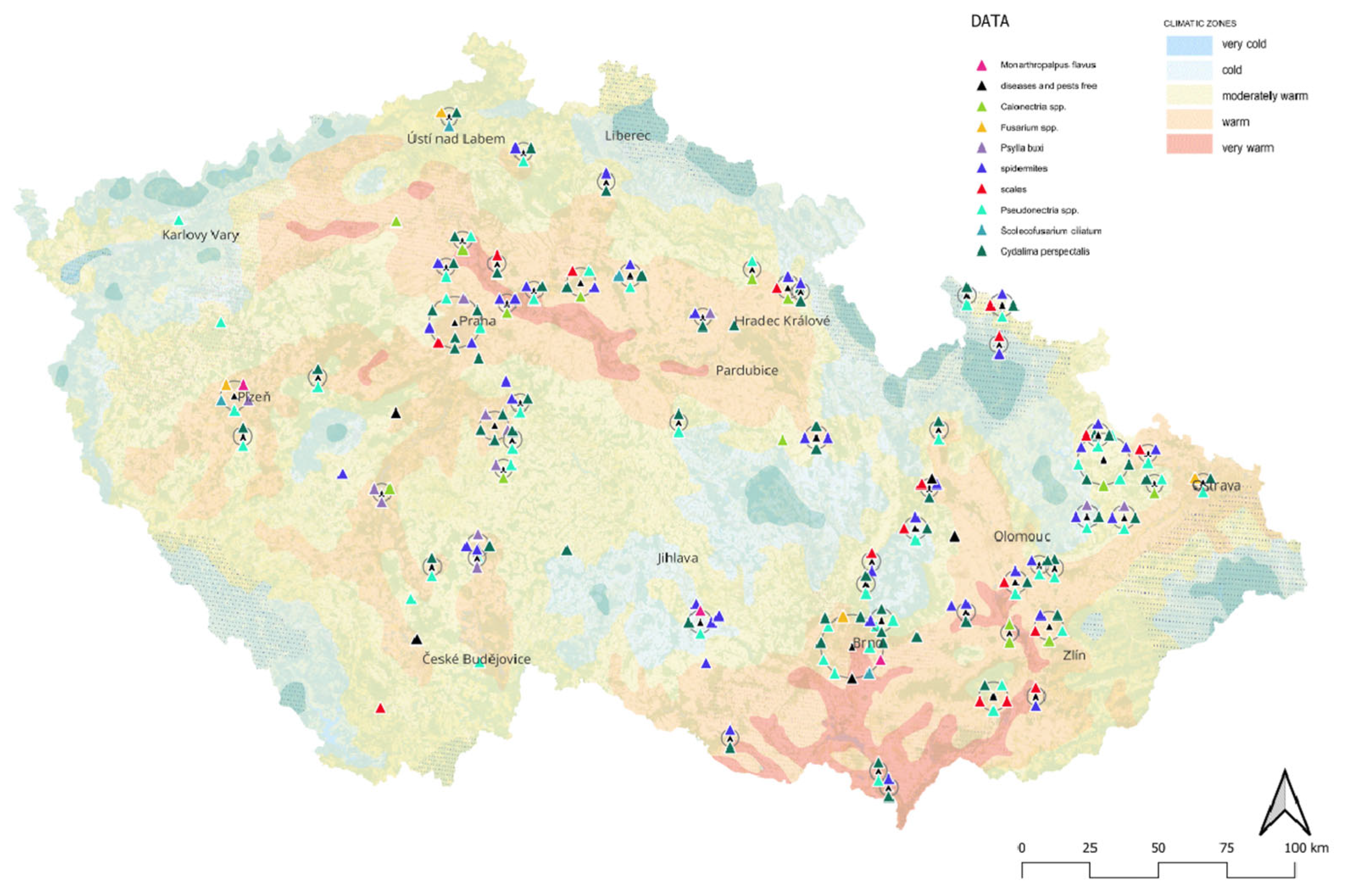


| Variant I “Minimalistic” |
|
| Variant II “Pampered” |
|
| Damage Degree | Volutella Blight | Boxwood Blight |
|---|---|---|
| 1 | healthy plants, no symptoms (0%) | healthy leaves and stems, no signs of infestation (0%) |
| 2 | loss of the leaf gloss, slight change in color, small spots on the edges of the leaves (1–5%) | individual brown spots on leaves, no leaf drop, no stem lesions (1–5%) |
| 3 | spots cover up to half of the leaf blade, leaves turn yellow (6–20%) | spots on leaves cover up to one-half of the blade, do not necrotize, occasional leaf drop, occasional stem lesions (6–20%) |
| 4 | leaves turn bronze, bark at the base of the stem cracks, occasionally peels off (21–35%) | drop of primarily infected leaves, formation of spots on new leaves, black lesions on a third of the stem (21–35%) |
| 5 | beginning of the dieback of the shoots, necrosis of the wood underneath the bark (36–50%) | leaf spots and stem lesions up to half of the plant, severe leaf drop, especially of the inner parts of the plants (36–50%) |
| 6 | dieback of infected shoots or entire plants (51–100%) | new spots occurring on newly grown leaves, stems with numerous long black lesions, almost defoliated plants (51–100%) |
| Cultivar | Time After Inoculation (Days) | The Presence of Symptoms (Score) | Time After Inoculation (Days) | The Presence of Symptoms (Score) | Time After Inoculation (Days) | The Presence of Symptoms (Score) |
|---|---|---|---|---|---|---|
| Buxus sempervirens | 4 | 1.09 ± 0.70 b | 8 | 2.36 ± 0.92 c | 18 | 2.82 ± 0.60 d |
| ‘Babylon Beauty’ | 4 | 1.18 ± 0.41 a | 8 | 1.36 ± 0.51 ab | 18 | 2.00 ± 0.63 bc |
| ‘Skylight’ | 4 | 1.82 ± 0.40 b | 8 | 1.91 ± 0.54 bc | 18 | 2.27 ± 0.47 bd |
| ‘Renaissance’ | 4 | 1.00 ± 0.00 a | 8 | 1.00 ± 0.00 a | 18 | 1.18 ± 0.40 a |
| ‘Heritage’ | 4 | 1.18 ± 0.40 a | 8 | 1.27 ± 0.47 ab | 18 | 1.36 ± 0.50 ac |
| ‘New Gen Independence’ | 4 | 1.14 ± 0.38 a | 8 | 1.29 ± 0.49 ab | 18 | 1.57 ± 0.53 abc |
| ‘New Gen Freedom’ | 4 | 2.20 ± 0.42 b | 8 | 2.10 ± 0.32 bc | 18 | 2.30 ± 0.48 bd |
| Buxus sempervirens—NC * | 4 | 1.00 ± 0.00 a | 8 | 1.00 ± 0.00 a | 18 | 1.00 ± 0.00 a |
| Site | Lednice | Studenec | Kroměříž | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Month | AMT (°C) | AMINT (°C) | NFD (day) | AMT (°C) | AMINT (°C) | NFD (day) | AMT (°C) | AMINT (°C) | NFD (day) |
| 2023 | 1 | 2.9 | −7.9 | 15.0 | 1.4 | −10.6 | 22.0 | 3.2 | −4.6 | 13.0 |
| 2 | 2.6 | −10.5 | 19.0 | 1.0 | −12.1 | 20.0 | 2.3 | −9.3 | 20.0 | |
| 3 | 6.6 | −7.5 | 16.0 | 4.6 | −7.9 | 20.0 | 6.5 | −6.4 | 14.0 | |
| 4 | 8.7 | −7.7 | 4.0 | 6.6 | −8.5 | 12.0 | 8.4 | −5.1 | 6.0 | |
| 5 | 14.6 | −0.5 | 1.0 | 12.6 | −1.1 | 3.0 | 13.7 | 0.5 | N | |
| 6 | 19.5 | 5.2 | N | 17.7 | 1.8 | N | N | N | N | |
| 7 | 22.7 | 8.0 | N | 20.7 | 5.8 | N | N | N | N | |
| 8 | 20.7 | 7.5 | N | 18.8 | 3.8 | N | 20.4 | 7.0 | N | |
| 9 | 18.4 | 6.7 | N | 16.3 | 3.0 | N | 17.7 | 6.9 | N | |
| 10 | 12.8 | −4.2 | 3.0 | 10.6 | −4.1 | 5.0 | 12.4 | −2.3 | 4.0 | |
| 11 | 5.6 | −6.9 | 11.0 | 3.8 | −9.8 | 18.0 | 5.7 | −6.6 | 12.0 | |
| 12 | 1.9 | −18.5 | 20.0 | 0.8 | −21.4 | 24.0 | 2.6 | −5.6 | 17.0 | |
| 2024 | 1 | 0.6 | −13.2 | 24.0 | −1.0 | −14.3 | 28.0 | 0.3 | −15.1 | 25.0 |
| 2 | 7.2 | −3.8 | 8.0 | 5.3 | −5.3 | 9.0 | 7.3 | −4.3 | 6.0 | |
| 3 | 9.0 | −5.2 | 9.0 | 6.8 | −7.0 | 14.0 | 8.9 | −5.0 | 8.0 | |
| 4 | 12.3 | −2.6 | 6.0 | 9.7 | −6.1 | 13.0 | 12.0 | −2.1 | 6.0 | |
| 5 | 16.8 | 3.0 | N | 14.5 | 0.9 | N | 16.6 | 2.6 | N | |
| 6 | 20.8 | 6.6 | N | 18.5 | 3.3 | N | 20.0 | 6.3 | N | |
| 7 | 23.3 | 8.4 | N | 20.6 | 5.8 | N | 22.3 | 9.1 | N | |
| 8 | 23.3 | 7.6 | N | 21.3 | 5.5 | N | 21.9 | 9.1 | N | |
| 9 | 16.5 | −0.1 | 1.0 | 14.9 | −1.7 | 2.0 | 16.1 | 1.5 | N | |
| 10 | 10.8 | −0.8 | 1.0 | 9.2 | −1.3 | 5.0 | 10.5 | −0.1 | 1.0 | |
| 11 | 3.1 | −5.3 | 19.0 | 1.8 | −7.3 | 23.0 | 3.6 | −3.6 | 18.0 | |
| 12 | 1.3 | −7.6 | 22.0 | 0.0 | −8.6 | 25.0 | 1.2 | −8.3 | 23.0 | |
| Variant | Tested Cultivar | NDVI |
|---|---|---|
| “Minimalistic” | BetterBuxus® ‘Babylon Beauty‘ | 0.54 ± 0.07 a |
| BetterBuxus® ‘Renaissance’ | 0.58 ± 0.06 b | |
| BetterBuxus® ‘Skylight‘ | 0.59 ± 0.07 bc | |
| BetterBuxus® ‘Heritage’ | 0.58 ± 0.08 bc | |
| NewGen® ‘Independence’ | 0.60 ± 0.08 c | |
| NewGen® ‘Freedom’ | 0.59 ± 0.07 bc | |
| Bxuxus sempervirens | 0.60 ± 0.08 c | |
| “Pampered” | BetterBuxus® ‘Babylon Beauty‘ | 0.55 ± 0.10 a |
| BetterBuxus® ‘Renaissance’ | 0.61 ± 0.06 bc | |
| BetterBuxus® ‘Skylight‘ | 0.61 ± 0.05 bc | |
| BetterBuxus®’Heritage’ | 0.61 ± 0.09 bc | |
| NewGen® ‘Independence’ | 0.59 ± 0.12 bc | |
| NewGen® ‘Freedom’ | 0.60 ± 0.08 bc | |
| Bxuxus sempervirens | 0.64 ± 0.05 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šafránková, I.; Souček, J.; Machanderová, M.; Salaš, P.; Burgová, J.; Holková, L. Determination of the Resistance of Tolerant Hybrids of Buxus to the Pathogen Cylindrocladium buxicola and the Effect of Nutrition and Climatic Conditions on Leaf Color. Horticulturae 2025, 11, 1256. https://doi.org/10.3390/horticulturae11101256
Šafránková I, Souček J, Machanderová M, Salaš P, Burgová J, Holková L. Determination of the Resistance of Tolerant Hybrids of Buxus to the Pathogen Cylindrocladium buxicola and the Effect of Nutrition and Climatic Conditions on Leaf Color. Horticulturae. 2025; 11(10):1256. https://doi.org/10.3390/horticulturae11101256
Chicago/Turabian StyleŠafránková, Ivana, Jiří Souček, Marie Machanderová, Petr Salaš, Jana Burgová, and Ludmila Holková. 2025. "Determination of the Resistance of Tolerant Hybrids of Buxus to the Pathogen Cylindrocladium buxicola and the Effect of Nutrition and Climatic Conditions on Leaf Color" Horticulturae 11, no. 10: 1256. https://doi.org/10.3390/horticulturae11101256
APA StyleŠafránková, I., Souček, J., Machanderová, M., Salaš, P., Burgová, J., & Holková, L. (2025). Determination of the Resistance of Tolerant Hybrids of Buxus to the Pathogen Cylindrocladium buxicola and the Effect of Nutrition and Climatic Conditions on Leaf Color. Horticulturae, 11(10), 1256. https://doi.org/10.3390/horticulturae11101256







